Isabelle Barthélémy , Research Assistant , Yemsoktheavy Khvan , Research Assistant , Tina Ly , Research Assistant , Suzanne Atkison , BPharm, MSc , Assistant Director , Denis Lebel , BPharm, MSc, FCSHP , Assistant Director , Jean-François Bussières , BPharm, MSc, FCSHP , Director
In Canada, the Food and Drug Act allows the distribution of drug samples to physicians, dentists, and pharmacists.1 Most provincial regulatory authorities do not prohibit the distribution of such samples in health care settings.2 However, drug samples are perceived differently by different health care stakeholders.2 In particular, the use of drug samples may bypass the optimal drug-use process in hospitals and retail pharmacies.3–5
The objective of this cross-sectional observational study was to compare the number of drug samples available in outpatient clinics in a mother–child university hospital centre in the province of Quebec in 2007, 2009, and 2012. In the study hospital, drug samples were not allowed in patient wards but were tolerated in outpatient clinics. Drug samples were monitored every 6 months by pharmacy staff, who made unannounced visits to the clinics. In addition to biannual monitoring, extensive audits were conducted periodically over 1- to 2-week periods. During the first extensive audit, in 2007, the number of units (e.g. tablets, vials) of drug samples in all outpatient clinics was counted. The audit was repeated in November 2009 and July 2012. For each audit period, the total numbers of both units and doses of drug samples were calculated, and the average number of doses was estimated for liquids (0.5 mL/dose) and topical agents (0.5 g/dose). The number of doses of drug samples per patient visit was also calculated, to indicate potential exposure of patients to samples.
In total, 31 locations (i.e., health care units) were identified in 21 outpatient clinics. A total of 14 221 units of drug samples were counted in 2007, 8080 units in 2009, and 6989 units in 2012 (see details in Table 1). Although the number of units decreased over time, the number of doses increased, from 78 955 in 2007 to 75 487 in 2009 and 91 000 in 2012 (breakdown by clinic not shown), mostly because of a higher proportion of topical drugs in the dermatology clinic. The number of doses of drug samples per patient visit remained stable: 0.40 in 2007, 0.38 in 2009, and 0.41 in 2012.
Table 1.
Profile of Drug Samples in a Mother–Child Teaching Hospital in 2007, 2009, and 2012

In 2012, only 19% of doses documented during the audit were listed on the official hospital drug formulary; in addition, 4% of the doses were expired. Despite implementation of a Web-based intranet form to declare drug samples received from industry sales representatives, most doses of drug samples had not been declared to the pharmacy by hospital staff.
The availability of drug samples in outpatient clinics at the study hospital has remained stable for the past 5 years. It may seem feasible to prohibit the distribution of samples locally in outpatient clinics, but in fact, it is difficult to do so when such distribution is not prohibited by the pertinent regulatory authorities. For instance, physicians and medical residents often work in multiple hospitals, and their regulations regarding drug samples may vary. We believe that drug samples do not contribute to better patient care and should only be dispensed by retail pharmacies through a structured approach, with documentation of doses dispensed in the patient’s record.
1. Food and Drug Regulations, C.R.C., c. 870. Ottawa (ON): Department of Justice; 2012 [cited 2012 Oct 05]. Available from: http://laws-lois.justice.gc.ca/eng/regulations/C.R.C.,_c._870/index.html
2. Prise de position conjointe : échantillons de médicaments [Joint position statement: drug samples]. Montréal (QC): Collège des médecins du Québec, Ordre des pharmaciens du Québec; 1997 [cited 2012 Oct 12]. Available from: www.opq.org/cms/Media/753_38_fr-CA_0_pp_echantillons_med.pdf
3. Soucy G, Bussières JF, Lebel D, Tardif L, Bailey B. Analyse proactive du risque associé à la distribution et à l’utilisation des échantillons de médicaments. Pharmactuel. 2008;41(5):310–4.
4. Soucy G, Bussières JF, Tardif L, Bailey B. Inventory of drug samples in a health care institution. Can J Hosp Pharm. 2009;62(4):298–306.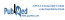
5. Tardif L, Bussières JF, Lebel D, Soucy G, Bailey B. A case study on the perceived advantages and disadvantages of using drug samples in a university hospital center. Ann Pharmacother. 2009;43(1):57–63.
Isabelle Barthélémy is also a DPharm intern at Université Claude Bernard Lyon 1, Villeurbanne, France. Yemsoktheavy Khvan and Tina Ly are also PharmD candidates with the Faculty of Pharmacy, Université de Montréal. Jean-François Bussières is also a Professor with the Faculty of Pharmacy, Université de Montréal.
Competing interests: None declared.
Canadian Journal of Hospital Pharmacy , VOLUME 66 , NUMBER 1 , January-February 2013