Pamela Fu , Glen Brown , Michael Legal , Stephen Shalansky
To promote heightened vigilance concerning antibiotic agents in Canadian hospitals, Accreditation Canada has implemented antibiotic stewardship as a required organizational practice.1 Antibiotic stewardship is the process of ensuring that only necessary and appropriate antibiotic agents are used and that their use is appropriate for each individual patient. It involves optimizing all steps of antibiotic therapy, including appropriate selection, dosing, route, and duration. Through antibiotic stewardship strategies, a hospital can optimize the use of antibiotics, reduce the risk of opportunistic infections, stabilize or reduce antibiotic resistance, promote patient safety, and reduce health care costs.1,2 According to the American Society of Health-System Pharmacists, pharmacists have the ability, while providing care to individual patients, to effectively participate in antibiotic stewardship through their activities as members of health care teams and committees and by using their knowledge and abilities to focus on proper antibiotic utilization.3,4
The current study was conducted within the Providence Health Care health authority, which comprised 2 acute care hospitals and several residential facilities in the lower mainland of British Columbia. In this health authority, pharmacist-initiated antibiotic stewardship practices have been in place for many years, despite the absence of a formal antibiotic stewardship program. No pharmacists or physicians were specifically assigned responsibility for ensuring that antibiotic use was appropriate for admitted patients. However, pharmacists at Providence Health Care were involved on a day-to-day basis in pharmaceutical care related to all aspects of drug therapy for admitted patients, including antibiotic therapy. In the course of providing care, pharmacists frequently performed antibiotic stewardship activities, such as making recommendations to narrow the spectrum of activity of prescribed therapy, adjusting dosages, stepping down from parenteral to oral therapy, stopping unnecessary antibiotics, and switching to more appropriate antibiotics. The Antibiotic Subcommittee of the health authority’s Pharmacy and Therapeutics Committee had established restrictions, procedures, and recommendations regarding the use of antibiotic agents. The Pharmacy Department had an expectation that all pharmacists would perform activities appropriate for ensuring the optimal use of antibiotic agents. The purpose of this study was to quantify the antibiotic stewardship activities performed by pharmacists at Providence Health Care before implementation of a formal antibiotic stewardship program. A literature search identified no studies quantifying antibiotic stewardship activities performed by pharmacists in any Canadian hospital.
The primary objective of the study was to quantify the various potential antibiotic stewardship activities related to target broad-spectrum antibiotics at St Paul’s Hospital and Mount Saint Joseph Hospital, the 2 acute care hospitals within Providence Health Care. The primary outcome was the proportion of patients included in the review who received the target antibiotics and experienced at least one antibiotic stewardship intervention by a pharmacist. The secondary objective was to determine the frequency of each type of antibiotic stewardship intervention.
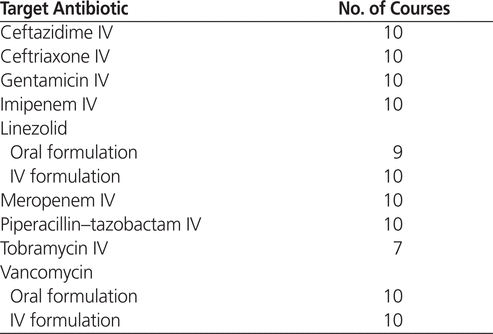
This study was a qualitative retrospective analysis of pharmacists’ documented antibiotic stewardship activities for a convenience sample of patients from St Paul’s Hospital and Mount Saint Joseph Hospital who received one of the target antibiotics (listed in Table 1). The target antibiotics were selected from all of the antibiotics used within Providence Health Care on the basis of their broad spectrum of activity, frequency of use, risk of toxicity, or high costs. Providence Health Care pharmacists were expected, as part of their pharmaceutical care duties, to intervene on any occasion of inappropriate use of these antibiotics, with the aim of reducing development of resistance, toxic effects, and costs. For each course of antibiotic therapy, pharmacists were expected to determine if the antibiotic was most appropriate for the known or presumed organism at the known or presumed site of infection. They were also expected to assess the dosage to optimize the potential for efficacy with the least toxicity. In addition, pharmacists were expected to initiate conversion to oral therapy, where appropriate, and to suggest discontinuation of antibiotic therapy when a patient’s clinical status indicated that continued antibiotic therapy was unwarranted. The selected antibiotics are subsequently referred to as “target antibiotics” (Table 1).
Table 1.
Target Antibiotics and Number of Treatment Courses Assessed

Treatment courses involving any of the target antibiotics in any eligible wards within the 2 hospitals were reviewed. Treatment courses that occurred during outpatient visits to the emergency department, in the operating rooms, on psychiatry wards, or in any ambulatory clinic were excluded. Only treatment courses longer than 24 h were eligible for evaluation of pharmacists’ interventions. The antibiotic treatment courses were identified retrospectively through a report generated by the health authority’s pharmacy distribution system, which contains the complete drug therapy record for every inpatient. To ensure that the results reflected a broad range of antibiotics and a wide range of pharmacists’ activities, the following restrictions were applied. A maximum of 2 treatment courses per ward were selected from each calendar week, to ensure evaluation over a sustained period. A maximum of 10 treatment courses for all target antibiotics combined were selected from any single ward, and a maximum of 5 treatment courses involving any one target antibiotic were selected per ward. Many hospital wards had more than 5 treatment courses for a given target antibiotic or more than 10 treatment courses in total, but the restrictions on selection aimed to broaden the assessment of pharmacists’ activities to maximize the period of analysis, the number of pharmacists in the cohort, and the number of hospital wards. Similarly, a maximum of 10 treatment courses were selected for any given individual antibiotic across all eligible wards. Data collection occurred for the period from October 1, 2012, through March 31, 2013. No data were collected for dates beyond March 31, 2013, even if the number of treatment courses for a given ward was less than 10. The data were collected retrospectively, with random selection of the specific antibiotic treatment courses meeting the inclusion and exclusion criteria by one of the investigators (P.F.), who was unfamiliar with both the patients and the pharmacists providing care.
For each patient receiving one of the target antibiotics, the pharmacy’s patient monitoring form was retrieved retrospectively following the patient’s discharge. The patient monitoring form is a document that Providence Health Care pharmacists use to communicate patient-specific drug therapy issues throughout the patient’s stay, across potentially numerous hospital locations and pharmacists. The form also serves as the clinical workload measurement tool to document pharmacists’ interventions when providing patient care. The hospital’s pharmacy administration expected that pharmacists would record details on the patient monitoring form each time they performed an intervention changing any aspect of drug therapy for any patient under their care. Pharmacy administration considered that there was good compliance with documentation of clinical activities by pharmacists during the period of patient care for which data were analyzed.
The patient monitoring forms were reviewed for documentation of any interventions related to the treatment courses selected for analysis. Specific interventions with the potential for value in antibiotic stewardship (Box 1) were selected for evaluation. In addition to reviewing the monitoring form, patients’ health records were reviewed to generate a description of the patient population, the rationale for antibiotic therapy, and the extent of documentation by pharmacists in the health care record. Patients’ health care records were also reviewed to confirm agreement with pharmacists’ interventions as documented on the patient monitoring form.
Box 1. Clinical Antibiotic Interventions Performed by PharmacistsInitiate antibiotic
Continue therapy
Discontinue therapy
Increase dose
Decrease dose
Clarify antibiotic order
Narrow the spectrum of activity
Step down (IV to oral)
Request drug levels
Pharmacokinetic calculation of dose
Monitor pharmacokinetics
Coordinate seamless care or apply for special authority
Obtain approved prescriber confirmation of appropriate therapy
Counsel patient
The study was approved by the health authority’s Research Ethics Board and the local university’s Research Ethics Board. Because this study was a qualitative, retrospective analysis of clinical interventions, the consent of patients and pharmacists was not required. More specifically, patients’ consent was not necessary because the study had no impact on individual patient care. Pharmacists’ consent was not required because the study methodology evaluated activities that, during the period of patient care for which data were analyzed, were already expected of the pharmacists by the institution. Informed consent was waived by both research ethics boards. There were no external sources of funding.
Of the 106 treatment courses included in the study, 82 were administered at St Paul’s Hospital and 24 at Mount Saint Joseph Hospital. This split reflected the number of acute care beds at the 2 facilities (360 at St Paul’s Hospital and 85 at Mount Saint Joseph Hospital). Based on the process for selecting treatment courses for analysis, each target antibiotic reached the maximum of 10 treatment courses, except for linezolid PO and tobramycin IV (Table 1). For the primary outcome, 80 (75%) of the 106 treatment courses had one or more interventions: 62 at St Paul’s Hospital and 18 at Mount Saint Joseph Hospital (equivalent frequency for the 2 institutions).
A total of 327 interventions were performed, an average of 3 interventions per treatment course. The most frequent type of intervention, continuation of therapy, accounted for 85 (26%) of the 327 interventions (Figure 1). Another 101 (31%) of the interventions involved pharmacokinetic monitoring (i.e., requesting drug levels, pharmacokinetic calculation of dosage, or monitoring of pharmacokinetics). Just under half of these pharmacokinetic interventions resulted in recommendations to increase or decrease the dose (42 [13%] of the interventions). Among the less common interventions, 13 (4%) involved initiating an antibiotic, 13 (4%) involved narrowing the spectrum of activity, 13 (4%) involved discontinuation of therapy, and 7 (2%) involved step-down from IV to oral therapy. Given that pharmacokinetic monitoring accounted for a major proportion of the interventions, it was not surprising that the top 3 antibiotics represented by the interventions were gentamicin IV (65 [20%]), vancomycin IV (65 [20%]), and tobramycin IV (52 [16%]) (Figure 2).
|
|
||
|
Figure 1. Frequency of each type of intervention ( n = 327). PK = pharmacokinetics, SA = special authority for medication, IV–PO step-down = step-down from intravenous to oral therapy. |
||
|
|
||
|
Figure 2. Frequency of interventions involving each antibiotic ( n = 327). IV = intravenous formulation, PO = oral formulation. |
||
The findings reported here suggest that pharmacists at Providence Health Care were frequently involved in antibiotic stewardship activities, even before a formal antibiotic stewardship program was created. No similar evaluation from a Canadian hospital could be found, which prevented comparison with results obtained by other investigators. We feel that these findings are truly representative of day-to-day, real-world activities in our health authority, because the methodology ensured a broad overview of pharmacists’ activities and patients’ treatments. The maximum number of treatment courses ( n = 10) was achieved for all target antibiotics except linezolid PO and tobramycin IV (Table 1), and the results were obtained from a large range of wards, which ensured that they reflected the activities of a large number of pharmacists.
Certain methodologic limitations warrant consideration. The study used a sampling technique to select only a limited number of patients who experienced the clinical pharmacy services of interest. This sampling technique used specific wards, interventions, and target antibiotics as inclusion criteria, in an attempt to ensure that the activities of a large number of pharmacists were assessed and to ensure that a variety of interventions was included. The study was structured to assess pharmacists’ antibiotic stewardship activities over a wide range of inpatient services. Evaluation of data for all patients receiving the target drugs over the study period would have provided a more complete picture of pharmacists’ stewardship activities, but this scope of analysis was not attempted because of resource limitations.
The study encompassed a variety of inpatient wards but excluded patients from the emergency department and the ambulatory clinics (including the home IV program). These exclusions were appropriate to the study purpose, which was to evaluate antibiotic stewardship activities involving inpatients in the study hospitals. The study method did not allow evaluation of pharmacists’ participation in antibiotic stewardship activities for outpatients. With the movement toward treating infections through programs such as the home IV program or rapid treatment pathways in the emergency department, it would be beneficial to include these 2 areas in future studies, as pharmacists play a key role in the care of ambulatory patients treated through these programs.
The choice of target antibiotics for this study was based on spectrum of activity, frequency of use, toxicities, and financial costs. The target drugs did not include any antibiotics from the fluoroquinolone class because of their lower frequency of use, the established resistance pattern to ciprofloxacin within the health authority, and the frequent use of only oral formulations of these drugs. Given these characteristics, it was anticipated that the need for interventions by pharmacists would be low. Other institutions with dissimilar patterns of fluoroquinolone use may find it beneficial to assess pharmacists’ antibiotic stewardship activities in relation to this drug class. Despite the exclusion of the fluoroquinolone class, the maximum number of treatment courses ( n = 10) was achieved for all but 2 of the target antibiotics (Table 1). For the purposes of evaluating baseline antibiotic stewardship practices, these results indicate that an adequate range of target antibiotics was included in the study.
Given that Providence Health Care serves the area as a tertiary hospital and a medical teaching facility, there were certain expectations of its pharmacists. Assessment of antibiotic regimens for appropriateness was one expectation. As specified by Accreditation Canada, an antibiotic stewardship program involves interprofessional collaboration and applicable education of health care providers.1 It is therefore another expectation of the Providence Health Care Pharmacy Department that pharmacists will educate other health care professionals during daily interactions with them in the course of providing patient care; however, the study method did not include documentation of antibiotic stewardship educational activities carried out by the pharmacists.
Specific interventions (Box 1) were predefined to ensure collection of data for pharmacists’ known antibiotic stewardship activities. A succinct definition of each intervention was used to facilitate data collection. The activities assessed were similar to the intervention activities covered by prospective auditing, a component of full antibiotic stewardship programs.1
The primary objective was to quantify the clinical interventions that pharmacists performed for patients receiving target antibiotics. However, the study did not assess the breakdown of types of interventions for each antibiotic used. A much larger sample size for each individual antibiotic would be necessary to provide meaningful results for individual antibiotics. If an institution wished to assess pharmacists’ actions for a specific antibiotic, this study method could be applied with a larger population.
Two factors contributed to continuation of therapy being the most prevalent intervention (Figure 1). First, at Providence Health Care, there is an automatic stop date of 5 days for IV antibiotics. The purpose of an automatic stop date is to ensure that antibiotics are reassessed in a timely fashion. Second, indications for the evaluated treatment courses frequently included disease states that require a long duration of therapy, such as bacteremia, exacerbation of cystic fibrosis, and Clostridium difficile diarrhea. The large proportion of interventions accounted for by continuation of therapy raises questions about the utility of the automatic stop date and the impact of this process on pharmacists’ workload. However, through the process of reviewing patient monitoring forms and charts, it was observed that re-evaluation of whether to continue therapy led to other interventions, such as initiation or discontinuation of antibiotic therapy, pharmacokinetic monitoring, and IV-to-oral step-down. The automatic stop dates therefore helped to flag those courses of antibiotic therapy that warranted evaluation for intervention. According to Accreditation Canada, the main goal of an antibiotic stewardship program is to optimize the use of antibiotics to achieve the best patient outcomes.1 Having an automatic stop date as a safety parameter prompted mandatory assessments, which frequently resulted in alteration of therapy.
Pharmacokinetic monitoring was another frequent intervention, which highlights this institution’s efforts in providing this service. An important component of antibiotic stewardship is not only to ensure that the antibiotic selected is appropriate for the indication, but also to ensure that the dosage is appropriate to maximize efficacy and minimize toxicity. The results presented here suggest that the pharmacists at this institution do both an assessment of antibiotic selection for indication and, where appropriate, a pharmacokinetic assessment of the dosage.
Interventions that occurred less frequently, such as initiating antibiotic use, narrowing the spectrum of activity, discontinuing therapy, and IV-to-oral step-down, may represent areas where interventions could be increased. However, many of the target antibiotics in this study did not have oral formulations as options for consideration. The addition of fluoroquinolones as a target group might have increased the frequency of IV-to-oral step-down interventions, although it is thought that interventions for fluoroquinolone therapy are needed only infrequently at this institution. The frequency of unaddressed need for narrowing the spectrum of activity or discontinuing therapy could not be estimated with the study methods used.
The interventions selected for review are considered important aspects of an antibiotic stewardship program. The broader components of a stewardship program, such as prospective formal education programs or long-term surveillance of sensitivity patterns, are not functions expected of front-line pharmacists at the study institution. At Providence Health Care, pharmacists are expected to educate other health care providers encountered during day-to-day care, but structured education programs are not an expectation. Similarly, the institution’s medical microbiology department conducts surveillance of resistance patterns.
Reviewing patient monitoring forms and patients’ health records may not have been the most comprehensive way to capture less frequently encountered antibiotic stewardship activities. It would be beneficial for Providence Health Care to establish documentation processes for antibiotic stewardship interventions, especially those that were observed less frequently in this study (e.g., initiation of antibiotic therapy, narrowing the spectrum of activity, IV-to-oral step-down).
Accreditation Canada recommends prospective audit and feedback for antibiotic therapy.1 This study showed that pharmacists at Providence Health Care were frequently involved with aspects of antibiotic stewardship before a structured antibiotic stewardship program had been established. However, evaluation of the types of interventions that pharmacists performed suggests potential areas for increased activity and intervention. These pharmacists should explore opportunities for interventions related to initiating antibiotics, narrowing the spectrum of therapy, and step-down from IV to oral therapy; they should also explore opportunities for establishing formal intraprofessional and interprofessional education programs. Institutions without a structured antibiotic stewardship program could consider using a method similar to the one described here to obtain a baseline quantitative measurement of their antibiotic stewardship activities and to identify areas of potential growth before, or as a component of, creating a formal antibiotic stewardship program.
1. Required organizational practices. Ottawa (ON): Accreditation Canada; 2012 [cited 2012 Jun 1]. Available from: www.accreditation.ca/uploadedFiles/ROP%20Handbook%20EN.pdf
2. Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77.
3. ASHP Council on Pharmacy Practice. ASHP statement on the pharmacist’s role in antimicrobial stewardship and infection prevention and control. Am J Health Syst Pharm. 2010;67(7):575–7.
4. Kaboli P. Realizing the pharmacoeconomic benefit of clinical pharmacy. Am J Health Syst Pharm. 2008;65(12):1123.
Competing interests: None declared.
Canadian Journal of Hospital Pharmacy , VOLUME 67 , NUMBER 4 , July-August 2014