Stability of Bortezomib 2.5 mg/mL in Vials and Syringes Stored at 4°C and Room Temperature (23°C)
Scott E
Walker
,
Lauren F
Charbonneau
,
Shirley
Law
ABSTRACT
Background:
Solutions of bortezomib 1.0 mg/mL for IV administration are reportedly stable for up to 42 days. Recent publications have reported that the safety profile of bortezomib is better with subcutaneous administration than with IV administration.
Objective:
To evaluate the stability of higher-concentration bortezomib solutions for subcutaneous administration (i.e., 2.5 mg/mL in 0.9% sodium chloride [normal saline or NS]).
Methods:
On study day 0, twelve 3.5-mg vials of powdered bortezomib were each reconstituted with 1.4 mL of NS to prepare solutions with concentration 2.5 mg/mL. Half of the solutions were subsequently stored in the original vials and half were transferred to syringes. Three of each type of container were stored in the refrigerator (4°C) and the other 3 of each type were stored at room temperature (23°C). Concentration analysis and physical inspection were completed on study days 0, 1, 2, 8, 12, 14, 19, and 21. The concentration of bortezomib was determined by a validated liquid chromatographic method with ultraviolet detection. The expiry date was determined according to the time to achieve 90% of the initial concentration, based on the fastest degradation rate calculated from the 95% confidence interval of the observed degradation rate.
Results:
The analytical method separated degradation products from bortezomib such that the concentration was measured specifically and accurately (with absolute deviations from known concentration averaging 2.99%), with intraday and interday reproducibility averaging 1.51% and 2.51%, respectively. During the study period, all solutions were observed to retain at least 95.26% of the initial concentration in both types of containers at both temperatures.
Conclusions:
Bortezomib (3.5 mg in manufacturer’s vial) reconstituted with 1.4 mL NS is physically and chemically stable for up to 21 days at 4°C or 23°C when stored in either the manufacturer’s original glass vial or a syringe. Subcutaneous injection of bortezomib represents a change in practice, and there is a potential safety concern if a solution of the increased concentration used for subcutaneous administration (2.5 mg/mL) is inadvertently used to prepare a dose intended for IV administration. Therefore, it is recommended that sites switching to subcutaneous administration of bortezomib eliminate 1.0 mg/mL IV solutions altogether or institute substantial barriers to prevent IV administration of the higher concentration of bortezomib.
KEYWORDS:
bortezomib
,
drug stability
,
oncology
,
IV therapy
RÉSUMÉ
Contexte :
Les solutions de bortézomib de 1,0 mg/mL pour administration intraveineuse sont jugées stables pour une période allant jusqu’à 42 jours. Des publications récentes indiquent que le profil d’innocuité du bortézomib est meilleur par administration sous-cutanée que par administration intraveineuse.
Objectif :
Évaluer la stabilité de solutions de bortézomib à concentration plus élevée pour l’administration sous-cutanée (c.-à-d., 2,5 mg/mL dans du chlorure de sodium à 0,9 % [solution physiologique salée ou SP])
Méthodes :
Au jour 0 de l’étude, on a reconstitué douze fioles de 3,5 mg de bortézomib sous forme de poudre avec 1,4 mL de SP par fiole afin d’obtenir des solutions d’une concentration de 2,5 mg/mL. La moitié de ces solutions a ensuite été entreposée dans les fioles d’origine et l’autre moitié a été placée dans des seringues. Trois seringues et trois fioles ont été entreposées au réfrigérateur (4 °C) alors que trois fioles et trois seringues ont été entreposées à la température ambiante (23 °C). Une analyse de la concentration ainsi qu’une inspection physique ont été effectuées aux jours 0, 1, 2, 8, 12, 14, 19 et 21 de l’étude. La concentration de bortézomib a été déterminée à l’aide d’une épreuve validée par chromatographie liquide avec détection ultraviolette. La durée de conservation a été établie en fonction du temps nécessaire pour atteindre 90 % de la concentration initiale, selon le taux de dégradation le plus rapide calculé grâce à l’intervalle de confiance à 95 % du taux de dégradation observé.
Résultats :
La méthode analytique a séparé le bortézomib de ses produits de dégradation de manière que la concentration a été mesurée de façon spécifique et précise (l’écart absolu par rapport à la concentration connue était en moyenne de 2,99 %), avec une reproductibilité moyenne intrajournalière et interjournalière respectivement de 1.51 % et de 2.51 %. Pendant l’étude, toutes les solutions conservaient au moins 95,26 % de la concentration initiale aux deux températures dans les fioles et les seringues.
Conclusions :
La solution obtenue à partir de la reconstitution du contenu d’une fiole de 3,5 mg de bortézomib (fiole du fabricant) avec 1,4 mL de SP est physiquement et chimiquement stable pendant une période allant jusqu’à 21 jours à des températures de 4 °C ou 23 °C lorsqu’elle est entreposée dans la fiole de verre d’origine du fabricant ou dans une seringue. L’injection sous-cutanée de bortézomib représente un changement dans la pratique. Une inquiétude demeure à propos d’un risque possible pour la sécurité des patients si une solution à concentration plus élevée (2,5 mg/mL), destinée à l’administration sous-cutanée, est utilisée par mégarde pour la préparation d’une dose à administrer par voie intraveineuse. Il est donc recommandé que les établissements qui se tournent vers l’administration sous-cutanée de bortézomib éliminent toutes les solutions de 1,0 mg/mL destinées à l’administration intraveineuse ou qu’ils mettent en place des obstacles importants pour éviter l'administration intraveineuse de solutions de bortézomib à concentration élevée.
MOTS CLÉS:
bortézomib
,
stabilité du médicament
,
oncologie
,
thérapie intraveineuse
[Traduction par l’éditeur]
INTRODUCTION
Bortezomib is indicated for the treatment of patients with previously untreated multiple myeloma who are unsuitable for stem cell transplantation, for the treatment of progressive multiple myeloma in patients who have received at least one prior course of therapy, or for the treatment of patients with mantle cell lymphoma that has relapsed or that was refractory to at least one prior course of therapy.1 It is available in Canada as 3.5 mg of sterile lyophilized powder in a 10-mL clear glass vial intended for reconstitution with 3.5 mL of 0.9% sodium chloride (normal saline [NS]) to prepare a 1.0 mg/mL solution.
When this drug was initially marketed, the manufacturer advised that the total storage time for reconstituted bortezomib must not exceed 8 h with exposure to usual indoor lighting.2 In 2008, Walker and others3 showed that bortezomib 1.0 mg/mL solutions intended for IV administration retained more than 95% of initial concentration for up to 42 days with storage at either 4°C or 23°C. In 2011, Moreau and others4 reported that among 222 patients with relapse of multiple myeloma, there was no significant difference in time to progression or 1-year overall survival with subcutaneous (SC) versus IV bortezomib. Adverse events were significantly less common with SC than IV administration, and the SC solution was well tolerated locally.4 To limit the volume injected, solutions for SC injection were prepared to a concentration of 2.5 mg/mL (3.5 mg bortezomib reconstituted with 1.4 mL of NS).4 Because SC administration achieved equal efficacy with fewer adverse events, the SC route should be the preferred method of administration, yet no data exist to support extended stability, and the current product monograph indicates that the reconstituted material may be stored for no more than 8 h.1
The objective of this study was to evaluate the stability of bortezomib over 21 days of storage at room temperature (23°C) or at 4°C in glass vials or polypropylene syringes after reconstitution of the manufacturer’s 3.5-mg vials with 1.4 mL of NS to produce 2.5-mg/mL solutions.
METHODS
Chromatographic Analysis
This study used the stability-indicating method of André and others,5 which was validated in the authors’ laboratory during the 2008 stability study of bortezomib.3 A modified separation method was revalidated in the authors’ laboratory before the current study began, according to accepted criteria.6–8 The liquid chromatographic system consisted of a solvent delivery pump (model P4000, Thermo Separation Products, Fremont, California), which pumped a mixture of 30% acetonitrile and 70% 0.05 mol/L potassium phosphate dibasic (catalogue no. 191431, MP Biomedicals, Solon, Ohio; lot 9761K). The pH of the buffer was adjusted to 6.8 with concentrated phosphoric acid (HPLC-grade, catalogue no. P286-1, Fisher Scientific, Fair Lawn, New Jersey; lot 076856) before it was mixed with acetonitrile. On each day, the strength of the mobile phase was adjusted to achieve a retention time for bortezomib of about 6.6 min through a 25 cm × 4.6 mm reverse-phase C18, 5-μm column (Supelco ABZ+, Sigma-Aldrich, Oakville, Ontario) at 1.0 mL/min. Two microlitres of each prepared sample, quality control sample, or standard was injected, in duplicate, directly onto the liquid chromatographic column using an autoinjector (Ultra WISP 715, Waters Scientific, Toronto, Ontario).
The column effluent was monitored with a variable-wavelength ultraviolet (UV) detector (UV6000, Thermo Separation Products, San Jose, California) at 270 nm. The signal from the detector was integrated and recorded with a chromatography data system (ChromQuest, version 5.0, Thermo Fisher Scientific Inc, Nepean, Ontario). The area under the bortezomib peak at 270 nm was subjected to least-squares linear regression, and the actual bortezomib concentration in each sample was determined by interpolation from the standard curve.
Assay Validation
Following set-up of the chromatographic system for bortezomib, the suitability of this method for use as a stability-indicating assay was tested by assaying samples that had been subjected to accelerated degradation of bortezomib with various concentrations of sodium hypochlorite. The contents of a 3.5-mg vial of bortezomib (bortezomib mannitol boronic ester for injection, Velcade, Janssen Ortho Inc; lot BIZSC00, expiry August 2014) were dissolved in 3.5 mL of distilled water to prepare a 1.0 mg/mL solution. To 100 μL of this solution, 5 μL of sodium hypochlorite (sodium hypochlorite 1%, Hygeol, Wampole Canada; lot 0B030A, expiry February 2014) of various concentrations (0.80%, 0.70%, 0.50%, 0.30%, 0.25%, 0.20%, 0.10%, 0.01%, and 0% [vehicle only]), prepared by dilution with distilled water, was added. Each mixture was vortexed and chromatographed immediately. Chromatograms from all samples were inspected for the appearance of additional peaks, and the bortezomib peak was compared between samples for changes in concentration, retention time, and peak shape (using electronic overlay and numeric calculation of tailing). UV spectral purity (200–365 nm, 6 nm bandwidth, deuterium lamp; UV6000, Thermo Separation Products, Fremont, California) of the bortezomib peak in a chromatogram of a sample degraded with sodium hypochlorite was compared with the spectrum of the authentic, undegraded sample of bortezomib in water obtained at time 0.
Following this first phase of evaluation and validation, the accuracy and reproducibility of standard curves were tested over 5 days, and system suitability criteria (theoretical plates, tailing, and retention time) were developed to ensure consistent chromatographic performance on each study day.9
Stability Study
On study day 0, twelve 3.5-mg vials of bortezomib (bortezomib mannitol boronic ester for injection, Velcade, Janssen Inc; lot BIZSC00, expiry August 2014) were each reconstituted with 1.4 mL of NS to prepare 2.5-mg/mL solutions. The contents of 6 of the vials were drawn into a corresponding number of 3-mL polypropylene syringes. Half of each type of container (3 vials and 3 syringes) were stored at room temperature (23°C ± 2°C), unprotected from fluorescent room light, and the other half (3 vials and 3 syringes) were stored in the refrigerator (4°C) without exposure to fluorescent lighting in the room.
Physical Stability
On study days 0, 1, 2, 8, 12, 14, 19, and 21, samples were drawn for concentration analysis and were inspected visually against white and black backgrounds for changes in colour and presence of particulate matter.
Bortezomib Analysis
On each study day (0, 1, 2, 8, 12, 14, 19, and 21), a 3.5-mg vial of bortezomib (Janssen Inc; lot BIZSC00, expiry August 2014) was reconstituted with 1.167 mL of distilled water to make a 3 mg/mL solution. On each study day this solution was further diluted to prepare standards with final concentrations of 3.000, 2.250, 1.125, 0.563, and 0.375 mg/mL. When combined with a blank, these standards served to construct a standard curve. In addition, 2 quality control samples with bortezomib concentrations of 0.75 and 1.5 mg/mL were prepared from the same stock solution. Two microlitres of each standard or quality control sample was chromatographed in duplicate without dilution. Intraday and interday errors were assessed by the coefficients of variation (CVs) of the peak areas of both quality control samples and standards.
On each study day (0, 1, 2, 8, 12, 14, 19, and 21), samples drawn from each of the 3 vials and 3 syringes stored at each temperature were assayed for bortezomib content. All samples initially contained a nominal concentration of 2.5 mg/mL of bortezomib. Two microlitres of each sample was injected directly onto the liquid chromatographic system without further preparation, in duplicate, to ensure the ability to distinguish between solutions that differed in concentration by 10% or more.10,11
Data Reduction and Statistical Analysis
After the CV of the assay was determined, a power calculation showed that duplicate sample analysis of 3 replicate samples combined with 7 study days had the ability to distinguish between concentrations that differed by at least 1% within each individual container.10,11 Means were calculated for replicate analyses. Mean results from different days for each assay were compared statistically to determine whether there was an association between observed result and time. Linear regression and multiple linear regression were used to determine whether there was an association between observed concentration and study day, type of container, or storage temperature. The 95% confidence interval (CI) for the percent remaining on the last study day was calculated for vials and syringes held at 4°C and 23°C. Analysis of variance was used to test differences in concentration on different study days, with different types of container, and at different storage temperatures. The 5% level was used as the a priori cut-off for significance. The concentration of bortezomib was considered “acceptable” or “within acceptable limits” if the lower limit of the 95% CI of concentration remaining was greater than 90% of the initial (day 0) concentration.
RESULTS
Accelerated Degradation and Assay Validation
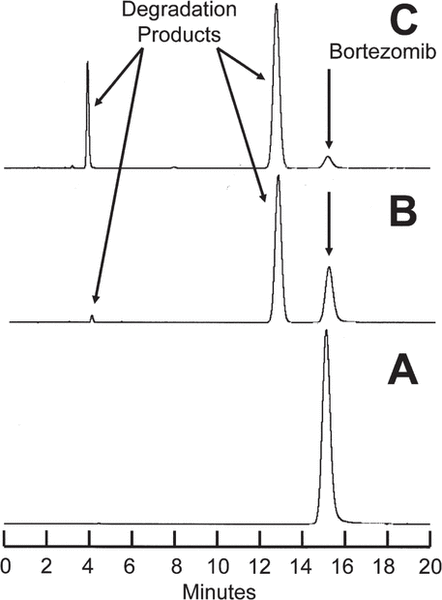
Degradation of bortezomib with sodium hypochlorite occurred quickly. At 23°C, a 1.0 mg/mL solution of bortezomib in water was degraded to 6.32% remaining when 5 μL of sodium hypochlorite at concentration 0.5% was added. Solutions containing lower concentrations of sodium hypochlorite degraded bortezomib more slowly. For example, when 5 μL of a 0.25% solution was added and the sample chromatographed immediately, 42.38% of the original concentration of bortezomib remained. These solutions were observed to contain degradation products of bortezomib, which eluted at 4.3 and 13.5 min (Figure 1). Additional peaks eluted at 29 min and between 4 and 9 min when concentrations of sodium hypochlorite above 0.4% were added. None of these degradation products interfered with quantification of bortezomib, and the UV spectrum of the bortezomib peak (200–365 nm) in a degraded sample was no different from the spectrum of the authentic, undegraded standard. The chromatograms of samples degraded with sodium hypochlorite in the current study were virtually identical with chromatograms produced by André and others5 using hydrogen peroxide as the degrading agent. Hydrogen peroxide and sodium hypochlorite produce all of the degradation products of bortezomib that can be produced by acid, base, and/or heat, as well as 2 other degradation products which eluted in our system at 13.5 and 29 min.
| |
|
|
|
Figure 1.
A: Chromatogram for bortezomib 2.5 mg/mL in water before addition of sodium hypochlorite. B: Chromatogram for bortezomib 2.5 mg/mL in water immediately after addition of 5 μL 0.25% sodium hypochlorite; 42% of the original amount of bortezomib remains. C: Chromatogram for bortezomib 2.5 mg/mL in water immediately after addition of 5 μL 0.5% sodium hypochlorite; 6% of the original amount of bortezomib remains. Degradation products appear at 4.3 and 13.5 min. Additional products (not evident in these images) appeared at 4.8, 8.7, and 29 min.
|
As a result of the chromatographic separation of these degradation products from bortezomib and the similarity of the UV spectrum (200–365 nm) between an authentic standard and bortezomib in a degraded sample, it was concluded that this analytical method was stability-indicating.6–8
Analysis of standard curves and quality control samples during validation indicated that the bortezomib concentration was measured accurately. Over the study period, standards and quality control samples showed an average absolute deviation of 2.99% from the expected concentration. Interday variation in sample concentration assessed through the standard deviation of the regression (
S
y.x in Table 1) and expressed as CV averaged 0.76%. Analytical reproducibility (as measured by CV) averaged 1.51% within a day and 2.51% between days. These results indicate that differences of 10% or more could be confidently detected within individual containers with acceptable error rates.10,11
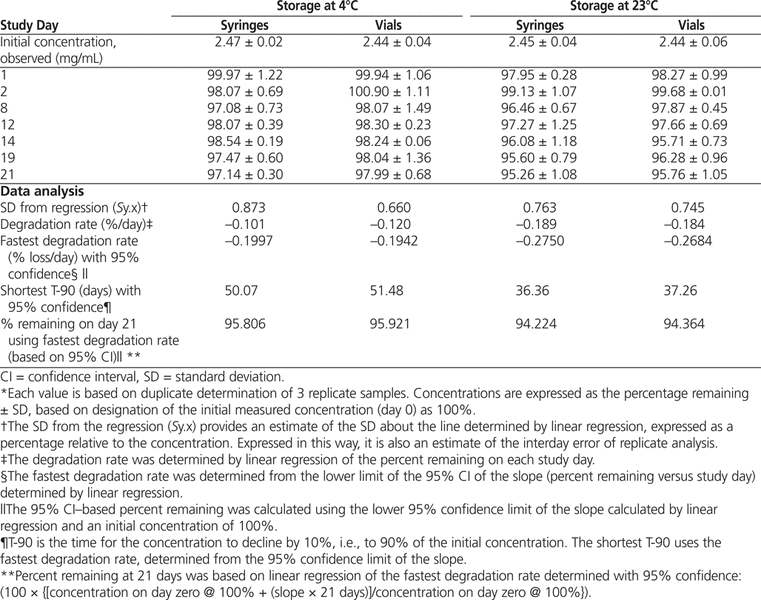
Table 1.
Percentage of Bortezomib Remaining after Storage*
Bortezomib Stability Study
All solutions in the original manufacturer’s glass vials were initially clear and colourless and remained so for the duration of the study. No particles were visible in any solution throughout the study period.
Over the study period, concentrations observed in all study samples at both temperatures retained at least 95.26% of their initial concentration (Table 1). Over the 21-day study period the bortezomib concentration varied between days (as assessed by standard deviation of regression and CV) by less than 1.0% for both vials and syringes at both room temperature and 4°C.
Analysis of variance revealed significant differences in percent remaining due to study day (
p
= 0.002) and temperature (
p
= 0.011), but not type of container (
p
= 0.22). The method was capable of detecting a 1% difference in concentration due to study day, storage temperature, or type of container.
Linear regression was also able to detect a significant linear trend for the concentration to change during the study period for vials (
p
= 0.008) and syringes (
p
= 0.046) stored at 4°C and for vials (
p
= 0.002) and syringes (
p
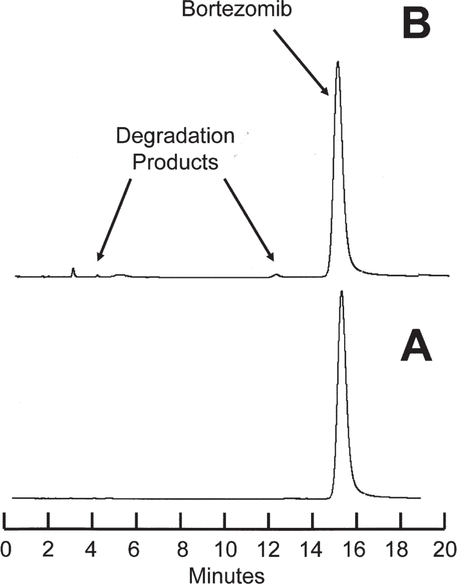
= 0.002) stored at room temperature. It was determined that after 21 days’ storage at 4°C, more than 95.8% of the initial concentration will remain in both vials and syringes, with 95% confidence. Similarly, after 21 days storage at room temperature, more than 94.2% of the initial concentration will remain in both vials and syringes, with 95% confidence. Inspection of chromatograms during the stability study (Figure 2) revealed small amounts of degradation products in solutions stored at room temperature for 21 days.
| |
|
|
|
Figure 2.
Chromatograms for bortezomib 2.5 mg/mL reconstituted with saline in the manufacturer’s original glass vial and stored at room temperature (23°C) during the stability study. A: Study day 0. B: After 21 days’ storage. Very small amounts of the degradation products observed during the acceleration study were observed in solutions stored at room temperature.
|
DISCUSSION
In this study, demonstration of a trend or a consistent decline in concentration during the study was considered more important than demonstrating a statistically significant difference in concentration between any 2 days. In fact, while significant differences between concentrations observed on any 2 study days could be observed (
p
< 0.002), these random fluctuations in concentration around the initial concentration were minor and not of practical importance and should be considered “noise” or experimental error. During the study period, concentrations observed in all study samples at both storage temperatures remained within 5.5% of the initial concentration. The concentration on day 21 as estimated by linear regression was within 5% of the initial (day 0) concentration, and the lower limit of the 95% CI of the percentage remaining on day 21 was within 6% of the initial (day 0) concentration.
Because only small changes in bortezomib concentration were detected under these storage conditions, assurance of the specificity of the analytical method is very important. The specificity of the analytical method was demonstrated during the accelerated degradation studies (Figure 1). In these studies, reduced bortezomib concentrations were measured as the concentration of apparent degradation products increased. The separation and detection of intact drug in the presence of degradation compounds must be assured before the method can be considered stability-indicating.6–8
Previous studies have shown that 1 mg/mL solutions of bortezomib in NS retain more than 95% of the initial concentration when stored at 4°C for 15 days,12 1 month,13 or 42 days.3 The current investigation has demonstrated similar stability of a 2.5 mg/mL solution over 21 days, indicating that concentration has little effect on stability. It is well accepted that appropriate extension of a drug’s expiry date can reduce wastage.14
SC injection of bortezomib represents a change in practice, and preparation of higher-concentration solutions represents a potential patient safety issue if a 2.5 mg/mL solution is mistaken for a 1.0 mg/mL solution and is inadvertently administered by the IV route. Therefore, we recommend that sites adopting SC administration of bortezomib should eliminate 1.0 mg/mL IV solutions altogether or should place significant barriers to IV administration of bortezomib to minimize the risk to patient safety.
We conclude that solutions of bortezomib 2.5 mg/mL, prepared from 3.5-mg vials reconstituted with 1.4 mL NS, are physically and chemically stable for up to 21 days at 4°C or room temperature in both syringes and the original manufacturer’s glass vial.
References
1. Velcade [product monograph]. Toronto (ON): Janssen Inc; 2012 Dec 18.
2. Velcade [product monograph]. Toronto (ON): Ortho-Biotech; 2005 Jan.
3. Walker SE, Milliken D, Law S. Stability of bortezomib reconstituted with 0.9% sodium chloride at 4°C and room temperature (23°C). Can J Hosp Pharm. 2008;61(1):14–20.
4. Moreau P, Pylypenko H, Grosicki S, Karamanesht L, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–40. Erratum in: Lancet Oncol. 2011;12(6):522.

5. André P, Cisternino S, Chiadmi F, Toledano A, Schlatter J, Fain O, et al. Stability of bortezomib 1-mg/mL solution in plastic syringe and glass vial. Ann Pharmacother. 2005;39(9):1462–6.

6. Trissel LA. Avoiding common flaws in stability and compatibility studies of injectable drugs. Am J Hosp Pharm. 1983;40(7):1159–60.
7. Trissel LA, Flora KP. Stability studies: five years later. Am J Hosp Pharm. 1988;45(7):1569–71.
8. Policy for publication of chemical stability study manuscripts. Can J Hosp Pharm. 1990;43(1):3–4.
9. Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, et al. Analytical methods validation: bioavailability, bioequivalence, and pharmacokinetic studies [conference report]. J Pharm Sci. 1992;81(3):309–12.

10. Frieman JA, Chalmers TC, Smith H Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 “negative” trials. N Engl J Med. 1978; 299(13):690–4.

11. Stolley PD, Strom BL. Sample size calculations for clinical pharmacology studies. Clin Pharmacol Ther. 1986;39(5):489–90.

12. Vanderloo JP, Pomplun ML, Vermeulen LC, Kolesar JM. Stability of unused reconstituted bortezomib in original manufacturer vials. J Oncol Pharm Pract. 2011;17(4):400–2.

13. Bolognese A, Esposito A, Manfra M, Catalano L, Petruzziello F, Martorelli MC, et al. An NMR study of the bortezomib degradation under clinical use conditions. Adv Hematol. 2009;2009:704928.


14. Walker SE, DeAngelis C, Iazzetta J, Gafni A. Chemotherapy waste reduction through shelf-life extension. Can J Hosp Pharm. 1994;47(1):15–23.
Scott E Walker
, MScPhm, is Director of Pharmacy and of the Division of Pharmacology, Sunnybrook Health Sciences Centre, and Associate Professor, Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Ontario.
Lauren F Charbonneau
, BSc(Pharm), is Manager of the Odette Cancer Centre Pharmacy, Sunnybrook Health Sciences Centre, Toronto, Ontario.
Shirley Law
, DipPharmTech, is a Research Assistant in Quality Control, Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, Ontario
Competing interests:
None declared.
Address correspondence to: Scott E Walker, Director of Pharmacy, Sunnybrook Health Sciences Centre, 2075 Bayview Avenue, Toronto ON M4N 3M5, e-mail:
scott.walker@sunnybrook.ca
Acknowledgements
This work was supported by an unrestricted educational grant from Janssen Canada Inc.
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
67
, NUMBER
2
,
March-April
2014