Karen Horon , Kefah Hayek , Carmel Montgomery
The use of abbreviations in medication orders has been identified as an underlying cause of serious, even fatal medication errors. The long-standing practice of using order-writing shortcuts has been found in medication orders and standing protocols and has even been legitimized in policies.1 The use of abbreviations in medication orders results in miscommunication, because staff members responsible for reading, interpreting, and processing these orders may not recognize or may misconstrue the abbreviations, which in turn results in misunderstanding of the intended meaning.2
The link between the use of dangerous abbreviations and critical incidents was shown by the Commonwealth of Pennsylvania Patient Safety Authority, which found, upon review of 103 critical incidents, that 56% involved the use of dangerous abbreviations and dose expressions (as reported during a teleconference entitled “Alphabet Soup: Hazardous to Your Health!” [sponsored by the Institute for Safe Medication Practices, February 4, 2005]).
The concept of reducing or even eliminating the use of dangerous abbreviations is not new. For almost 30 years, the US Institute of Safe Medication Practices (ISMP) has received a steady stream of reported errors, some of them causing critical incidents, due to misinterpretation of a handful of dangerous abbreviations. The ISMP (US), the Institute for Safe Medication Practices Canada, the National Coordinating Council for Medication Error Reporting and Prevention, the Health Quality Council of Alberta, Accreditation Canada, and the Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations) have all aimed to establish safe practices by advocating for prohibition of a short list of dangerous abbreviations and dose expressions.
Despite widespread advocacy by these key organizations seeking a reduction in the use of dangerous abbreviations and dose designations, such use continues. Nursing and pharmacy staff are often put in the difficult and risky position of processing orders that contain dangerous abbreviations and dose designations. The alternative to processing such orders is either clarifying each order or refusing to process the orders, both of which also put patients at risk.
The former Capital Health—Edmonton & Area, which consisted of 12 acute care hospitals and 1 rehabilitation hospital, embarked on a patient safety initiative to reduce the use of dangerous abbreviations and dose designations. At the time of the initiative, none of the sites were using computerized physician order entry. Instead of enforcement through clarification or refusal to process medication or total parenteral nutrition (TPN) orders that contained dangerous abbreviations or dose designations, intensive and focused educational strategies were used to gain compliance. A 75% reduction in the use of prohibited abbreviations, relative to baseline, was the established goal.
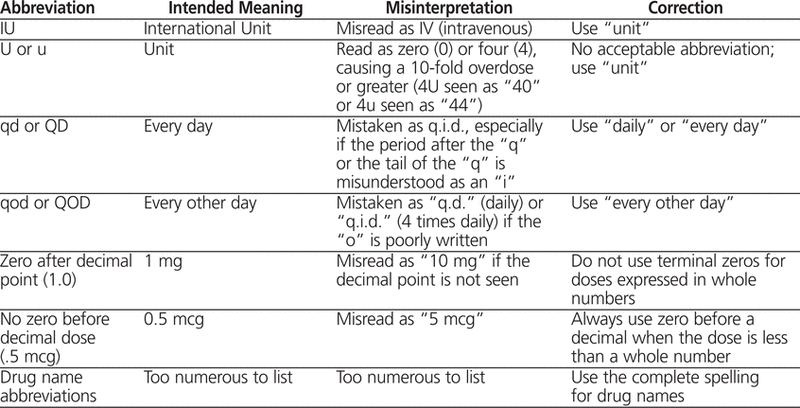
Although no abbreviation in medication orders is acceptable, 7 abbreviations were considered to be the most dangerous, according to the Joint Commission and ISMP, and were selected for prohibition in Capital Health—Edmonton & Area (Table 1). In 2004, the Drugs and Therapeutics Committee and the Medical Advisory Council approved the list for implementation.
Table 1.
Abbreviations Prohibited by Capital Health—Edmonton & Area

A working group was established to develop a policy, as well as communication and education materials, and to implement the list of prohibited abbreviations. The working group consisted of representatives from nursing, pharmacy, and quality and patient safety, along with a physician champion.
Because enforcement strategies were not considered a safe option, the following intensive and focused educational interventions were developed and delivered and/or distributed between August 1 and September 30, 2005:
description and rationale for the prohibited abbreviations initiative
communication about the initiative to internal and external health care providers, educators, and students
development and distribution of a list of the prohibited abbreviations and dose designations and the correct terminology to replace prohibited terms
examples of medication abbreviations and why they pose a risk to patient safety
provision of a tool kit for each patient care area, containing posters (Figure 1), frequently asked questions, stickers for the bookmark in medical charts (Figure 2 and described in more detail below), and self-adhesive notes (Figure 3)
in-service sessions for medical, pharmacy, and nursing staff and dietitians
electronic availability of a PowerPoint presentation (Microsoft Canada, Mississauga, Ontario) with speaking notes, for additional education sessions and for self-study
regular reporting of audit results to all staff, including medical staff, as well as to the Drugs and Therapeutics Committee and the Medical Advisory Council
|
|
||
|
Figure 1. Poster for unit-level display. Poster background was bright green. See Appendix 1 (available at www.cjhp-online.ca/index.php/cjhp/issue/view/88/showToc) for the colour version of this poster. ©2005–2012 Alberta Health Services |
||
|
|
||
|
Figure 2. Sticker for bookmark in medical chart. Background was bright green. See Appendix 2 (available at www.cjhp-online.ca/index.php/cjhp/issue/view/88/showToc) for the colour version of this sticker. ©2005–2012 Alberta Health Services |
||
|
|
||
|
Figure 3. Self-adhesive sticker. Background was bright green. See Appendix 3 (available at www.cjhp-online.ca/index.php/cjhp/issue/view/88/showToc) for the colour version of this sticker. ©2005–2012 Alberta Health Services |
||
One-time communication to educational facilities took the form of a memorandum, which included the approved list of prohibited abbreviations, as well as a suggestion to include a section on prohibited abbreviations in course work. Letters cosigned by the Capital Health—Edmonton & Area vice president of medical affairs and the University of Alberta dean of medicine were sent to each physician, medical resident, and undergraduate medical student in Capital Health—Edmonton & Area. Internal communication documents were distributed widely among nursing and pharmacy staff and dietitians.
The stickers for medical chart bookmarks (Figure 2) were created to serve as a visual reminder to prescribers, encouraging them to avoid using the prohibited abbreviations and dose designations. One of these stickers was placed permanently in a standard position in each patient’s chart. For inpatient units, the bookmark sticker was placed on the plastic page divider of the chart, so that when the chart was opened, the sticker would appear to the left of the current patient care orders. For outpatient units and other areas that did not use a binder for patient charts (such as clinics and the emergency department), the bookmark sticker was placed in or on an area of the unit’s choosing, to serve as a reminder to prescribers when writing patient care orders. Enough bookmark stickers were provided to ensure availability of one for each chart on each patient care unit. The bookmark stickers were coated, such that they would remain legible when charts were wiped down for infection control purposes.
Small self-adhesive notes, preprinted with the term “Prohibited Abbreviation” and an arrow (Figure 3), were intended for use by anyone who noticed a prohibited abbreviation or dose designation while processing a medication or TPN order (handwritten or preprinted). The removable sticky note was placed directly on the order within the chart, with the arrow pointing to the prohibited abbreviation. The intent was to provide a nonconfrontational, nonpermanent means of notifying the prescriber that a prohibited abbreviation had been used and as a reminder to avoid its use when writing future orders. The prescriber could subsequently remove the sticky note. The tool kits distributed to each patient care unit had several pads of these custom-printed self-adhesive notes.
The stickers for chart bookmarks and the self-adhesive notes were available for use when the list of prohibited abbreviations was implemented on October 1, 2005.
In-service education included delivery of more than 80 face-to-face presentations by members of the working group, targeting mainly medical and nursing staff across the region. Each presentation illustrated the risks to patient safety associated with the prohibited abbreviations and dose designations and featured examples of prohibited abbreviations and how they could be misinterpreted. All in-service presentations were completed by September 30, 2005, before implementation of the list of prohibited abbreviations.
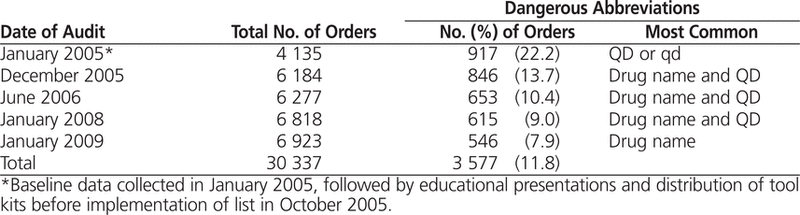
During the 4-year evaluation period, at 4 specific time points, a total of 26 202 medication orders were reviewed for the presence of prohibited abbreviations. For each of these audits, as well as the baseline audit, all medication orders received on 1 day from 3 of the largest sites in Capital Health—Edmonton & Area were reviewed.
The first set of data was collected in January 2005, to measure baseline compliance with the established list of prohibited abbreviations and dose designations. This baseline audit revealed that 22.2% of medication orders (range 20%–23% for the 3 sites) involved the use of prohibited abbreviations or designations (Table 2). The first compliance audit, 3 months after completion of the intensive education effort (December 2005), showed a reduction in the use of prohibited abbreviations from the baseline value of 22.2% to 13.7%. Subsequent compliance audits showed further decreases in the use of prohibited abbreviations and designations, with an overall reduction of 64.4% from baseline over the 4-year period, even as the number of orders audited increased (Table 2).
Table 2.
Results of Compliance Audits for All Medication Orders from 3 Acute Care Sites over a 4-Year Period
Also included in the first compliance audit was 1 week’s worth of TPN orders ( n = 180) from the same 3 acute care sites. By the time of the first compliance audit, in December 2005, there was a 98.5% decrease (from 36.8% to 0.6%) in the use of prohibited abbreviations (Table 3). Given these results and the focused education that was provided to a relatively small number of dieticians, TPN orders were not included in subsequent audits.
Table 3.
Results of Baseline and Compliance Audits for Total Parenteral Nutrition (TPN) Orders at 3 Acute Care Sites

Over the 4-year evaluation period, the overall reduction in the use of prohibited abbreviations on medication and TPN orders was 81.5%. Use of the prohibited abbreviations and dose designations on the short list (Table 1) declined from baseline for all but “trailing zero” (Table 4). Notably, the percentage of audited orders with “QD” (or “qd”) decreased from 14.3% at baseline to 1% at the end of the evaluation period. Use of drug name abbreviations decreased from 5.2% to 4.3%.
Table 4.
Use of Prohibited Abbreviations Before and After Implementation

The provision of quality patient care is directly dependent upon optimal communication among health care providers.3
In this study, the baseline 1-day audit revealed that 917 orders from 3 sites contained one or more of the prohibited abbreviations listed in Table 1. Assuming roughly 300 noncompliant orders per day per site, enforcement (either by calling each prescriber to seek clarification or refusing to process the order until the prescriber wrote out the abbreviation in full) would have disrupted patient care and caused a breakdown in relationships among medical, nursing, and pharmacy staff. It should be noted, however, that during this initiative, nursing and pharmacy staff did clarify orders with abbreviations if they felt it was necessary.
Over the study period, intensive, targeted, and well-received education, along with reminder tools, reduced the incidence of prohibited abbreviations in medication and TPN orders by 64.4% and 98.5%, respectively. The overall reduction in the use of prohibited abbreviations was 81.5%, which far exceeded the goal of 75%. This success was thought to be largely attributable to the reminder tools. In particular, the bookmark stickers for patients’ charts served as reminders to prescribers before and during order-writing. Anecdotal reports indicated that the self-adhesive notes were an effective yet nonconfrontational means of reminding prescribers to avoid a prohibited abbreviation when writing the next order. Relationships among clinicians were preserved, and patients were not put at further risk by slowing or stopping the processing of orders.
Frequency of use decreased for all prohibited abbreviations and dose designations except the trailing zero and no leading zero. Even though use of both of these dose designations increased over the study period, their overall use remained very low (< 1%). The use of “QD” or “qd” showed the greatest decrease over time. This result was impressive, given that “QD” (or “qd”) was the most common among all of the prohibited abbreviations in the baseline audit. Also impressive was the consistent reduction in use of prohibited abbreviations over the 4-year period. Regular communication throughout the evaluation period involved sharing audit results with the Drugs and Therapeutics Committee and the Medical Advisory Council after each audit and including a one-line congratulatory note on staff members’ pay cheques following 2 of the 4 audits. There were no formal continuing education sessions after implementation of the list in October 2005, although all of the information and educational materials remained posted online (on the institutional intranet) for staff and physicians.
The drug name abbreviations appearing on medication orders from the fourth compliance audit (January 2009) were further analyzed to determine if they included any of the 20 error-prone drug name abbreviations that ISMP has deemed unsafe4 (Table 5).
Table 5.
Frequency of Orders in Final Audit (January 2009) that Contained Any of the 20 Error-Prone Drug Name Abbreviations Identified by Institution for Safe Medication Practices4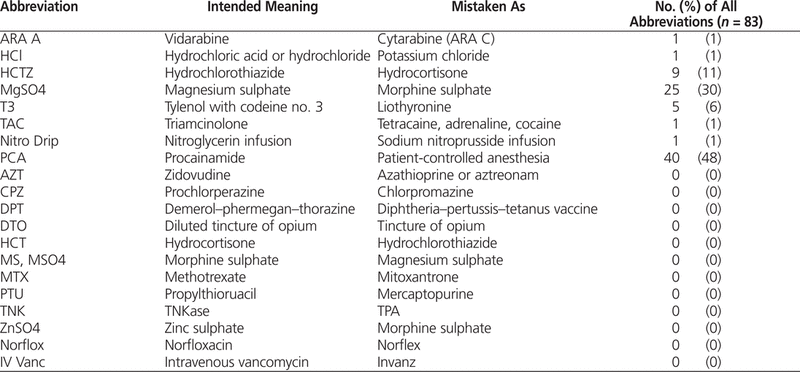
Of the 296 drug name abbreviations noted during the audit, less than one-third (83 or 28%) represented abbreviations included in the ISMP list of error-prone drug name abbreviations (Table 5), and they accounted for only 8 of the 20 abbreviations in this list. The biggest contributor to these numbers was the abbreviation “PCA”, which is often used to abbreviate procainamide. In the chart audits for Capital Health—Edmonton & Area, however, PCA was exclusively used to abbreviate “patient controlled analgesia”. According to Accreditation Canada, neither use of this abbreviation is acceptable. The remaining 12 of the 20 error-prone drug name abbreviations identified by ISMP were not found in the orders audited from Capital Health—Edmonton & Area. Conversely, the ISMP list did not include 213 other drug abbreviations found in the audit. The most common of these were “pip/tazo” and “KCl”. Neither of these has so far been reported to have contributed to morbidity or mortality from misinterpretation, so it is probably unnecessary to recommend that these abbreviations be added to the ISMP list.
In anticipation of Accreditation Canada’s Required Organizational Practice to adopt the ISMP “Do Not Use” abbreviations list, the results of the latest audit (in January 2009) were also further analyzed to determine the frequency of the additional 9 dangerous abbreviations and dose designations that would need to be prohibited in Capital Health—Edmonton & Area. No communication or education strategies were applied before this additional analysis. The 9 abbreviations and designations (“μg”, “OD”, “OS”, “OU”, “cc”, “D/C”, “@”, “>”, and “<”) were used in 12% of the audited orders (Figure 4). “D/C” was the most frequently used and is risky because it has 2 commonly intended but quite different meanings: “discharge” and “discontinue”. In most of the audited orders, “D/C” was used to abbreviate “discontinue”. Using “D/C” for either intended meaning should be prohibited.
|
|
||
|
Figure 4. Frequency of use of 9 additional abbreviations that would be required to fully adopt the “Do Not Use” list of the Institute for Safe Medication Practices, as identified during January 2009 audit ( n = 836). |
||
Prohibited abbreviations in the medication orders from one of the sites were analyzed to determine the designation of prescribing staff who used the abbreviations. The following categories of prescribers were audited: pharmacists, nurse practitioners, medical residents, and physicians. Unfortunately, signatures on the orders were so illegible that almost 60% of orders containing a prohibited abbreviation could not be audited to determine the staff member involved. Of the approximately 40% of medication orders for which signatures were legible, 26% of those containing a prohibited abbreviation had been written by medical residents. This is not surprising, as medical residents write the majority of orders at the hospital audited.
In 2009, the Accreditation Canada Required Organizational Practice on dangerous abbreviations came into effect.5 Although the list of prohibited abbreviations will have to grow to encompass Accreditation Canada requirements, targeted educational strategies will still be used, rather than enforcement, to reduce and ultimately eliminate the use of dangerous abbreviations.
1.
Abushaiga ME, Zaran FK, Bach DS, Smolarek RT, Farber MS. Educational interventions to reduce the use of unsafe abbreviations.
Am J Health Syst Pharm
2007;64(11):1170–1173.
2.
Brunetti L, Santell JP, Hicks RW. The impact of abbreviations on patient safety.
Jt Comm J Qual Patient Saf
2007;33(9):576–583.
3.
Walsh KE, Gurwitz JH. Medical abbreviations: writing little and communicating less.
Arch Dis Child
2008;93(10):816–817.
4. ISMP’s list of error-prone abbreviations, symbols, and dose designations. Horsham (PA): Institute for Safe Medication Practices; 2004 [cited 2009 Jul 3]. Available from: www.ismp.org/tools/errorproneabbreviations.pdf
5. 2010 report on required organizational practices: results from Canadian health organizations . Ottawa (ON): Accreditation Canada; 2010.
Canadian Journal of Hospital Pharmacy , VOLUME 65 , NUMBER 4 , July-August 2012