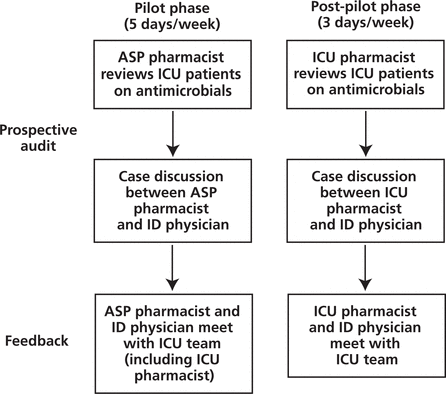
Figure 1. Prospective audit and feedback model within an antimicrobial stewardship program (ASP). ICU = intensive care unit, ID = infectious diseases.
Valerie Leung , Suzanne Gill , Jaclyn Sauve , Kelly Walker , Carmine Stumpo , Jeff PowisABSTRACT
Background:
Promoting the appropriate use of antimicrobials is a core value of antimicrobial stewardship. Prospective audit and feedback constitute an effective strategy for reducing the cost and use of antimicrobials, as well as their adverse effects, such as infection with Clostridium difficile.
Objective:
To evaluate the antimicrobial stewardship program in the intensive care unit at the authors’ hospital, in order to determine the cost and utilization of antimicrobials, as well as the rate of nosocomially acquired C. difficile infection.
Methods:
An infectious diseases team, consisting of a physician and a pharmacist, performed prospective audit and feedback during a pilot study (April to June 2010). The team met with the intensive care unit team daily to discuss optimization of therapy. The cost and utilization of antimicrobial drugs, as well as rates of C. difficile infection, were compared between the pilot period and the same period during the previous year (April to June 2009). For 3 months after the pilot phase (i.e., July to September 2010), the strategy was continued 3 days per week.
Results:
After introduction of the antimicrobial stewardship program, there was a significant reduction in the cost of antimicrobial drugs: $27 917 less than during the same period in the previous year, equivalent to a reduction of $15.45 (36.2%) per patient-day ($42.63 versus $27.18). Utilization of broad-spectrum antipseudomonal antimicrobial agents was also significantly lower, declining from 63.16 to 38.59 defined daily doses (DDDs) per 100 patient-days (reduction of 38.9%). After the pilot period, the rate declined further, to 28.47 DDDs/100 patient-days. During the pilot period, there were no cases of C. difficile infection, and in the post-pilot period, there was 1 case (overall rate 0.42 cases/1000 patient-days). This rate was lower than (but not significantly different from) the rate for April to September 2009 (1.87 cases/1000 patient-days). There were no differences in mortality rate or severity of illness.
Conclusion:
The antimicrobial stewardship program in this community hospital was associated with significant decreases in antimicrobial costs and in utilization of antipseudomonal antimicrobial agents and a nonsignificant decrease in the rate of C. difficile infection. Knowledge exchange, peer-to-peer communication, and decision support, key factors in this success, will be applied in implementing the antimicrobial stewardship program throughout the hospital.
KEYWORDS: antimicrobial stewardship , prospective audit and feedback , Clostridium difficile
RÉSUMÉ
Contexte :
La promotion de l’utilisation appropriée des antimicrobiens est l’une des pierres angulaires de la gestion responsable des antimicrobiens. Les vérifications prospectives et la rétroaction constituent une stratégie efficace pour réduire le coût et l’utilisation des antimicrobiens ainsi que leurs effets indésirables, comme l’infection à Clostridium difficile .
Objectifs :
Examiner le programme de gestion responsable des antimicrobiens à l’unité de soins intensifs de l’hôpital des auteurs, afin de déterminer le coût et l’utilisation des antimicrobiens de même que le taux d’infections nosocomiales à C. difficile .
Méthodes :
Une équipe des maladies infectieuses formée d’un médecin et d’un pharmacien ont effectué une vérification prospective et une rétroaction durant l’étude pilote (avril à juin 2010). L’équipe a rencontré l’équipe de l’unité de soins intensifs quotidiennement pour discuter de l’optimisation du traitement. Le coût et l’utilisation des antimicrobiens de même que les taux d’infections à C. difficile compilés pour la période de l’étude ont été comparés à ceux obtenus durant la même période l’année précédente (avril à juin 2009). Pendant les trois mois suivant l’étude pilote (c.-à-d. de juillet à septembre 2010), la stratégie a été poursuivie à raison de trois jours par semaine.
Résultats :
On a observé après la mise en place du programme de gestion responsable des antimicrobiens une diminution significative du coût des antimicrobiens : 27 917 $ de moins qu’à la même période de l’année précédente, soit une réduction de 15,45 $ (36,2 %) par journée-patient (42,63 $ contre 27,18 $). Le recours à des antibactériens antipseudomonas à large spectre a également grandement diminué, passant de 63,16 à 38,59 doses journalières définies ( Defined Daily Dose [ DDD ]) par 100 journées-patients (réduction de 38,9 %). Après l’étude pilote, ce taux a continué de baisser, passant à 28,47 DDD par 100 journées-patients. Durant l’étude pilote, aucun cas d’infection à C. difficile n’a été observé et durant la période suivant l’étude pilote, un cas a été observé (taux global de 0,42 cas par 1000 journées-patients). Ce taux s’est avéré inférieur (quoique non significativement différent) au taux observé entre avril et septembre 2009 (1,87 cas par 1000 journées-patients). Aucune différence dans le taux de mortalité ou la gravité de la maladie n’a été notée.
Conclusions :
Le programme de gestion responsable des antimicrobiens de cet hôpital communautaire a été associé à des diminutions significatives du coût des antimicrobiens et de l’utilisation des antimicrobiens antipseudomonas et à une diminution non significative du taux d’infections à C. difficile . Le partage des connaissances, l’échange entre les pairs et les outils d’aide à la décision, des facteurs clés du succès de ce programme, seront utilisés dans la mise en œuvre du programme de gestion responsable des antimicrobiens à l’échelle de l’hôpital.
MOTS CLÉS: gestion responsable des antimicrobiens , vérification prospective et rétroaction , Clostridium difficile
[Traduction par l’éditeur]
A variety of international and local groups advocate for judicious use of antibiotics. In 2007, the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) published evidence-based guidelines for developing an institutional program to enhance antibiotic stewardship.1 These guidelines included 2 core strategies (prospective audit with intervention and feedback; formulary restriction and preauthorization), as well as recommendations for supplemental activities.
The process of prospective audit and feedback is an active intervention whereby an infectious diseases physician or clinical pharmacist reviews each patient’s antimicrobial therapy and communicates feedback on how to improve therapy to the prescriber.1 Randomized controlled trials have shown that this process is effective in reducing inappropriate use of antimicrobials. The outcomes of these trials have included overall reduction in the inappropriate use of antimicrobials,2 fewer days of parenteral therapy,3,4 decreased costs,3,5 and decreased rates of Clostridium difficile infection and other nosocomial infections.4
Although prospective audit and feedback programs are effective, they are also resource-intensive. Comprehensive programs in large teaching centres, as recently described by Dresser and Nelson,6 may not be feasible in community hospitals because of limitations in the availability and allocation of resources. Despite these challenges, antimicrobial stewardship remains an important initiative for community hospitals. This paper describes the implementation and evaluation of an antimicrobial stewardship initiative in a 490-bed community teaching hospital located in East Toronto, Ontario.
The main objectives of the antimicrobial stewardship program at this hospital were to reduce the use of and expenditures on antimicrobials and to reduce rates of nosocomially acquired C. difficile infection. Therefore, for the purposes of this evaluation, utilization and costs of antimicrobials and rates of C. difficile infection were monitored. Infection with C. difficile was chosen as a primary outcome because it is an important unintended consequence of antimicrobial use that is associated with poor outcomes and health care costs. Other nosocomial infections caused by drug-resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA) are also of interest. However, the impact of an antimicrobial stewardship program on antimicrobial resistance patterns would not be observable by the end of a 3-month pilot project, so such outcomes were not included in this study. A secondary objective was the rate of acceptance of recommendations by prescribers.
A 3-month pilot project (April 1 to June 30, 2010) was conducted on a designated patient care unit where prospective audit and feedback would be the core strategy for antimicrobial stewardship. The hospital’s 12-bed combined medical and surgical intensive care unit (ICU) was selected for the pilot project, as requested by the ICU care team. The recent trend of escalating antimicrobial use in the ICU also made this patient care unit an attractive location for the pilot project.
Before commencement of the pilot project, educational material highlighting the importance of an antimicrobial stewardship program was developed, and in-service sessions for ICU nursing and pharmacy staff were provided. An infectious diseases physician also met with the ICU care team to highlight the importance of the program and to develop a process for prospective audit and feedback.
An infectious diseases team consisting of a physician (J.P.) and a pharmacist carried out prospective audit and feedback in the ICU. During the pilot period, the role of the antimicrobial stewardship pharmacist was covered on a rotating basis by 4 pharmacists (V.L., S.G., J.S., K.W.), 2 of whom also covered the ICU rotation (J.S., K.W.). On usual working days (Monday to Friday), the designated antimicrobial stewardship pharmacist used a standardized data collection tool to independently review all patients in the ICU who were receiving antimicrobials. The pharmacist then met with the infectious diseases physician to discuss and review these individual cases. Then, the pharmacist and the physician met in person with the ICU care team, including the ICU pharmacist, to review patients receiving antimicrobials and to provide feedback on how to optimize antimicrobial therapy in these cases.
Following the initial 3-month pilot period, a modified prospective audit and feedback strategy was continued. In the post-pilot phase, the antimicrobial stewardship role was fulfilled by the pharmacist covering the ICU rotation on a modified 3-day schedule in the ICU (Monday, Tuesday, and Thursday) (Figure 1).
|
|
||
|
Figure 1. Prospective audit and feedback model within an antimicrobial stewardship program (ASP). ICU = intensive care unit, ID = infectious diseases. |
||
Recommendations made by the antimicrobial stewardship team to the ICU care team were defined a priori as the following: dose adjustment for renal or hepatic impairment, dose optimization for indication, optimization of duration of therapy, discontinuation of therapy, de-escalation of therapy, or change to a broader-spectrum agent or a different agent for empiric coverage. An example of dose optimization for indication was recommending an increase in the dose of metronidazole for suspected cases of C. difficile infection. Recommendations related to optimizing the duration of therapy were suggestions to increase or decrease the length of therapy for a specific indication, for example, recommending a prolonged course of treatment for empyema or recommending a shorter duration of therapy for hospital-acquired pneumonia in a clinically stable patient with signs and symptoms of improvement. Conversely, a recommendation to discontinue therapy would be made when there was no clear cause of infection requiring treatment. One common example of this type of recommendation was discontinuing antimicrobial therapy in patients who had no clinical signs and symptoms of urinary tract infection. The purpose of recommendations to de-escalate therapy was to streamline empiric therapy on the basis of results of culture and sensitivity testing and clinical criteria. Finally, recommendations to change to a broader-spectrum or different agent captured cases in which empiric therapy was thought to be inadequate or where a different agent was thought to be better for the clinical situation.
Expenditures for and utilization of antimicrobials, as well as frequency of nosocomially acquired C. difficile infection, were the primary outcomes in this study. Data on expenditures and utilization were collected for the 3-month pilot period (April 1 to June 30, 2010) and were then compared with data for the same 3 months in the previous year (April 1 to June 30, 2009). This specific comparator period was selected to avoid confounding of the data by seasonal variations in antimicrobial usage and patient mix. The same metrics were also collected during the post-pilot phase (July 1 to September 30, 2010) to confirm that the modified strategy remained effective. Data for cases of hospital-acquired C. difficile infection were collected for a 1-year period before implementation of the antimicrobial stewardship program and during both the pilot and post-pilot phases. Mortality and severity of illness were monitored for ICU patients throughout the pilot and post-pilot periods. Outcomes were compared before and after implementation of the stewardship program using the χ2 test for categorical variables and unpaired t tests for continuous variables.
Data for antimicrobial expenditures were obtained from the institution’s financial database and are presented as antimicrobial costs per patient-days. Specifically, the defined daily dose (DDD) of the World Health Organization was used to capture antimicrobial utilization.7 The DDDs were calculated from the pharmacy’s monthly inventory data, according to the number of doses dispensed, and are presented as DDDs per 100 patient-days. Nosocomially acquired cases of C. difficile infection were identified by the institution’s Infection Prevention and Control team, which used the standard definition of the Ontario Ministry of Health and Long-Term Care (MOHLTC). Deaths of ICU patients were captured from the MOHLTC Critical Care Information System database. The complexity of cases was assessed with the Multi-Organ Dysfunction Score, also obtained from the Critical Care Information System database.
During the pilot period (April 1 to June 30, 2010), there were a total of 1127 patient-days in the ICU. The antimicrobial stewardship team made a total of 142 recommendations, for which the overall acceptance rate was 94% (Table 1). The most common recommendations were to discontinue therapy or optimize the duration of therapy.
Table 1.
Types of Recommendations Made by the Antimicrobial Stewardship Team*
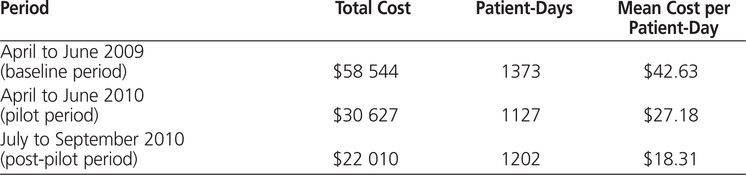
Reductions in antimicrobial costs were realized after implementation of the antimicrobial stewardship program (Table 2). Compared with the same period in 2009, when there were 1373 patient-days in the ICU, there was an absolute reduction of $27 917 in costs for antimicrobials. When adjusted for patient volume, this translated to a decrease of $15.45 (36.2%) in average antimicrobial cost per patient-day ($42.63 versus $27.18). In the post-pilot phase (July 1 to September 30, 2010), during which there were 1202 patient-days, the average antimicrobial cost was $18.31 per patient-day. The average antimicrobial costs decreased significantly after implementation of antimicrobial stewardship ( p = 0.024).
Table 2.
Comparison of Antimicrobial Costs in the Intensive Care Unit Before and After Implementation of Prospective Audit and Feedback
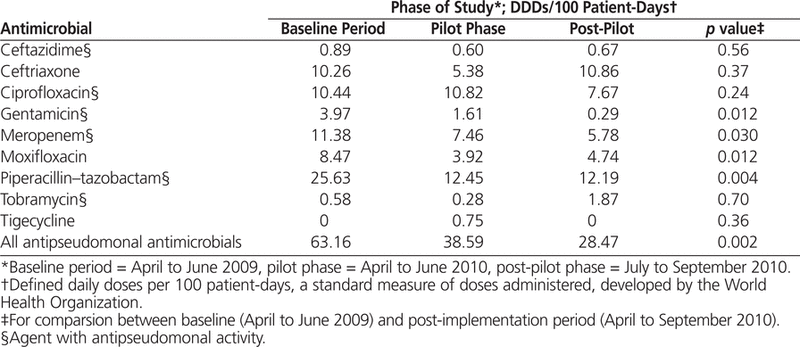
Data on the utilization of specific antimicrobials are presented in Table 3. During the pilot phase, utilization of broad-spectrum antipseudomonal antimicrobials was 38.59 DDDs/100 patient-days, less than the 63.16 DDDs/100 patient-days during the baseline period. This translated to an absolute reduction of 24.57 DDDs/100 patient-days (38.9%). In the post-pilot phase, utilization of broad-spectrum anti-pseudomonal antimicrobials was 28.47 DDDs/100 patient-days. After introduction of the stewardship program, there were significant decreases in the use of gentamicin, piperacillin–tazobactam, meropenem, moxifloxacin, and antipseudomonal antimicrobials (Table 3).
Table 3.
Comparison of Utilization of Broad-Spectrum and Antipseudomonal Antimicrobials
The baseline rate of C. difficile infection in the ICU for the 1-year period preceding implementation of the stewardship program was 1.42 cases/1000 patient-days (based on 8 cases). More specifically, the rate between April 1 and September 30, 2009, was 1.87 cases/1000 patient-days (based on 5 cases). During the pilot phase, there were no cases of C. difficile infection, and during the 3 month post-pilot phase, there was 1 case, which represented a post-implementation rate of 0.42/1000 patient-days. Altogether, these data represent a non-significant decrease in hospital-acquired C. difficile infection after implementation of the stewardship program ( p = 0.19).
No adverse effects on patient mortality were noted after implementation of the antibiotic stewardship program. The baseline mortality rate in the ICU (from April 1 to September 30, 2009) was 14.6% (95% confidence interval [CI] 12.5%–17.5%), similar to the rate of 14.5% (95% CI 12.4%–17.4%) after implementation (from April 1 to September 30, 2010). Severity of illness as measured by the mean Multi-Organ Dysfunction Score was also similar at baseline and after implementation of antimicrobial stewardship: 2.90 (95% CI 2.42–3.57) versus 2.86 (95% CI 2.50–3.39).
Previous studies of comprehensive antimicrobial stewardship programs have demonstrated decreases in antimicrobial use of 22% to 37%.2–4 This ICU pilot study showed that implementing a prospective audit and feedback strategy in a community hospital is feasible and that substantial reductions in antimicrobial use can be achieved.
Various measures for evaluating an antimicrobial stewardship program were described in the IDSA–SHEA guidelines.1 At the institution where the current study was conducted, a combination of process and outcome measures was selected because of the relatively short duration of the pilot study and technological constraints. A long-term goal of this program is to evaluate the impact of a reduction in antimicrobial use on antimicrobial resistance. However, reductions in antimicrobial resistance lag behind reductions in antimicrobial use, so it would have been impractical to attempt evaluation of any effect on resistance rates after only 6 months of follow-up.
Although there were limitations to the financial analysis, financial data on drug expenditures represent an important metric for a hospital’s administration. At this institution, antimicrobials make up roughly 27% of the drug budget for the ICU; therefore, reductions in expenditures for this group of drugs can have a substantial impact on the overall ICU drug budget. During the 6 months over which the stewardship program was implemented, antimicrobial costs were projected at roughly $102 000, yet actual expenditures were only $52 637. This represents a 48% difference, which would translate to an annualized saving of $98 726 in the ICU alone. This difference represents about 14% of the hospital’s overall antimicrobial budget ($700 000 in 2009/2010).
Unpredictable fluctuations in drug acquisition costs represent a major limitation in basing an evaluation on financial data alone. Therefore, the antimicrobial stewardship program was also evaluated in terms of DDDs per 100 patient-days, on the basis of pharmacy charge data for selected antimicrobials. During the pilot and post-pilot periods, there were no releases of generic versions of antimicrobials that would account for the reduction in costs observed after introduction of the stewardship program. This institution uses computerized physician order entry and electronic medication administration records; however, it has yet to develop an automated method of extracting data for DDDs per 100 patient-days or days of therapy according to the doses administered to patients. Although calculating DDDs per 100 patient-days by the method used in this study may not accurately capture patients’ exposure to the drugs (which is more accurately estimated with days of therapy), we felt it was important to have a metric measuring antimicrobial utilization, independent of changes in numbers of patients or drug acquisition costs. Despite these limitations, we are confident that data for both costs and utilization indicate that the antimicrobial stewardship program had a positive impact within this organization.
This evaluation of the antimicrobial stewardship program was observational in nature, which might have led to potential biases influencing the validity of the results. Staff in the ICU were aware of the intervention, as well as the outcomes being measured. As a result, they may have modified their behaviours simply because an evaluation was in progress. This form of bias is common to quasi-experimental research.8 We attempted to account for the impact of the antimicrobial stewardship program by evaluating the interventions made through this program. The number of interventions implemented strongly suggests that the observed reductions in cost and utilization of antimicrobials resulted from the program, not from behaviour modification related to the evaluation.
Implementing an antimicrobial stewardship program in a midsized community hospital presented unique challenges that may be different from those that would be experienced in a large teaching centre. Initially, no funding was available for the pilot, so the plan was to implement the pilot project while simultaneously developing a business case to request funding. An antimicrobial stewardship committee consisting of representatives from Infection Control, Information Technology Services, and Pharmacy was created as a subgroup reporting to the Pharmacy and Therapeutics Committee. The pilot study proceeded with in-kind resources provided by Pharmacy, Infection Control, and the physician lead, while the administrative team considered the business case. Hospital funding for a physician (0.5 full-time equivalent [FTE]) and a pharmacist (1.0 FTE) was made available to the project partway through the pilot period. This funding was provided to support a hospital-wide antimicrobial stewardship program.
The IDSA and the SHEA recommended that a core member of the multidisciplinary antimicrobial stewardship team be a clinical pharmacist with infectious diseases training.1 Like many community hospitals, the institution where this study was conducted did not have an individual with this type of expertise on staff, nor was there a pharmacist covering the inpatient Infectious Disease Service before implementation of the antimicrobial stewardship program. Since the pilot study was initiated with in-kind resources, the antimicrobial stewardship role was fulfilled on a rotational basis by 4 pharmacists with various clinical and educational backgrounds, none of whom had formal infectious diseases training. The hospital’s current model of ICU pharmacist coverage, whereby 2 pharmacists rotate on a weekly to biweekly basis, allowed these pharmacists to cover the antimicrobial stewardship role during off-service weeks. The other 2 pharmacists covering the stewardship rotation were the Infectious Disease/HIV clinic pharmacist and the clinical manager. Where possible, the role was rotated on a weekly basis to facilitate continuity. During the pilot phase, 2 pharmacists attended an antimicrobial stewardship certification program (presented by the not-for-profit educational organization MAD-ID [Making a Difference in Infectious Diseases]), the clinical manager took full responsibility for analyzing metrics associated with antimicrobial cost and utilization, and the infectious diseases physician was responsible for reporting patient outcomes such as mortality and nosocomial infection rates. This distribution of roles allowed us to carry out antimicrobial stewardship with existing resources and expertise. This unique approach also promoted knowledge exchange among the 4 pharmacists, which we felt was important to the sustainability of the program beyond the pilot period. In the post-pilot phase, prospective audit and feedback were carried out on a modified 3-day per week schedule by the on-service ICU pharmacist, which allowed reallocation of both physician and pharmacist resources to expand the antimicrobial stewardship program beyond the ICU.
Aside from the unique model as described here, we identified 2 other key learning points that were pivotal to the success of the antimicrobial stewardship program in our hospital. The first was staff acceptance, an extremely important element in building a sustainable stewardship program. We used a peer-to-peer approach to educate nursing, pharmacy, and physician staff about the pilot program. For example, in-service sessions for ICU nursing staff were conducted by an infection control practitioner with a nursing background, as well as by the ICU pharmacists, with whom nursing staff already had a working relationship. The antimicrobial stewardship physician, with assistance from the stewardship pharmacist, conducted the prospective audit and feedback with the intensivists in a face-to-face setting. The clinical manager provided regular project updates to pharmacists during staff meetings. Traditional channels such as the hospital newsletter were also used. We believe that peer-to-peer communication strategies were especially useful, contributing to a high rate of acceptance of recommendations in this pilot program and increasing the profile of the antimicrobial stewardship program within the institution.
The second key learning point was related to decision support. Because the hospital was using computerized physician order entry and electronic medication administration records, a number of ideas on how to leverage the system emerged during the pilot phase of the antimicrobial stewardship program. For example, we implemented an electronic antimicrobial rounding report, which enabled documentation of stewardship recommendations. We also created an automated alert to notify prescribers when antimicrobials are ordered for a patient with a recent history of C. difficile infection. As we expand the program across the institution, we will need to become more efficient at directing resources and are currently exploring the use of order entry fields such as indication and duration of therapy to help the antimicrobial stewardship team identify priority cases in which prospective audit and feedback will have the greatest impact.
Because of the relatively short evaluation timeframe of the antimicrobial stewardship program, there were several limitations worthy of discussion. After 6 months, the impact of the program on important outcomes such as antimicrobial resistance remained unknown. Our plan is to review resistance rates in the ICU at least 1 year after implementation. Although we were encouraged by the further decreases in antimicrobial cost and utilization in the post-pilot phase and believe that these results support our modified strategy, our expectation, based on previous studies, is that both of these measures will eventually revert to the levels observed during the baseline period. In addition, it is not possible to predict whether the lower rates of C. difficile infection will be sustained over time and how much effect antimicrobial stewardship might have on this type of infection, since such infections are subject to other factors related to infection control.
It is possible for community hospitals to successfully implement a prospective audit and feedback strategy for antimicrobial stewardship. The ICU antimicrobial stewardship program described here was a sustainable initiative with demonstrated success beyond the pilot phase. As we move toward wider implementation of stewardship across other acute care areas in the hospital, we will purposefully incorporate knowledge exchange, peer-to-peer communication, and decision support to create a sustainable program.
1.
Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society of Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.
Clin Infect Dis
2007;44(2):159–177.
2.
Solomon DH, Van Houten L, Glynn RJ, Baden L, Curtis K, Schrager H, et al. Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center.
Arch Intern Med
2001;161(15):1897–1902.
3.
Fraser GL, Stogsdill P, Dickens JD Jr, Wennberg DE, Smith RP Jr, Prato BS. Antibiotic optimization. An evaluation of patient safety and economic outcomes.
Arch Intern Med
1997;157(15):1689–1694. Erratum in:
Arch Intern Med
1997;157(21):2487.
4.
Carling P, Fung T, Killion A, Terrin N, Barza M. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years.
Infect Control Hosp Epidemiol
2003;24(9):699–706.
5.
LaRocco A Jr. Concurrent antibiotic review programs—a role for infectious diseases specialists at small community hospitals.
Clin Infect Dis
2003;37(5):742–743.
6. Dresser L, Nelson S. Practice spotlight: pharmacists in an antimicrobial stewardship program. Can J Hosp Pharm 2010;63(4):328–329.
7. DDD: definition and general considerations. Oslo (Norway): WHO Collaborating Center for Drug Statistics Methodology; [updated 2009 Dec 17; cited 2011 Sep 21]. Available from: www.whocc.no/ddd/definition_and_general_considera/
8.
Kohli E, Ptak J, Smith R, Taylor E, Talbot EA, Kirkland KB. Variability in the Hawthorne effect with regard to hand hygiene performance in high-and low-performing inpatient care units.
Infect Control Hosp Epidemiol
2009;30(3):222–225.
The authors thank the Pharmacy and Infection Control departments at Toronto East General Hospital for supporting this study and Angela Wang, Information Technology Department, for assistance with retrieval of electronic data.
Canadian Journal of Hospital Pharmacy , VOLUME 64 , NUMBER 5 , September-October 2011