Anais DelicourtResearch Assistant, Jean-François Bussières, BPharm, MSc, FCSHPDirector, Denis Lebel, BPharm, MSc, FCSHPAssistant Director
The practice of monitoring serum vancomycin concentrations in children remains controversial because of pharmacokinetic variability within and between patients and a lack of guidance from the literature.1–8 Two questions remain unanswered: Should we be measuring the level of this drug in children, and what therapeutic ranges should be targeted?1
At the time of writing (late 2010), the initial vancomycin dosage at the Centre hospitalier universitaire (CHU) Sainte-Justine in Montréal, Quebec, ranged from 10 to 15 mg/kg per dose every 6 or 8 h, with each dose administered slowly by infusion over 1 h. For all patients, trough and peak vancomycin levels are usually measured in association with the third or fourth dose, with the trough being determined immediately before administration and the peak 1 h after infusion is complete. The dosage is then adjusted to achieve the target therapeutic ranges (i.e., 5–10 μg/mL for the trough and 20–40 μg/mL for the peak), according to the pharmacist’s recommendations, which are based on a one-compartment model (Sawchuk–Zaske method) and calculated pharmacokinetic parameters. A local retrospective drug utilization review of vancomycin was conducted in 2010, using data for 30 patients with 5 days or more of therapy. Measured serum concentrations for trough and peak fell within the specified therapeutic ranges in 60% and 33% of the cases, respectively, up to the fifth day of vancomycin treatment (Delicourt A, Lavoie A, Touzin K, Therrien R, Lebel D. A retrospective study of vancomycin therapeutic drug monitoring in pediatrics. Manuscript submitted for publication).
To characterize therapeutic drug monitoring practices for vancomycin in pediatric patients across Canada, we surveyed 13 Canadian pediatric centres in May 2010 using SurveyMonkey. The questionnaire was to be completed by a single clinical pharmacist at each hospital, on the basis of the person’s opinion and pursuant to the centre’s usual practice. Data related to neonatology were excluded. Twelve of the 13 centres responded, for a response rate of 92%.
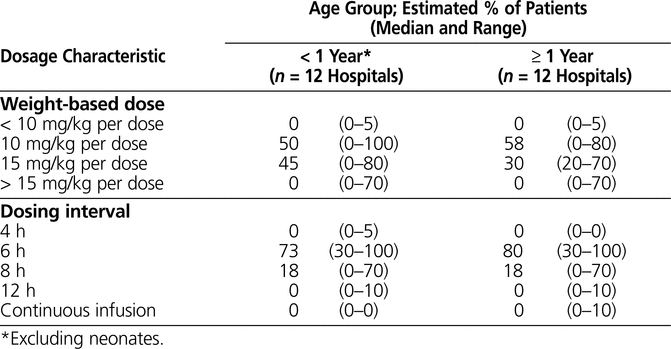
The initial vancomycin dosage used for children varied among the respondents, and the majority of children received the drug every 6 h (Table 1). Eight of the 12 respondents reported that their hospitals did not have a maximum initial dose. Among the remaining hospitals, the maximum dose was 60 mg/kg per day at 3 centres and 100 mg/kg per day at 1 centre.
Table 1 Initial Vancomycin Dosages and Intervals Used for Pediatric Care in Canada
After dose adjustment, a new target trough, with or without measurement of peak levels, was prescribed for a median of 80% (range 30% to 95%) of patients at each hospital (n = 9 respondents). Trough level was measured for a median of 100% (range 50% to 100%) of patients at each hospital (n = 11 respondents), and peak level was measured for a median of 10% (range 0% to 100%) of patients (n = 12 respondents). Of the 12 centres surveyed, 7 reported that they measured peak levels in fewer than 10% of patients.
Although some authors believe that the controversy regarding therapeutic monitoring for vancomycin is over, the results of this survey indicate that pediatric practice varies widely across Canada. Vancomycin is a time-dependent antibiotic. As such, to ensure efficacy, its concentration at the site of action must be maintained, between consecutive doses, at a level higher than the minimum inhibitory concentration. In addition, the relation between toxicity and maximum serum concentration has not been clearly demonstrated.9 Although there are few conclusive data on the superiority of one method over another (i.e., monitoring of peak and trough levels versus monitoring trough levels only), a majority of children receiving vancomycin therapy do not have samples drawn for monitoring of peak levels. In answer to the questions that we posed at the beginning of this letter, we believe that monitoring of peak levels should be limited to life-threatening situations (e.g., meningitis) and patients with altered volume of distribution. Also, the targeted therapeutic range for the trough should be 5 to 15 mg/mL.
More generally, clinicians should balance their actions between efficiency and safety and should periodically reconsider their practice. Such evaluation of clinical practice should include periodic review of the literature, local drug utilization reviews, and benchmarking. Revised guidelines for vancomycin monitoring (i.e., 15 mg/kg per dose in most clinical situations, with monitoring of peak levels limited to exceptions) were adopted by our pharmacy and therapeutics committee, and practice will be monitored over the next 12 months.
1. Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009;66(1):82–98. Erratum in: Am J Health Syst Pharm 2009;66(10):887.
2. Seay RE, Brundage RC, Jensen PD, Schilling CG, Edgren BE. Population pharmacokinetics of vancomycin in neonates. Clin Pharmacol Ther 1994;56(2):169–175. Erratum in: Clin Pharmacol Ther 1995;58(2):142.
3. Tan WH, Brown N, Kelsall AW, McClure RJ. Dose regimen for vancomycin not needing serum peak levels? Arch Dis Child Fetal Neonatal Ed 2002;87(3):F214–F216.
4. Glover ML, Cole E, Wolfsdorf J. Vancomycin dosage requirements among pediatric intensive care unit patients with normal renal function. J Crit Care 2000;15(1):1–4.
5. de Hoog M, Schoemaker RC, Mouton JW, van der Anker JN. Vancomycin population pharmacokinetics in neonates. Clin Pharmacol Ther 2000;67(4):360–367.
6. Jimenez-Truque N, Thomsen I, Saye E, Creech CB. Should higher vancomycin trough levels be targeted for invasive community-acquired methicillin-resistant Staphylococcus aureus infections in children? Pediatr Infect Dis J 2010;29(4):368–370.
7. Crumby T, Rinehart E, Carby MC, Kuhl D, Talati AJ. Pharmacokinetic comparison of nomogram-based and individualized vancomycin regimens in neonates. Am J Health Syst Pharm 2009;66(2):149–153.
8. Taketomo CK, Hodding JH, Kraus DM. Pediatric dosage handbook. 16th ed. Hudson (OH): Lexi-Comp Inc; 2009.
9. Miles MV, Li L, Lakkis H, Youngblood J, McGinnis P. Special considerations for monitoring vancomycin concentrations in pediatric patients. Ther Drug Monit 1997;19(3):265–270.
Anais Delicourt is also a pharmacy intern with the Université de Nantes, Nantes, France. Jean-François Bussières is also a clinical professor in the Faculty of Pharmacy at Université de Montréal, Montréal, Quebec.
Canadian Journal of Hospital Pharmacy, VOLUME 64, NUMBER 2, 2011