Charlotte Kistner , Mary H H Ensom , Diane Decarie , Gillian Lauder , Roxane R CarrABSTRACT
Background
Naloxone may be administered in conjunction with morphine to reduce the risk of opioid-induced pruritis. Combining these drugs for coadministration may be beneficial, but little is known about their physical compatibility and stability in combined solutions.
Objective:
To describe the physical compatibility and stability of morphine sulphate and naloxone hydrochloride (at various concentrations) in IV admixtures.
Methods:
The physical compatibility and stability of admixtures of morphine 1000 μg/mL and naloxone 4 μg/mL, 12.5 μg/mL, and 25 μg/mL in 0.9% sodium chloride were studied. For each concentration of naloxone, one bag was stored at room temperature (22°C) for 72 h and one bag was stored under refrigeration (4°C) for 30 days. For all preparations, physical characteristics, including pH, colour, and formation of precipitate, were evaluated. The samples were also analyzed by a stability-indicating high-performance liquid chromatographic method. Stability was defined as the retention of at least 90% of the initial concentration.
Results:
No notable changes in pH or colour and no macroprecipitation were observed in any of the preparations after storage at 22°C for up to 72 h or at 4°C for up to 30 days. All preparations maintained more than 90% of the initial concentrations of morphine and naloxone at the end of the respective study periods. The calculated lower limit of the 95% confidence interval also indicated that 90% or more of the initial concentration remained at the end of each study period.
Conclusion:
Admixtures of morphine sulphate and naloxone hydrochloride were stable for 72 h at room temperature and for 30 days with refrigeration.
KEYWORDS: naloxone , morphine , compatibility
RÉSUMÉ
Contexte :
La naloxone peut être administrée en concomitance avec la morphine pour réduire le risque de prurit induit par les opioïdes. L’association de ces médicaments pour leur administration concomitante peut être bénéfique, mais on ne sait que peu de choses sur leur compatibilité physique et leur stabilité dans des solutions combinées.
Objectif :
Décrire la compatibilité physique et la stabilité du sulfate de morphine et du chlorhydrate de naloxone (à diverses concentrations) mélangés dans des solutions pour administration i.v.
Méthodes :
La compatibilité physique et la stabilité des mélanges de morphine à 1000 μg/mL et de naloxone à 4 μg/mL, 12,5 μg/mL et 25 μg/mL dans du chlorure de sodium à 0,9 % ont été étudiées. Pour chaque concentration de naloxone, on a entreposé un sac à la température ambiante (22 °C) pendant 72 heures et un autre au réfrigérateur (4 °C) pendant 30 jours. Les propriétés physiques, notamment le pH, la couleur et la formation de précipité, ont été évaluées pour toutes les préparations. Les échantillons ont aussi été analysés à l’aide d’une épreuve validée mesurant la stabilité par chromatographie liquide haute performance. La stabilité a été définie comme étant la rétention d’au moins 90 % de la concentration initiale des agents.
Résultats :
Aucun changement notable du pH ou de la couleur et aucune formation de macroprécipité n’ont été observés dans l’ensemble des préparations qui ont été conservées à une température de 22 °C pendant un maximum de 72 heures ou à une température de 4 °C pendant un maximum de 30 jours. Toutes les préparations ont conservé plus de 90 % de leurs concentrations initiales de morphine et de naloxone à la fin de leurs périodes d’étude respectives. La limite inférieure de l’intervalle de confiance à 95 % indiquait également que 90 % ou plus de la concentration initiale subsistait à la fin de chaque période d’étude.
Conclusion:
Les mélanges de sulfate de morphine et de chlorhydrate de naloxone sont demeurés stables pendant 72 heures à la température ambiante et pendant 30 jours lorsqu’ils étaient réfrigérés.
MOTS CLÉS: naloxone , morphine , compatibilité
[Traduction par l’éditeur]
Morphine, an opioid analgesic, is well established for controlling moderate to severe pain in children and adults.1–8 One common adverse effect of morphine is pruritus. In a small study of 25 adult patients receiving morphine by patient-controlled analgesia (PCA) for postoperative pain, 40% were reported to have pruritus.2 Pruritus has been reported to occur in 9% to 77% of pediatric patients receiving continuous morphine infusions,8,9 with some requiring discontinuation of the infusion because the pruritus was intolerable.8 Pruritus secondary to morphine by IV infusion is postulated to be mediated centrally via opioid receptors.10,11 Although intradermal injection of morphine causes a local histamine reaction, release of histamine is not believed to be responsible for the itching caused by opioids administered spinally or systemically.10
Most existing therapeutic approaches to treatment of opioid-induced pruritus are unreliable, are often ineffective, and may increase the risk of adverse drug reactions.12–14 For example, naltrexone was more effective than placebo in reducing the incidence of pruritus (reduction of 34% to 62%), but this drug also decreases analgesia.15,16 Studies of nalbuphine in adults have generated conflicting data: some studies reported no reduction in pruritus,13,17 whereas others showed a lower incidence of pruritus with nalbuphine than with placebo (13% versus 78%, respectively)18 and lower pruritus scores (by 1 to 2 points on a 4-point scale).14 In children, however, nalbuphine has been shown to increase drowsiness14,17 and is ineffective in treating pruritus.19 Droperidol has been found to decrease the incidence of pruritus, but it also increases somnolence relative to placebo.20,21 Studies of propofol at subanesthetic doses have had conflicting outcomes, with either decreased or increased pruritus and increased risk of oversedation and respiratory depression.22–24 Other agents, such as IV nalmefene, epidural epinephrine, intramuscular hydroxyzine, IV diphenhydramine, epidural clonidine, epidural prednisone, and IV ondansetron, have been tested but were ineffective in reducing opioid-induced pruritus.12,25,26
Naloxone is a pure opioid antagonist that competes for and displaces opioids at opioid receptor sites without having any effect on the receptors themselves. This drug has been reported to reduce the incidence of opioid-induced pruritus among adult patients receiving morphine by postoperative PCA, from 55% with placebo to 25% with naloxone.27 No respiratory depression was observed with naloxone, and no differences in respiratory rate, sedation scores, blood pressure, heart rate, or oxygen saturation between naloxone and placebo groups were reported.27 In children, naloxone “piggy-backed” into patients’ morphine PCA infusions reduced the incidence of pruritus from 77% in the placebo group to 20% in the naloxone group.9 In an underpowered pilot study ( n = 16 patients) examining co-infusion of morphine with low-dose (0.25 μg/kg per hour) or high-dose (1 μg/kg per hour) naloxone, trends suggested that the high-dose group had a lower incidence of pruritus than the low-dose group.28 The authors of that study suggested further randomized controlled studies comparing naloxone with placebo.28 Together, these data indicate that naloxone may be effective in reducing opioid-induced pruritus without increasing adverse effects or affecting the quality of analgesia in patients receiving continuous morphine infusions. Notably, in all of these studies, naloxone was used as a preventive strategy and was started at the same time as the opioid infusion, rather than being used to treat established pruritus.
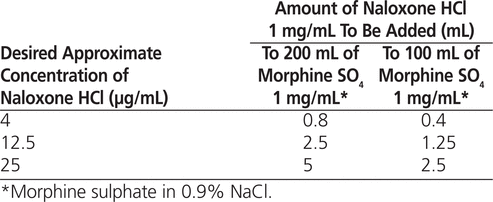
Combining morphine and naloxone at standard concentrations in a single container may be beneficial, as the naloxone dose would increase or decrease proportionally with changes in the morphine dose, and the patient would receive a bolus of naloxone along with a bolus of morphine. Combining the drugs may also decrease the risk of infusion rate errors29,30 and might save time and resources by avoiding the need to mix and hang more than one solution. Published data have confirmed the Y-site compatibility of morphine sulphate (SO 4 ) 4 mg/mL with naloxone hydrochloride (HCl) 16 μg/mL from separate infusion pumps run into the same IV site.31 However, to the authors’ knowledge, based on a search of tertiary compatibility references and the primary literature (i.e., MEDLINE and Embase databases), there have been no studies investigating the chemical and physical compatibility of morphine and naloxone in the same minibag at the standard concentrations used at the authors’ institution. The standard concentrations at the authors’ institution are morphine SO 4 1 mg/mL in 0.9% sodium chloride (NaCl) for PCA and naloxone HCl 4 μg/mL in 0.9% NaCl for prevention of itching or urinary retention during epidural administration of morphine. For the stability study reported here, 3 concentrations of naloxone (4 μg/mL, 12.5 μg/mL, and 25 μg/mL) were tested. Having information about multiple concentrations would allow clinicians to adjust the morphine dosage according to the patient’s clinical situation, with options as to the amount of naloxone being co-infused to treat pruritus.
The purpose of this study was to determine the compatibility of morphine SO 4 and naloxone HCl when combined and stored in minibags at room temperature and under refrigeration.
Stock solutions of morphine SO 4 and naloxone HCl were prepared from commercially available (premixed) solutions. These stock solutions were prepared in a laminar flow hood using aseptic technique. All solutions, both premixed solutions and admixtures prepared immediately before the experiment, were contained in polyvinyl chloride minibags (Baxter Corporation, Toronto, Ontario; lot UR12112127).
Morphine SO 4 was acquired premixed 100 mg/100 mL (concentration 1000 μg/mL) in 0.9% NaCl (Baxter Corporation; lots T66043 and T65135). A total of nine 200-mL bags of morphine were prepared; for each 200-mL bag, the contents of 2 premixed 100-mL bags were transferred into one empty minibag. The appropriate amount of naloxone HCl 1 mg/mL (Sandoz Canada, Boucherville, Quebec; lot AR2903) was then added to generate each test concentration, as described in Table 1 (three 200-mL minibags for each concentration). Because volumes of naloxone 1 mg/mL were added to the prepared 200-mL bags of morphine in 0.9% NaCl, and because the original 100-mL bags contained saline overfill, the final concentrations were approximate. The nominal concentrations were as follows:
naloxone 4 μg/mL and morphine 1000 μg/mL (1 mg/mL)
naloxone 12.5 μg/mL and morphine 1000 μg/mL
naloxone 25 μg/mL and morphine 1000 μg/mL
Table 1
Admixture of Naloxone-Containing Solutions

The physical characteristics of each solution were evaluated qualitatively at the time of preparation and then every day for the first 2 weeks and every other day for the next 2 weeks (until day 30). Each solution was examined for changes in colour (against white and black backgrounds) and for formation of precipitate. Minibags containing the combined solutions were stored at 4°C or at 22°C; those stored at 4°C were allowed to equilibrate to 22°C before sampling. Three aliquots from each sample from the combination solution minibags were then collected to determine the pH. The pH meter (model 8000, VWR International, Mississauga, Ontario) was calibrated with commercially available standards at the beginning of each test session. Immediately following the physical observations, samples (1.5 mL each) were transferred to 2-mL polypropylene vials (VWR International; lot 325792596) and immediately stored at −85°C. Throughout the study period, the same individual (C.K.) performed assessments of physical characteristics and collected the samples. Samples were stored frozen until analysis by a stability-indicating high-performance liquid chromatography (HPLC) – ultraviolet detection method. Precision of the assay was evaluated by intraday and interday validation methods. Accuracy of the assay was calculated as the mean deviation between nominal and observed concentrations. Means, standard deviations (SDs), coefficients of variation (CVs), and accuracy were calculated. Acceptable limits were defined a priori as up to 10% for CVs and 90% or better for accuracy.
A 6-point calibration curve was prepared, with 2 blanks (water and 0.9% NaCl [Baxter Corporation, Mississauga, Ontario; lot WOF30A1]) at the beginning of each run to ensure no carry-over from one run to the next. Standards were prepared by combining commercially available morphine SO 4 1 mg/mL (Sandoz Canada; lot AR6494) and naloxone HCl 1 mg/mL (diluted in 0.9% NaCl to a concentration of 0.1 mg/mL) as required to obtain the following concentrations: 300 and 10 μg/mL, respectively; 250 and 6 μg/mL, respectively; 200 and 5 μg/mL, respectively; 150 and 2.5 μg/mL, respectively; 100 and 1.5 μg/mL, respectively; and 50 and 0.5 μg/mL, respectively. The internal standard was prepared by diluting ketamine HCl 25 mg/mL (Sandoz Canada; lot AP8595) in 0.9% NaCl to a concentration of 250 μg/mL and then adding this internal standard to the prepared standards to a final concentration of 25 μg/mL. Each standard solution was passed through a 0.45-μm microfilter to prevent injection of impurities onto the column. The range of the standard curve encompassed the test concentrations of morphine and naloxone diluted for HPLC analysis (as described below). The intraday variation was determined by running morphine at concentrations of 80, 180, 200, and 220 μg/mL and naloxone at concentrations of 0.7, 2.0, 4.0, and 8.0 μg/mL in quadruplicate throughout a single day. The interday variation was determined by running the same analyte concentrations for each drug in quadruplicate for 4 days.
On the day of analysis, the study samples were thawed, mixed by vortex mixer for 10 s, and prepared by combining 200 μL of each study sample with 100 μL of ketamine and 700 μL of 0.9% NaCl for the samples stored at 22°C and 4°C. The theoretical final concentrations of morphine and naloxone injected onto the column were 200 and 0.8, 200 and 2.5, and 200 and 5.0 μg/mL, respectively. The final concentration of ketamine was 25 μg/mL. Each solution was passed through a 0.45-μm microfilter before injection of a 15-μL sample onto the column.
All solvents were HPLC-grade and were filtered before use. All reagents and chemicals with expiry dates were used before their respective expiry dates.
The HPLC instrumentation (Waters Alliance System model 2690, Waters Ltd, Mississauga, Ontario) consisted of a delivery pump, an automatic injector equipped with a 200-μL injector, a Symmetry C18, 3.5-μm (100 mm × 4.6 mm) silica column (Waters Alliance System Ltd; lot 01953019514077), a C18 5-μm (3.9 mm × 20 mm) guard column (Waters Alliance System Ltd; lot 02493202091), and an ultraviolet detector set at 203 nm. The mobile phase developed in the authors’ laboratory on the basis of previous work consisted of a gradient mixture of 5.2%–25%–5.2% acetonitrile (VWR International, Edmonton, Alberta; lot 49027), 5.2%–27.5%–5.2% methanol (Fisher Scientific, Richmond, British Columbia; lot 0869535), and 89.6%–47.5%–89.6% 5 mmol/L potassium phosphate buffer (Sigma Aldrich, Oakville, Ontario; lot 107K0100) pH 3.5, which was run over a period of 6.5 min.32 The flow rate fluctuated between 1.0 and 1.5 mL/min.
A combined solution of morphine 1000 μg/mL and nalox-one 25 μg/mL was prepared in 0.9% NaCl. The pH, initially 4.8, was adjusted to 9.0 with 2N sodium hydroxide, and the sample was incubated for 72 h at 90°C. The sample was cooled to room temperature and the pH readjusted to 4.8 with 1N hydrochloric acid. The sample was then adjusted with 0.9% NaCl to concentrations of morphine 200 μg/mL and naloxone 5 μg/mL, exposed to daylight for 72 h, and then filtered and injected onto the column. The chromatogram obtained for the degradation was compared with a chromatogram obtained from the standard curve to determine any changes in concentrations, retention time, and shape of the peak.
Means, SDs, CVs, and accuracy were calculated for samples analyzed in triplicate (experimental samples) or quadruplicate (validation standards). The percentage of the initial concentration of morphine and naloxone remaining was calculated for each sample, and stability of each drug was defined as maintenance of at least 90% of the initial concentration. The percentage of each drug remaining at the end of the study period after storage at room temperature and 4°C was calculated from the concentrations measured at the end of the study period, as determined by linear regression and the concentrations calculated at time 0, according to the following formula: concentration at end of study period ÷ concentration at time 0 × 100%. The 95% confidence interval (CI) of the amount remaining at the end of the study period was calculated from the lower limit of the 95% CI of the slope of the curve relating concentration and time, determined by linear regression via computer analysis according to the following formula: lower limit of the 95% CI of the concentration at the end of study period ÷ concentration at time 0 × 100%.
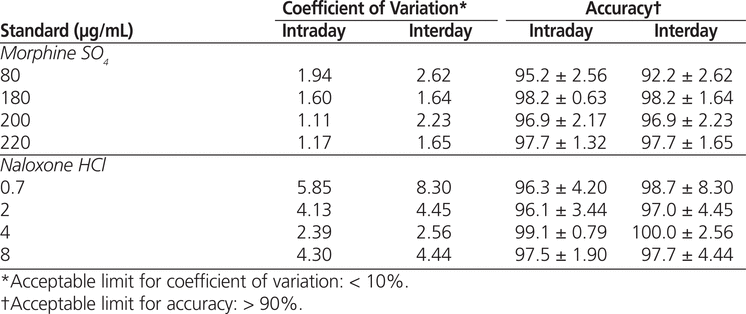
Regression analysis of the peak area ratio of morphine and naloxone to ketamine (internal standard) versus the concentration of each morphine and naloxone standard demonstrated linearity over the range of concentrations, with coefficients of determination ( r 2) greater than 0.996 ( n = 4) for morphine and greater than 0.997 ( n = 4) for naloxone. The intraday and interday CVs for both drugs were less than 10% and therefore within acceptable limits (Table 2). Intraday and interday accuracy were also within acceptable limits (greater than 90%) (Table 2). The detection limits were 0.25 μg/mL for morphine and 0.15 μg/mL for naloxone. The retention times were 1.65 min for morphine, 4.00 min for naloxone, and 2.98 min for ketamine (Figure 1, top).
Table 2
Intraday and Interday Coefficients of Variation and Accuracy for Morphine Sulphate and Naloxone Hydrochloride in Combined Solutions
|
|
||
|
Figure 1 Top: Chromatogram for undegraded admixture, showing retention times of 1.65 min for morphine, 4.00 min for naloxone, and 2.98 min for ketamine. Middle: Chromatogram for degraded preparation shows shortening of the morphine retention time (to 1.33 min) and 23.3% reduction in the morphine peak, as well as disappearance of the naloxone peak. Bottom: Overlay of top and middle chromatograms shows no interfering peaks. AU = arbitrary absorbance units. |
||
When a combined sample of morphine and naloxone was subjected to degradation, the morphine retention time was shortened to 1.33 min and the peak was reduced by 23.3%. The naloxone peak was undetectable after degradation (Figure 1, middle). No interfering peaks were detected (Figure 1, bottom). Therefore, the method was deemed capable of indicating stability.
No notable changes in colour or macroprecipitation occurred in any of the samples over the duration of the study. For samples mixed and stored in minibags at room temperature for 72 h or at 4°C for 30 days, the pH varied little (Table 3).
Table 3
Measured pH of Combined Solutions of Morphine Sulphate and Naloxone Hydrochloride Stored at Room Temperature (22°C) or Under Refrigeration (4°C)*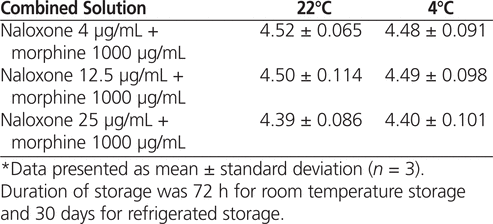
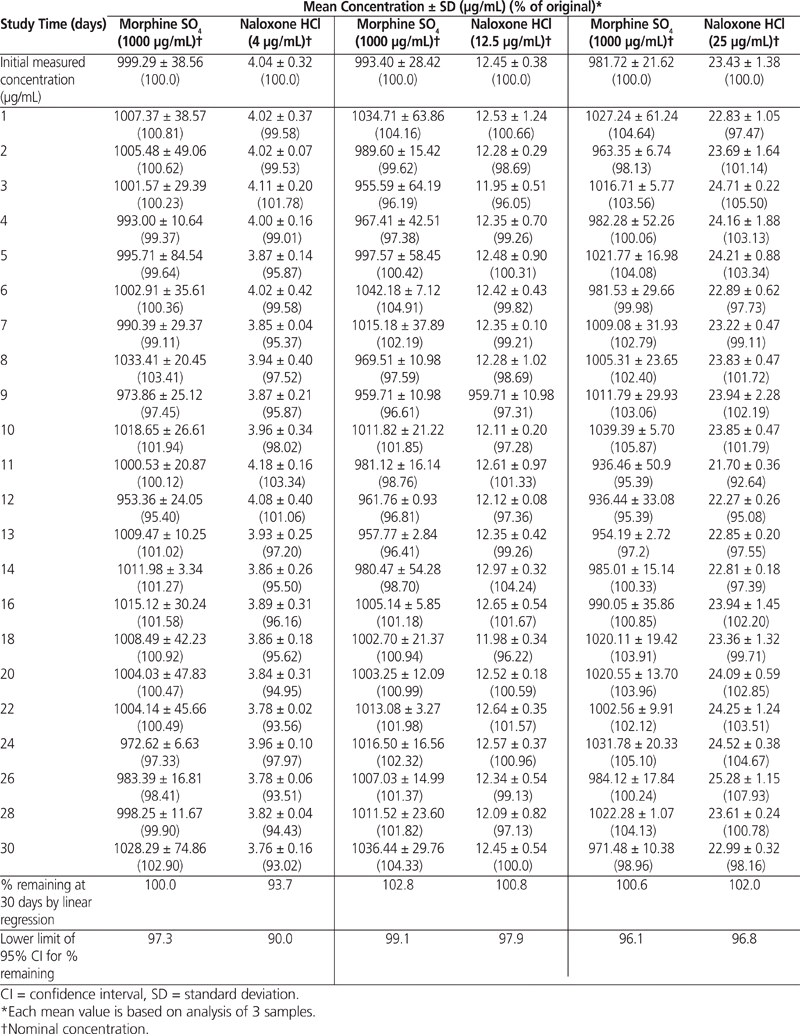
The 95% CI of the slope determined by linear regression indicated that the concentrations of all of the morphine–naloxone admixtures stored at room temperature in minibags for up to 72 h maintained at least 90% of the initial concentrations: 92.6% and 90.6%, respectively, for morphine 1000 μg/mL – naloxone 4 μg/mL; 97.2% and 91.7%, respectively, for morphine 1000 μg/mL – naloxone 12.5 μg/mL; and 101.6% and 90.0%, respectively, for morphine 1000 μg/mL – naloxone 25 μg/mL (Table 4). The solutions stored at 4°C in IV minibags for up to 30 days also maintained at least 90% of their initial concentrations: 97.3% and 90.0%, respectively, for morphine 1000 μg/mL – naloxone 4 μg/mL; 99.1% and 97.9%, respectively, for morphine 1000 μg/mL –naloxone 12.5 μg/mL; and 96.1% and 96.8%, respectively, for morphine 1000 μg/mL – naloxone 25 μg/mL (Table 5).
Table 4
Morphine Sulphate and Naloxone Hydrochloride Remaining After Storage at Room Temperature (22°C)
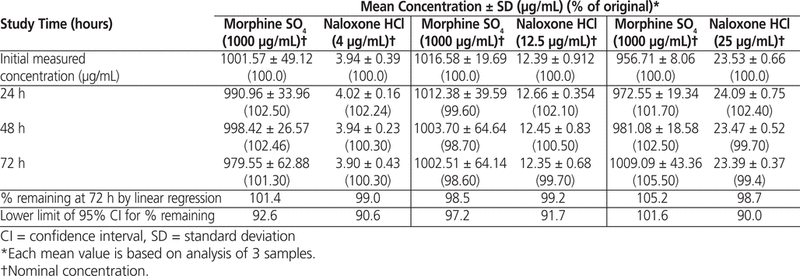
Table 5
Morphine Sulphate and Naloxone Hydrochloride Remaining After Refrigerated Storage (4°C)
The infusion of naloxone along with morphine may be beneficial in reducing opioid-induced pruritus. Co-infusion of the 2 drugs as an admixture may have additional benefits in terms of both safety and efficacy, in providing the naloxone bolus along with the morphine bolus.
On the basis of the 95% CI of the slope determined by linear regression, it is predicted that morphine 1000 μg/mL combined with naloxone 4, 12.5, or 25 μg/mL in 0.9% NaCl will maintain at least 90% of initial concentration for 3 days (72 h) at 22°C and 30 days at 4°C. Stability appeared to be better under refrigeration: in some cases, the concentration of naloxone declined to nearly 90% of initial concentration by day 3 at room temperature, but concentrations of this drug remained above 90% at day 30 with refrigeration. On the basis of these data, morphine 1000 μg/mL can be admixed with any of the 3 tested concentrations of naloxone, and the admixture can be hung for up to 3 days at room temperature, as per current guidelines at the authors’ institution. Admixtures of morphine and naloxone can be prepared in advance using sterile technique and conditions and stored under refrigeration for up to 30 days.
Certain limitations of this study may be due to storage of samples at –85°C until the time of the batch analysis. It was assumed that degradation of morphine and naloxone would not occur at this low temperature and that there would be no volume losses due to freeze-drying during storage. It was also assumed that errors due to serial analysis would be greater than any errors associated with batch analysis. Another potential limitation was the lack of evaluation for possible contamination of the minibags after the 30-day period of storage under refrigeration. Other potential limitations are related to the degradation methods: although the samples were exposed to heat and daylight, no additional methods such as acid hydrolysis and oxygenation were applied. Furthermore, the composition of the degradation products was not analyzed.
According to serial qualitative, pH, and HPLC analyses, solutions of morphine SO 4 1000 μg/mL admixed with naloxone HCl 4, 12.5, or 25 μg/mL in 0.9% NaCl in minibags for storage at 22°C and 4°C were compatible for 3-day and 30-day periods, respectively. Given that this trial has demonstrated the physical compatibility and stability of these 2 agents, the next step will be a clinical trial to determine if co-infusion of an admixture of naloxone HCl with morphine SO 4 is effective in preventing opioid-induced pruritus without decreasing the quality of analgesia.
1 Furyk JS, Grabowski WJ, Black LH. Nebulized fentanyl versus intravenous morphine in children with suspected limb fractures in the emergency department: a randomized controlled trial. Emerg Med Australas. 2009;21(3):203–9.

2 Hong D, Flood P, Diaz G. The side effects of morphine and hydromorphone patient-controlled analgesia. Anesth Analg. 2008;107(4):1384–9.

3 Berde CB, Lehn BM, Yee JD, Sethna NF, Russo D. Patient-controlled analgesia in children and adolescents: a randomized, prospective comparison with intramuscular administration of morphine for postoperative analgesia. J Pediatr. 1991;118(3):460–6.

4 Doyle E, Robinson D, Morton NS. Comparison of patient-controlled analgesia with and without a background infusion after lower abdominal surgery in children. Br J Anaesth. 1993;71(5):670–3.

5 Bozkurt P, Kaya G, Yeker Y, Altintas F, Bakan M, Hacibekiroglu M, et al. Effectiveness of morphine via thoracic epidural vs intravenous infusion of postthoracotomy pain and stress response in children. Paediatr Anaesth. 2004;14(9):748–54.

6 Sadhasivam S, Boat A, Mahmoud M. Comparison of patient-controlled analgesia with and without dexmedetomidine following spine surgery in children. J Clin Anesth. 2009;21(7):493–501.

7 Poe-Kochert C, Tripi PA, Potzman J, Son-Hing JP, Thompson GH. Continuous intravenous morphine infusion for postoperative analgesia following posterior spinal fusion for idiopathic scoliosis. Spine. 2010;35(7):754–7.
8 Howard RF, Lloyd-Thomas A, Thomas M, Williams DG, Saul R, Bruce E, et al. Nurse-controlled analgesia (NCA) following major surgery in 10,000 patients in a children’s hospital. Paediatr Anaesth. 2010;20(2):126–34.

9 Maxwell LG, Kaufmann SC, Bitzer S, Jackson EV Jr, McGready J, Kost-Byerly S, et al. The effects of a small-dose naloxone infusion on opioid-induced side effects and analgesia in children and adolescents treated with intravenous patient-controlled analgesia: a double-blind, prospective, randomized, controlled study. Anesth Analg. 2005;100(4):953–8.

10 Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, et al. Itch: scratching more than the surface. QJM. 2003;96(1):7–26.

11 Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33(2):149–60.

12 Kjellberg F, Tramèr MR. Pharmacological control of opioid-induced pruritus: a quantitative systematic review of randomized trials. Eur J Anaesthesiol. 2001;18(6):346–57.
13 Morgan PJ, Mehta S, Kapala DM. Nalbuphine pretreatment in cesarean section patients receiving epidural morphine. Reg Anesth Pain Med. 1991; 16(2):84–8.
14 Penning JP, Samson B, Baxter AD. Reversal of epidural morphine-induced respiratory depression and pruritus with nalbuphine. Can J Anaesth. 1988; 35(6):599–604.

15 Abboud TK, Afrasiabi A, Davidson J, Zhu J, Reyes A, Khoo N, et al. Prophylactic oral naltrexone with epidural morphine: effect on adverse reactions and ventilatory responses to carbon dioxide. Anesthesiology. 1990;72(2):233–7.

16 Abboud TK, Lee K, Zhu J, Reyes A, Afrasiabi A, Mantilla M, et al. Prophylactic oral naltrexone with intrathecal morphine for cesarean section: effects on adverse reactions and analgesia. Anesth Analg. 1990; 71(4):367–70.

17 Parker RK, Holtmann B, White PF. Patient-controlled epidural analgesia: interactions between nalbuphine and hydromorphone. Anesth Analg. 1997; 84(4):757–63.
18 Wang JJ, Ho ST, Tzeng JI. Comparison of intravenous nalbuphine infusion versus naloxone in the prevention of epidural morphine-related side effects. Reg Anesth Pain Med. 1998;23(5):479–84.
19 Nakatsuka N, Minogue SC, Lim J, Montgomery CJ, Court CA, Malherbe S, et al. Intravenous nalbuphine 50 μg·kg–1 is ineffective for opioid-induced pruritus in pediatrics. Can J Anaesth. 2006;53(11):1103–10.

20 Horta ML, Horta BL. Inhibition of epidural morphine-induced pruritus by intravenous droperidol. Reg Anesth. 1993;18(2):118–20.
21 Horta ML, Ramos L, Gonçalves Zda R, de Oliveira MA, Tonellotto D, Teixeira JP, et al. Inhibition of epidural morphine-induced pruritus by intravenous droperidol. The effect of increasing the doses of morphine and of droperidol. Reg Anesth. 1996;21(4):312–7.
22 Törn K, Tuominen M, Tarkkila, Lindgren L. Effects of sub-hypnotic doses of propofol on the side effects of intrathecal morphine. Br J Anaesth. 1994;73(3):411–2.

23 Warwick JP, Kearns CF, Scott WE. The effect of subhypnotic doses of propofol on the incidence of pruritus after intrathecal morphine for caesarean section. Anaesthesia. 1997;52(3):270–5.

24 Grattidge P. Nausea and vomiting after major arthroplasty with spinal anaesthesia including morphine: a randomised trial of subhypnotic propofol infusion as prophylaxis. Acta Anaesthesiol Scand. 1998;42(1):124–7.

25 Lin TF, Yeh YC, Yen YH, Wang YP, Lin CJ, Sun WZ. Antiemetic and analgesic-sparing effects of diphenhydramine added to morphine intravenous patient-controlled analgesia. Br J Anaesth. 2005;94(6):835–9.

26 Yeh HM, Chen LK, Lin CJ, Chan WH, Chen YP, Lin CS, et al. Prophy-lactic intravenous ondansetron reduces the incidence of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg. 2000;91(1):172–5.
27 Gan TJ, Ginsberg B, Glass PS, Fortney J, Jhaveri R, Perno R. Opioid-sparing effects of a low-dose infusion of naloxone in patient-administered morphine sulfate. Anesthesiology. 1997;87(5):1075–81.

28 Koch J, Manworren R, Clark L, Quinn CT, Buchanan GR, Rogers ZR. Pilot study of continuous co-infusion of morphine and naloxone in children with sickle cell pain crisis. Am J Hematol. 2008;83(9):728–31.

29 Apkon M, Leonard J, Probst L, DeLizio L, Vitale R. Design of a safer approach to intravenous drug infusions: failure mode effects analysis. Qual Saf Health Care. 2004;13(4):265–71.

30 Bell DS, Clements RS, Perentesis G, Roddam R, Wagenknecht L. Dosage accuracy of self-mixed vs premixed insulin. Arch Int Med. 1991; 151(11):2265–9.
31 Trissel LA, Leissing NC. Trissel’s tables of physical compatibility. Lake Forest (IL): Multimatrix; 1996.
32 Ensom MHH, Décarie D, Leung K et al. Stability of hydromorphone–ketamine solutions in glass bottles, plastic syringes, and IV bags for pediatric use. Can J Hosp Pharm. 2009;62(2):112–8.
Competing interests: None declared.
Canadian Journal of Hospital Pharmacy , VOLUME 66 , NUMBER 3 , May-June 2013