Stability of Pantoprazole Sodium in Glass Vials, Polyvinyl Chloride Minibags, and Polypropylene Syringes
Ronald F
Donnelly
ABSTRACT
Background:
Pantoprazole sodium, a proton-pump inhibitor, is approved for the short-term treatment of several types of ulcer, Zollinger–Ellison syndrome, and gastroesophageal reflux disease.
Objective:
To determine the physical compatibility and chemical stability of ethylenediaminetetra-acetic acid (EDTA)–free pantoprazole in glass vials, polypropylene syringes, and polyvinylchloride (PVC) minibags, after storage at 2°C to 8°C with protection from light or at 20°C to 25°C with exposure to light.
Methods:
Solutions of pantoprazole 4 mg/mL reconstituted in 0.9% sodium chloride (normal saline [NS]) were stored in glass vials at 20°C to 25°C. Similar solutions were transferred to polypropylene syringes and stored at 2°C to 8°C. Stock solution was further diluted, in 5% dextrose in water (D5W) or NS, to 0.4 or 0.8 mg/mL, and samples were then packaged in PVC minibags for storage at 2°C to 8°C or at 20°C to 25°C. Samples collected on days 0, 2, 3, 7, 14, 21, and 28 were analyzed in duplicate with a stability-indicating high-performance liquid chromatography assay.
Results:
Pantoprazole 4 mg/mL was stable (i.e., retained at least 90% of initial concentration) for 3 days when stored in glass vials at 20°C to 25°C or for 28 days when stored in polypropylene syringes at 2°C to 8°C. Pantoprazole 0.4 mg/mL diluted in D5W and stored in PVC minibags was stable for 2 days at 20°C to 25°C or for 14 days at 2°C to 8°C. At 0.8 mg/mL, pantoprazole in D5W was stable for 3 days at 20°C to 25°C or 28 days at 2°C to 8°C. Pantoprazole diluted to either 0.4 or 0.8 mg/mL in NS and stored in PVC minibags was stable for 3 days at 20°C to 25°C or 28 days at 2°C to 8°C.
Conclusions:
The present study confirmed or extended previously reported expiry dates for pantoprazole sodium packaged in glass vials, polypropylene syringes, and PVC minibags.
KEYWORDS:
pantoprazole sodium
,
injection
,
dextrose
,
normal saline
,
stability
,
high-performance liquid chromatography
,
glass vials
,
polypropylene syringes
,
polyvinylchloride bags
RÉSUMÉ
Contexte :
L’emploi du pantoprazole sodique, un inhibiteur de la pompe à protons, est approuvé dans le traitement à court terme de divers types d’ulcère, du syndrome de Zollinger-Ellison et du reflux gastro-œsophagien.
Objectif :
Déterminer la compatibilité physique et la stabilité chimique de solutions de pantoprazole sodique exemptes d’acide éthylène diamine tétra acétique (EDTA) conditionnées dans des fioles de verre, des seringues de polypropylène et des minisacs de polychlorure de vinyle (PVC) après avoir été entreposées à 2–8 °C à l’abri de la lumière ou à 20–25 °C exposées à la lumière.
Méthodes :
Des solutions de pantoprazole sodique à 4 mg/mL reconstitué dans du chlorure de sodium à 0,9 % (solution physiologique salée [SP]) ont été entreposées dans des fioles de verre à 20–25 °C. Des solutions similaires ont été transférées dans des seringues de polypropylène, puis conservées à 2–8 °C. La solution de base a été à nouveau diluée soit dans du dextrose à 5 % dans l’eau (D5E), soit dans une SP, à 0,4 ou à 0,8 mg/mL, et des échantillons ont été conditionnés dans des minisacs de PVC entreposés à 2–8 °C ou à 20–25 °C. Les échantillons recueillis aux jours 0, 2, 3, 7, 14, 21 et 28 ont été analysés en double au moyen d’une épreuve validée par chromatographie liquide haute performance mesurant la stabilité.
Résultats :
Les solutions de pantoprazole à 4 mg/mL sont demeurées stables (conservant au moins 90 % de la concentration initiale) pendant trois jours lorsqu’elles étaient entreposées dans des fioles de verre à 20–25 °C ou pendant 28 jours lorsqu’elles étaient conservées dans des seringues de polypropylène à 2–8 °C. Les solutions de pantoprazole à 0,4 mg/mL diluées dans du D5E et conservées dans des minisacs de PVC sont demeurées stables pendant deux jours à 20–25 °C ou pendant 14 jours à 2–8 °C. À une concentration de 0,8 mg/mL, les solutions de pantoprazole dans du D5E sont demeurées stables pendant trois jours à 20–25 °C et pendant 28 jours à 2–8 °C. Les solutions de pantoprazole diluées à une concentration de 0,4 ou de 0,8 mg/mL dans une SP et conservées dans des minisacs de PVC sont demeurées stables pendant trois jours à 20–25 °C ou pendant 28 jours à 2–8 °C.
Conclusions :
Cette étude confirme ou prolonge les durées de conservation publiées précédemment pour les solutions de pantoprazole sodique conditionnées dans des fioles de verre, des seringues de polypropylène et des minisacs de PVC.
MOTS CLÉS:
pantoprazole sodique
,
injection
,
dextrose
,
solution physiologique salée
,
stabilité
,
chromatographie liquide haute performance
,
fioles de verre
,
seringues de polypropylène
,
sacs de polychlorure de vinyle
[Traduction par l’éditeur]
INTRODUCTION
Pantoprazole sodium is a proton-pump inhibitor that can be sed for short-term treatment of duodenal ulcer, gastric ulcer, gastroesophageal reflux disease, and pathologic gastrointestinal hypersecretory conditions associated with Zollinger-Ellison syndrome, and for the prevention of gastrointestinal lesions induced by nonsteroidal anti-inflammatory drugs and duodenal ulcer associated with
Helicobacter pylori
. For patients who are unable to take medications orally, pantoprazole may be given by direct IV administration or as an IV infusion.
In Canada, pantoprazole sodium for injection is formulated with and without ethylenediaminetetra-acetic acid (EDTA). The 2 formulations have previously been studied by Walker and others1,2 and Carpenter and others.3 In one study, pantoprazole sodium 4 mg/mL (with EDTA) packaged in polypropylene syringes was stable for 4 days when stored under refrigeration (3°C to 5°C) or at room temperature (23°C to 25°C).4 A search of the literature revealed no information about the stability of pantoprazole sodium 4 mg/mL packaged in glass vials, and one manufacturer (Sandoz Canada Inc) recommends discarding the unused EDTA-free solution after 6 h.5 Another manufacturer of the EDTA-free product (Pharmascience Inc) recommends using the solution within 24 h of initial puncture of the vial.6
Stability studies may have a variety of aims, including reductions in cost, wastage, and workload. At the authors’ institution, more than 1000 vials of pantoprazole are used monthly; therefore, extending the expiry date of the various forms of unused solution would be beneficial.
This study was undertaken to generate physical compatibility and chemical stability data for solutions of EDTA-free pantoprazole sodium 4 mg/mL stored in glass vials at room temperature with exposure to light, with the ultimate goal of extending the expiry timeframe beyond the manufacturer’s recommendation of 6 h. In addition, the study was designed to confirm previous stability data (with possible extension of the expiry timeframe) for pantoprazole sodium packaged in polypropylene syringes with storage under refrigeration and protection from light. The study also examined the stability of EDTA-free pantoprazole sodium diluted to 0.4 or 0.8 mg/mL in either 5% dextrose in water (D5W) or 0.9% sodium chloride (normal saline [NS]) and packaged in polyvinylchloride (PVC) bags, with storage for 3 days at room temperature with exposure to light or for 28 days under refrigeration with protection from light.
METHODS
Sample Preparation
Stock solutions were prepared by reconstituting a total of 33 commercial vials of EDTA-free pantoprazole sodium for injection (Sandoz Canada Inc, Boucherville, Quebec; lot AD6461, expiry October 2010) with NS (Baxter Corp, Mississauga, Ontario; lot W9C17A1, expiry September 2010), 10 mL per vial, according to the manufacturer’s instructions. All samples were analyzed before these expiry dates. Three of these vials were stored at room temperature (20°C to 25°C) with exposure to light. Stock solution from 6 vials was combined and then divided among three 20-mL polypropylene syringes (Becton and Dickinson Inc, Deerfield, New Jersey; lot 8353531, expiry December 2013) with subsequent storage under refrigeration (2°C to 8°C) with protection from light. Stock solution was further diluted with either D5W or NS, to either 0.4 or 0.8 mg/mL, and was then transferred to a total of 24 PVC minibags. Three bags of each concentration–diluent combination were stored under refrigeration with protection from light or at room temperature with exposure to light.
Physical Compatibility
To monitor the solutions for physical compatibility, samples collected at various time points (as described below) were inspected for clarity against a black background and for colour change against a white background using a 4× illuminated magnifying glass. The pH was determined on each day of analysis with a calibrated pH meter (Accumet 25, Fisher Scientific Ltd, Nepean, Ontario). The meter was calibrated before determination of pH using standardized buffers with pH 7 (Fisher Scientific Ltd; lot SC7134746, expiry May 31, 2009) and pH 10 (Fisher Scientific Ltd; lot 082707, expiry May 2010).
Chemical Stability
High-Performance Liquid Chromatography System
The mobile phase for the high-performance liquid chromatography (HPLC) system was similar to that described by Walker and others1 but was modified to contain 35% acetonitrile (instead of 40%) with 65% phosphate buffer pH 7.1. The phosphate buffer was prepared from 0.05 mol/L sodium phosphate (dibasic) USP (Fisher Scientific Ltd; lot 974557) in HPLC-grade water. The pH of the final mixture was adjusted to 7.1 with concentrated (85%) HPLC-grade phosphoric acid (Fisher Scientific Ltd; lot 082037). The solid phase for the HPLC system was a C18 5-μm, 4.6 × 250 mm column (Luna, Phenomenex Inc, Torrance, California; lot 441292-2). A flow rate of 1 mL/min was delivered with an isocratic delivery pump (model LC-10AS, Shimadzu Corporation, Kyoto, Japan), and the column effluent was monitored at 290 nm with a photodiode array detector (model SPD-M20A, Shimadzu Corporation). Injection volumes of 100 μL were applied to the column with an auto-injector (model Sil-10A
XL
, Shimadzu Corporation). Data were collected and analyzed with Class-VP software (version 7.1, Shimadzu Corporation).
Assay Validation
Forced-degradation samples were used to measure the specificity of the assay method. A stock solution of pantoprazole sodium (Sandoz Canada Inc; lot AD6461, expiry October 2010) was prepared by weighing 30 mg of material and dissolving it in 30 mL of HPLC-grade water. A 10-mL volume of this stock solution was acidified by adjusting the pH to 4.4 with 0.1N hydrochloric acid (Fisher Scientific Ltd; lot 084273). An alkaline degradation sample was prepared by adjusting the pH of a 10-mL volume of the stock solution to about 12.2 with 5N sodium hydroxide (Fisher Scientific Ltd; lot SC6135444). To create an oxidized sample, 0.5 mL of 30% hydrogen peroxide (Fisher Scientific Ltd; lot 043211) was combined with 9.5 mL of stock solution.
At time zero, a portion of the stock solution prepared in HPLC-grade water was diluted 1:10 with mobile phase and was analyzed chromatographically. The acidic and alkaline degradation samples were incubated at 50°C in a hot water bath, and the oxidized sample was stored at 23°C. After 1, 5, 25, 73, 96, 122, and 1128 h, samples were collected from the degradation solutions, diluted 1:10 with mobile phase, and analyzed chromatographically.
Multiwavelength (220 and 290 nm) and ultraviolet (UV) spectral (200–350 nm) analyses were used to determine the purity of all pantoprazole peaks in the degradation samples. Correlation coefficients of the UV spectra were determined by comparing the pantoprazole peak from the degradation samples with the pantoprazole sodium peak in the reference material (Toronto Research Chemicals Inc, Toronto, Ontario; catalogue no. P183000, lot 1-SWM-19-1).
Intraday variation was based on the average area under the curve of 5 replicate samples injected at 3 separate time points on a single day and is reported as the coefficient of variation (CV). The slopes, linear coefficients, and average area under the curve for samples collected on 5 separate days were used to determine the interday variation of the method. Three recovery samples, analyzed in duplicate, obtained on 5 separate days were used to measure the accuracy of the method. The sensitivity of the assay was also determined.
Five known degradation or impurity products of pantoprazole sodium (all obtained from Toronto Research Chemicals Inc) were analyzed for potential interference with the parent compound: pantoprazole
N
-oxide (catalogue no. P183010, lot 11-AZC-121-1), pantoprazole sulphide (catalogue no. P183020, lot 11-SWM-22-1), pantoprazole sulphide
N
-oxide (catalogue no. P183030, lot 11-AZC-115-1), pantoprazole sulphone (catalogue no. P183015, lot 11-AZC-47-1), and pantoprazole sulphone
N
-oxide (catalogue no. P183013, lot 11-AZC-60-1).
Stability Study
A single 5-mL sample was collected from each container (as described in the “Sample Preparation” section above) immediately after packaging and was placed into a clean glass test tube. These samples were designated as day 0 samples. Subsequently, for containers stored at room temperature, samples were collected on days 2 and 3, whereas containers stored under refrigeration were sampled on days 7, 14, 21, and 28. Each sample was analyzed on the day of collection.
On each analysis day, an accurate stock solution (about 1 mg/mL) of pantoprazole sodium was prepared from reference material (Toronto Research Chemicals Inc; catalogue no. P183000, lot 1-SWM-19-1), and a standard curve was prepared by analyzing diluted samples of the stock solution (0.025, 0.05, 0.10, 0.15, and 0.20 mg/mL with 35% acetonitrile). The linearity of all standard curves was assessed by least-squares regression analysis. On each day of analysis, sample solutions were diluted either 1:40 (for 4 mg/mL solutions), 1:8 (for 0.8 mg/mL solutions), or 1:4 (for 0.4 mg/mL solutions) with 35% acetonitrile and then assayed. An internal standard (100 μL of benzocaine 0.2 mg/mL) was added to each diluted sample solution before analysis. Solutions were considered to be chemically stable if the percentage of initial concentration remained above 90%.
RESULTS
Physical Compatibility
No precipitate was apparent when samples were observed under 4× magnification. The samples were colourless at the time of preparation but developed a slight yellow tinge over the course of the study. This change in colour was more evident in the more concentrated solutions. The pH changed only slightly over the study period, with a trend toward greater acidity with time (maximum change in pH −0.79; change in mean pH over time for glass vials, from 9.52 to 9.49; for syringes, from 9.59 to 9.46; for PVC bags with D5W diluent, from 8.94 to 8.30; and for PVC bags with NS diluent, from 9.26 to 8.47).
Chemical Stability
HPLC Assay Validation
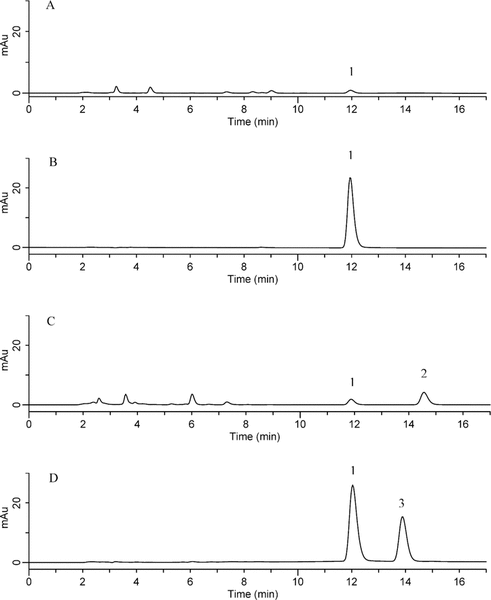
The concentration of the acidic degradation sample decreased to about 4% of the original after 25 h of heating (Figure 1A). Exposure of pantoprazole to alkaline conditions and heating caused a reduction in concentration of about 14% after 1128 h (Figure 1B). After 73 h exposure to oxidation, only 7.1% of the original concentration remained (Figure 1C). Figure 1D shows the chromatogram for pantoprazole 0.4 mg/mL in D5W after 28 days of storage under refrigeration with protection from light. Two new minor peaks appeared during the 28 days of storage. None of the degradation peaks interfered with the parent peak or the peak for the internal standard. The purity of the pantoprazole peaks in the degradation samples was confirmed by multiwavelength and UV spectral analysis. The correlation coefficient was greater than 0.990 for spectral comparison of the parent compound with the reference material.
| |
|
|
|
Figure 1.
A: Chromatogram of an acid-degraded sample of pantoprazole sodium (pH adjusted to 4.4) after heating for 25 h. B: Chromatogram of an alkaline-degraded sample of pantoprazole sodium (pH adjusted to 12.2) after heating for 1128 h. C: Chromatogram of an oxidized sample of pantoprazole sodium after 73 h at room temperature. D: Chromatogram of pantoprazole sodium diluted to 0.4 mg/mL in 5% dextrose in water after refrigeration for 28 days with protection from light. Key to numbering of peaks: peak 1 is pantoprazole sodium, peak 2 is a degradation product, and peak 3 is the internal standard. AU = absorbance units.
|
The intraday CV of 1.30% was based on a comparison of area ratios of pantoprazole sodium to internal standard determined at 0, 5, and 24 h. The interday CVs (for samples collected on 5 separate days) were 2.25% for slope, 0.05% for linear coefficients, and 1.19% for average area. The average recovery ± standard deviation from samples of known concentration was 100.3% ± 1.2%. The calculated sensitivity of the assay for pantoprazole sodium was 10.3 ng.
None of the peaks of the known degradation and impurity compounds interfered with the peak for pantoprazole sodium. The elution times for these peaks were 6.0 min for pantoprazole
N
-oxide, 2.6 min for pantoprazole sulphide, 11.5 min for pantoprazole sulphide
N
-oxide, 15.8 min for pantoprazole sulphone, and 6.9 min for pantoprazole sulphone
N
-oxide, whereas pantoprazole sodium eluted at 12.1 min and the internal standard at 14.0 min.
Stability Study
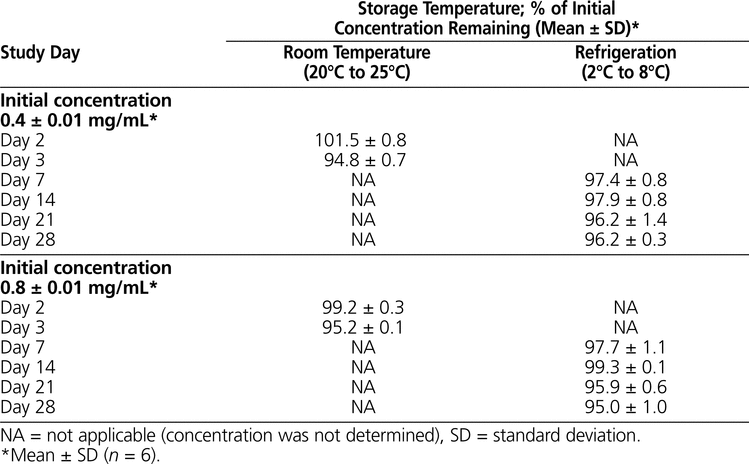
The results of the determination of chemical stability of pantoprazole sodium are summarized in Tables 1 to 4. Pantoprazole sodium 4 mg/mL stored in glass vials at room temperature with exposure to light was stable for 3 days (Table 1). Pantoprazole sodium 4 mg/mL stored in polypropylene syringes under refrigeration with protection from light was stable for 28 days (Table 2). Pantoprazole sodium prepared in D5W at 0.4 mg/mL and stored in minibags was stable for 2 days at room temperature with exposure to light or 14 days under refrigeration with protection from light (Table 3). The more concentrated solution of pantoprazole sodium prepared in D5W (0.8 mg/mL) and stored in minibags was stable for 3 days at room temperature with exposure to light or 28 days under refrigeration with protection from light. Pantoprazole sodium diluted in NS at 0.4 or 0.8 mg/mL and stored in minibags was stable for 3 days at room temperature with exposure to light or 28 days under refrigeration with protection from light (Table 4). As the samples of pantoprazole sodium degraded during the study, 2 new peaks appeared in the chromatograms, with retention times similar to those of 2 peaks in the samples created by forced degradation (Figure 1D).
Table 1.
Stability of Pantoprazole Sodium 4 mg/mL in 0.9% Sodium Chloride after Storage in Glass Vials at Room Temperature (20°C to 25°C) with Exposure to Light
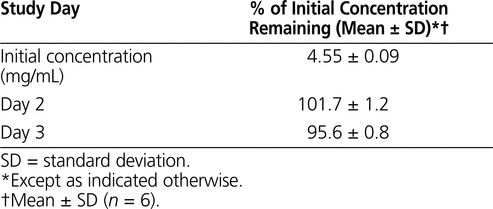
Table 2.
Stability of Pantoprazole Sodium 4 mg/mL in 0.9% Sodium Chloride after Storage in Polypropylene Syringes under Refrigeration (2°C to 8°C) with Protection from Light
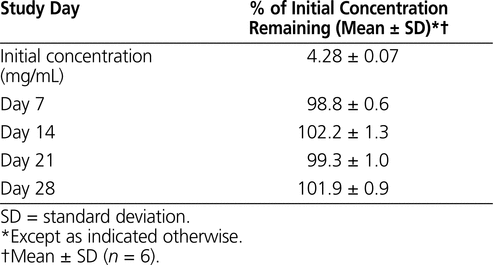
Table 3.
Stability of Pantoprazole Sodium in 5% Dextrose in Water after Storage in Polyvinylchloride Minibags
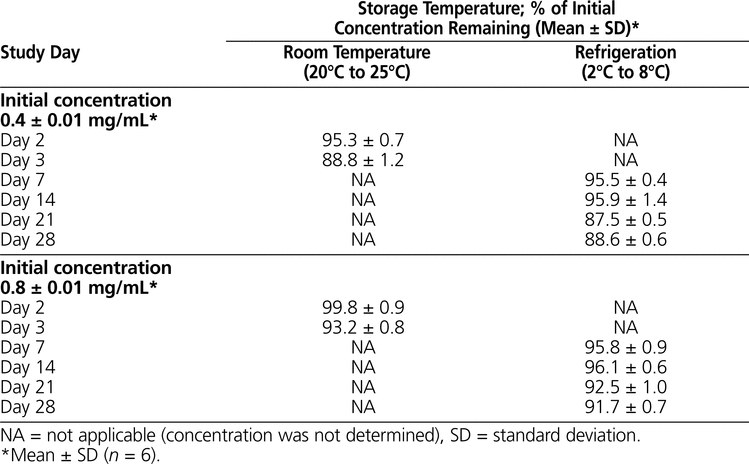
Table 4.
Stability of Pantoprazole Sodium in 0.9% Sodium Chloride after Storage in Polyvinylchloride Minibags
DISCUSSION
To the author’s knowledge, this is the first reported study of the stability of pantoprazole sodium stored in glass vials, although one manufacturer5 recommends using the reconstituted solution and any further dilutions within 6 h of initial puncture of the vial stopper.
Walker and others1 investigated the stability of pantoprazole sodium, in a formulation containing EDTA, when further diluted with either D5W or NS and packaged in PVC bags. These authors suggested a shorter expiry timeframe than is reported here, because they allowed the solutions to be stored at room temperature for a certain amount of time after refrigerated storage, which led to faster degradation. In another study,2 the same authors examined the stability of EDTA-free pantoprazole sodium obtained from another manufacturer and found that solutions diluted to 0.4 and 0.8 mg/mL in NS were stable for 48 h at 23°C or 14 days at 4°C. In that study, the concentration remained at 100% after 14 days, which supports the finding reported here that both concentrations of pantoprazole prepared in NS were stable for 28 days at 5°C. Carpenter and others3 found that an EDTA-free product was stable for 48 h when diluted to 0.4 mg/mL with either D5W or NS and stored at 22°C. The duration of stability of 0.4 mg/mL solutions prepared in D5W was the same in that study and the one reported here (2 days at room temperature); the duration of stability of solutions prepared in NS was 1 day longer in the current study (3 days at room temperature) than in the earlier study, at which point about 94% of the original concentration remained. In the study by Johnson,4 the concentration of pantoprazole sodium in polypropylene syringes was greater than 99% of the original concentration after 4 days of storage at either 3°C to 5°C or 23°C to 25ºC. In the study reported here, similar solutions were stable for 28 days when stored under refrigeration.
In this study, pantoprazole seemed to be more stable in NS than in D5W at both concentrations (0.4 and 0.8 mg/mL). Both Walker and others1 and Carpenter and others3 observed the same trend. In both the study by Walker and others1 and the study reported here, stability was concentration-dependent, with more concentrated solutions being more stable at both storage temperatures.
All solutions were initially colourless when viewed under 4× magnification against a white background; however, over time the solutions developed a slight yellow shade, which was more apparent in the more concentrated solutions. Johnson4 reported a similar finding. Neither study found any association between the colour change and a decrease in concentration of the pantoprazole.
CONCLUSIONS
Pantoprazole sodium (Sandoz Canada Inc, 4 mg/mL) stored in glass vials was stable for 3 days at room temperature (20°C to 25°C) with exposure to light. When packaged in polypropylene syringes, pantoprazole (4 mg/mL) was stable for 28 days with storage under refrigeration (2°C to 8°C) with protection from light. Pantoprazole diluted to 0.4 mg/mL in D5W and stored in PVC minibags was stable for 2 days at room temperature (20°C to 25°C) with exposure to light or 14 days under refrigeration (2°C to 8°C) with protection from light. Pantoprazole 0.8 mg/mL in D5W had beyond-use dates of 3 days at room temperature (20°C to 25°C) with exposure to light and 28 days under refrigeration (2°C to 8°C) with protection from light. Pantoprazole diluted to 0.4 and 0.8 mg/mL in NS and stored in PVC minibags was stable for 3 days at room temperature (20°C to 25°C) with exposure to light or 28 days under refrigeration (2°C to 8°C) with protection from light.
Institutions should base the final expiry date for pantoprazole on their own results for sterility testing and aseptic validation, as well as the chemical stability of the drug.
References
1.
Walker S, Iazzetta J, Law S. Extended stability of pantoprazole for injection in 0.9% sodium chloride or 5% dextrose at 4°C and 23°C.
Can J Hosp Pharm
2009;62(2):135–141.
2.
Walker SE, Iazzetta J, Law S. Stability of pantoprazole in 0.9% sodium chloride (NS) at 4°C and room temperature (24°C) [abstract]. C
an J Hosp Pharm
2005;58 Suppl 2:S34.
3.
Carpenter JF, McNulty MA, Dusci LJ, Ilett KF. Stability of omeprazole sodium and pantoprazole sodium diluted for intravenous infusion.
J Pharm Tech
2006;22:95–98.
4.
Johnson CE. Stability of pantoprazole in 0.9% sodium chloride injection in polypropylene syringes.
Am J Health Syst Pharm
2005;62(22): 2410–2412.

5.
Pantoprazole sodium for injection [product monograph]. Boucherville (QC): Sandoz Canada Inc; 2008 Feb 17.
6.
Pantoprazole sodium for injection [product monograph]. Montreal (QC): Pharmascience Inc; 2009 May 5.
Ronald F Donnelly
, MSc(Chem), BSc(Pharm), is Product Development Pharmacist with the Department of Pharmaceutical Sciences, The Ottawa Hospital (Civic Campus), Ottawa, Ontario
Acknowledgements
This project was funded by an unrestricted research grant from Sandoz Canada.
Address correspondence to: Ronald F Donnelly, Department of Pharmaceutical Sciences, The Ottawa Hospital, 1053 Carling Avenue, Ottawa ON K1Y 4E9, e-mail:
rdonnelly@ottawahospital.on.ca
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
3
,
May-June
2011