Mário L de Lemos , PharmD, MSc(Oncol), Nadine Badry , BSc(Pharm), Editor, Linda Hamata , BSc(Pharm), Staff Pharmacist
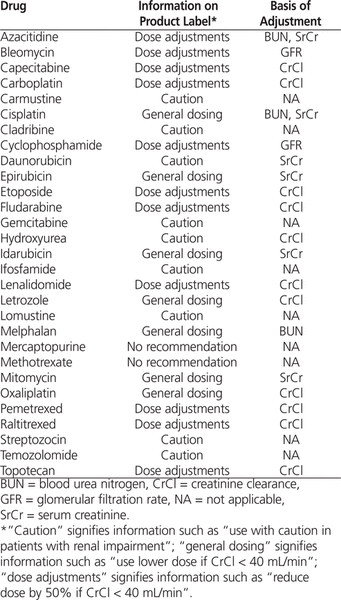
Creatinine clearance (CrCl) is often used to determine initial dosing of renally excreted antineoplastic drugs. CrCl is typically estimated on the basis of serum creatinine, for which the Cockcroft–Gault formula is commonly used.1 It has been argued that dosing based on this formula should be held as the “gold standard” because it is the basis of information on product labels.2,3 To verify this claim, we reviewed the product labels of 29 antineoplastic drugs available in Canada for which dose adjustment is required for patients with impaired renal function (Table 1).
Table 1.
Types of Renal Dosing Information for Individual Drugs

For 11 (38%) of the drugs, the labels provided a general caution or no recommendation at all (Table 2), whereas for 7 (24%), renal function was described in terms of serum creatinine or blood urea nitrogen. As such, the labels for only 11 (38%) of the drugs provided specific information on renal dose adjustments (Table 2), and for only 2 drugs (capecitabine and pemetrexed) did the labels actually mention the Cockcroft–Gault formula.
Table 2.
Summary of Renal Dosing Information
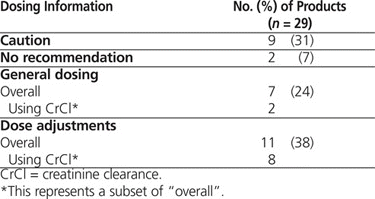
Overall, the product labels of 18 (62%) of the reviewed drugs provided limited information on renal dosing or did not recommend use of CrCl for dose adjustments. Therefore, it seems reasonable to question the insistence on using only the Cockcroft–Gault formula for dosing of drugs in patients with renal impairment, except where specific supporting data are available (e.g., for capecitabine, pemetrexed).
1. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
2. Holweger K, Lipp HP, Dietz K, Hartmann JT, Bokemeyer C. Novel algorithm for more accurate calculation of renal function in adults with cancer. Ann Pharmacother. 2008;42(12):1749–57.
3. Kleber M, Cybulla M, Bauchmüller K, Ihorst G, Koch B, Engelhardt M. Monitoring of renal function in cancer patients: an ongoing challenge for clinical practice. Ann Oncol. 2007;18(5):950–8.
Competing interests: None declared.
Canadian Journal of Hospital Pharmacy , VOLUME 66 , NUMBER 4 , July-August 2013