Alexia Janes, Cynthia Tanguay, Nicolas J Caron, Jean-François BussièresABSTRACT
Background:
Occupational exposure to hazardous drugs may lead to adverse reproductive effects. There is no safe exposure limit for health care professionals.
Objectives:
To monitor levels of cyclophosphamide, ifosfamide, and methotrexate contamination in oncology pharmacy and patient care areas in Canadian health care institutions.
Methods:
The study was conducted in 2014. Hospitals with at least 50 acute care beds were invited to participate. At each participating centre, 12 standardized sites (6 in pharmacy areas and 6 in patient care areas) were sampled. The samples were analyzed for the presence of cyclophosphamide, ifosfamide, and methotrexate by ultra-performance liquid chromatography tandem mass spectrometry technology. The limits of detection were 0.36 pg/cm2 for cyclophosphamide, 0.95 pg/cm2 for ifosfamide, and 0.97 pg/cm2 for methotrexate. Descriptive statistical analyses were performed to determine the median, 75th percentile, and maximum levels.
Results:
Fifty-one hospitals participated in this descriptive study, and a total of 584 samples were quantified. Overall, 294 (50%) of the samples were positive for cyclophosphamide, 125 (21%) for ifosfamide, and 54 (9%) for methotrexate. The most frequently contaminated sampling sites in pharmacy areas were the front grille inside the hood and the floor in front of the hood and, in patient care areas, the armrest and outpatient clinic counter. The 75th percentiles for surface concentration were 10.8 pg/cm2 for cyclophosphamide, 1.59 pg/cm2 for ifosfamide, and below the limit of detection for methotrexate.
Conclusions:
Relative to 3 other multicentre studies conducted in Quebec over the past few years, the proportion of positive samples remained constant. Nonetheless, the 75th percentile surface concentration of antineoplastic drugs has been decreasing and seems to have reached a plateau. Local (country-specific or region-specific) and attainable goals for surface contamination with hazardous drugs should be set annually, so long as no health-based limit is known.
KEYWORDS: occupational exposure, environmental monitoring, antineoplastic agents, cyclophosphamide, ifosfamide, methotrexate
RÉSUMÉ
Contexte :
L’exposition professionnelle à des médicaments dangereux peut causer des effets indésirables sur la reproduction. Aucune limite d’exposition sécuritaire n’est établie pour les professionnels de la santé.
Objectifs :
Évaluer les taux de cyclophosphamide, d’ifosfamide et de méthotrexate dans la pharmacie d’oncologie et dans les unités de soins des établissements de santé canadiens.
Méthodes :
L’étude s’est déroulée en 2014. Les hôpitaux disposant d’au moins 50 lits de soins de courte durée ont été invités à participer. Dans chacun des établissements participants, des échantillons ont été prélevés dans 12 zones prédéterminées : 6 dans les pharmacies et 6 dans les unités de soins. On a ensuite analysé les échantillons par chromatographie liquide à très haute performance couplée à la spectrométrie de masse en tandem afin de détecter la présence de cyclophosphamide, d’ifosfamide et de méthotrexate. Le seuil de détection était de 0,36 pg/cm2 pour la cyclophosphamide, de 0,95 pg/cm2 pour l’ifosfamide et de 0,97 pg/cm2 pour le méthotrexate. Des analyses statistiques descriptives ont été effectuées afin de déterminer la médiane, le 75e percentile et les taux maximums.
Résultats :
Au total, 51 hôpitaux ont participé à cette étude descriptive et 584 échantillons ont été quantifiés. Dans l’ensemble, 294 (50 %) échantillons étaient positifs pour la cyclophosphamide, 125 (21 %) pour l’ifosfamide et 54 (9 %) pour le méthotrexate. Les zones les plus fréquemment contaminées étaient : en pharmacie, la grille avant dans la hotte et le sol devant la hotte; dans les unités de soins, les accoudoirs et le comptoir des cliniques de consultation externe. Le 75e percentile de la concentration de surface était de 10,8 pg/cm2 pour la cyclophosphamide, 1,59 pg/cm2 pour l’ifosfamide et sous le seuil de détection pour le méthotrexate.
Conclusions :
Comparativement à trois autres études multicentriques menées au Québec au cours des dernières années, la proportion de prélèvements positifs demeure la même. Toutefois, le 75e percentile de la concentration de surface d’antinéoplasiques a diminué et semble avoir atteint un plateau. Des objectifs locaux (pour le pays ou selon les régions) et réalisables de contamination de surface par des médicaments dangereux devraient être établis chaque année, et ce, tant qu’aucune limite fondée sur les critères liés à la santé ne sera pas déterminée.
MOTS CLÉS: exposition professionnelle, surveillance environnementale, antinéoplasiques, cyclophosphamide, ifosfamide, méthotrexate
Occupational exposure to hazardous drugs can lead to adverse effects on health care workers, including nurses, pharmacists, pharmacy technicians, and support workers.1 In particular, exposure to hazardous drugs has been shown to lead to adverse reproductive outcomes.2,3 To raise awareness about this issue, the US National Institute for Occupational Safety and Health (NIOSH) published an alert on the prevention of occupational exposure to hazardous drugs in 2004.4 Since then, NIOSH has been updating its list of hazardous drugs every 2 years. In addition to antineoplastic drugs, other drugs that are considered hazardous include immunosuppressants, antipsychotics, antidepressants, and hormones. In the 2014 NIOSH list of hazardous drugs, the majority of drugs (97/184 [53%]) are antineoplastic drugs.5
Following the 2004 NIOSH alert,4 many organizations reviewed their guidelines for the safe handling of hazardous drugs. For instance, the American Society of Health-System Pharmacists published new guidelines in 2006.6 The United States Pharmacopeial Convention is also developing a new guideline (USP General Chapter <800>) that will contain specific requirements for compounding both antineoplastic and nonantineoplastic hazardous drugs.7 In Quebec, the Association paritaire pour la santé et la sécurité du travail du secteur des affaires sociales (a “joint, sector-based association dedicated to promoting occupational health and safety prevention and supporting health and social service sector workers and institutions”) published a safe handling guide in 2008.8 In addition, the Ordre des pharmaciens du Québec recently published a new guideline for compounding sterile hazardous drugs.9 This guideline recommends that chemical contamination on work surfaces be assessed twice per year.
Since 2008, the authors’ group has performed 3 multicentre studies of environmental contamination in Quebec hospitals.10–12 For a more recent investigation, reported here, the scope of study was expanded to include Canadian centres outside Quebec. The aim of this study was to monitor environmental contamination with cyclophosphamide, ifosfamide, and methotrexate in oncology pharmacy and patient care areas in Canadian hospitals. The secondary objective was to describe temporal trends.
Directors of pharmacy departments in Canadian hospitals with at least 50 acute care beds were contacted by e-mail. Any hospital that handled one or more of the 3 target antineoplastic drugs was eligible to participate in the study. Pharmacy directors in Quebec hospitals (n = 58) were contacted on December 20, 2013, and a reminder was sent on January 10, 2014. Pharmacy directors in hospitals in other provinces (n = 137) were contacted on January 10, 2014 (there was no systematic follow-up for potential respondents outside Quebec).
Each participating hospital was expected to apply local policies and procedures for compounding, administration, surface cleaning, waste management, and other aspects of drug handling. All participating hospitals were equipped with laminar air flow cabinets in the pharmacy, for sterile compounding. Each hospital assumed the cost of analysis for its own samples. Participants were asked whether they were using a closed-system transfer device (CSTD) for preparing antineoplastic drugs in the pharmacy (i.e., “a drug transfer device that mechanically prohibits the transfer of environmental contaminants into the system and the escape of hazardous drug or vapor concentrations outside the system”4). Participants were also asked whether they removed the outer packaging of antineoplastic drugs in the pharmacy after receipt and whether they cleaned the vials of antineoplastic drugs in the pharmacy after receipt. After the study was completed, each participating centre received a report comparing its results with global results from all participating centres. The authors’ research group previously set the overall 75th percentile of surface concentration as a goal for every participating centre; after the conclusion of the study, centres were encouraged to target sampling sites with values above this goal for corrective measures.
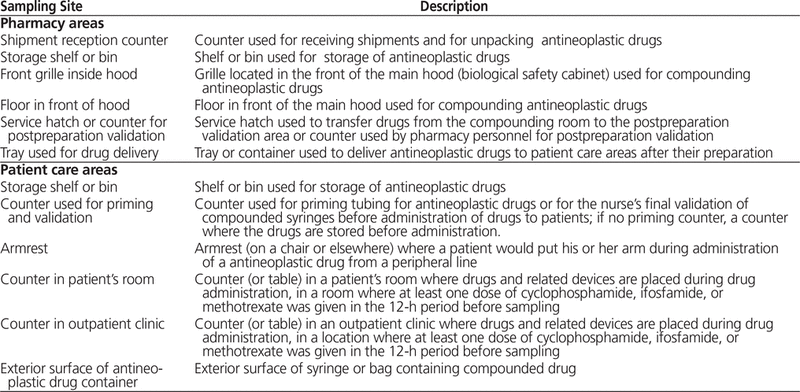
The sampling technique was described previously.10–12 Twelve standardized measurement sites, 6 in pharmacy areas and 6 in patient care areas, were selected for sampling (Table 1). These sites were identical with those targeted in the 2008–2010, 2012, and 2013 studies.10–12 Each participating hospital was given a description and photographs of the standardized sampling sites. The photographs provided were taken at the investigators’ hospital. Each participating hospital was also asked to provide a picture of the actual sites used for sampling. Samples were collected by one research assistant (A.J.) from the research team (for nearby hospitals) or by a trained employee from the participating hospital (for remote hospitals). A video of the sampling technique was provided for training of employees. For each sample, a standardized surface of about 600 cm2 (20 cm × 30 cm) was sampled with one 6 cm × 8 cm Wypall X60 wipe (Kimberly Clark Professional, Newton Square, Pennsylvania). The wipe was moistened with 1 mL of sampling solution (10% methanol and 90% 5 mmol/L ammonium acetate). Sites were sampled at the end of a workday or in the morning, before surfaces were washed. The sampling technique, an adaptation of the technique described by Larson and others,13 was developed by the Institut national de santé publique du Québec.
Table 1. Description of 12 Standardized Sites to be Sampled at Each Centre
Sampling wipes were stored at a temperature between 2°C and 8°C in 50-mL polypropylene tubes. Before analysis, 10 mL of extracting solution and internal standards were added to each tube. Each tube was mechanically stirred for 10 min, and an aliquot of the solution was removed for analysis. For each sample, 3 antineoplastic drugs (cyclophosphamide, ifosfamide, methotrexate) were quantified by ultra-performance liquid chromatography tandem mass spectrometry (Acquity UPLC chromatographic system coupled with Xevo TQ-S tandem mass spectrometer; Waters, Milford, Massachusetts). Chromatography was carried out on a C18 Acquity UPLC BEH column (2.1 × 50 mm, 1.7 μm; Waters, Milford, Massachusetts) with a gradient from 10/90 to 60/40 of methanol / 5 mmol/L ammonium acetate over 2 min. Overall, mean recovery from surfaces was 79%, and the intra-assay coefficient of variation was 22%. Recovery from surfaces ranged from 67% to 89% for stainless steel (coefficient of variation 5%–8%), from 79% to 102% for melamine (coefficient of variation 1%–3%), from 89% to 92% for plastic (coefficient of variation 4%–6%), and from 37% to 82% for linoleum (coefficient of variation 7%–22%).
Results expressed in nanograms per millilitre (ng/mL) were converted to nanograms per square centimetre (ng/cm2). These values were then multiplied by 11 (the dilution factor) and divided by 600 cm2 (the surface area sampled) to obtain the final results, which were expressed in picograms per square centimetre (pg/cm2). The limit of detection was 0.36 pg/ cm2 (19.8 pg/mL) for cyclophosphamide, 0.95 pg/cm2 (52 pg/mL) for ifosfamide, and 0.97 pg/cm2 (53 pg/mL) for methotrexate. The limit of quantification was 1.21 pg/cm2 (65.9 pg/mL) for cyclophosphamide, 3.17 pg/cm2 (173 pg/mL) for ifosfamide, and 3.25 pg/cm2 (177 pg/ mL) for methotrexate. The limit of detection was used as the reporting limit.
The proportion of positive samples was calculated. A sample was considered positive for a particular drug if the value was above the limit of detection. Descriptive statistical analyses (minimum, median, 75th percentile, 90th percentile, maximum) were carried out. For calculations, concentrations that fell between the limit of detection and the limit of quantification were assigned a value corresponding to the limit of quantification divided by 2,14 and concentrations that fell below the limit of detection were assigned a value corresponding to the limit of detection divided by 2.15
Results from the hospitals that participated in the 3 earlier studies10–12 and the current study were used for comparisons over time.
Subanalyses were performed according to hospitals’ working practices, i.e., use of a CSTD, removal of packaging, and cleaning of vials after initial receipt. The effect of CSTD use was evaluated for pharmacy sampling sites corresponding to steps performed during and after compounding (i.e., hood, floor, service hatch, delivery tray). The effect of packaging removal and cleaning of vials was evaluated for pharmacy sampling sites corresponding to steps performed after receipt of drugs (i.e., storage shelf, hood, floor, service hatch, delivery tray). Results were compared with a Kolmogorov–Smirnov test for 2 unpaired samples. A p value less than 0.05 was considered significant. For these subanalyses, only the results for cyclophosphamide contamination were used, as they were deemed representative of the current situation; there was too little surface contamination with ifosfamide and methotrexate to allow similar subanalyses.
A total of 51 Canadian hospitals participated in this study: 34 (59%) of the 58 Quebec hospitals and 17 (12%) of the 137 centres from other Canadian provinces. The respondents from provinces other than Quebec were from Manitoba, New Brunswick, Nova Scotia, Ontario, and Prince Edward Island.
Two-thirds of the participating centres (34/51 [67%]) were teaching hospitals. Nearly all participating centres (49/51 [96%]) provided information about their working practices. Of these, 12 (24%) used a CSTD: ChemoClave System (ICU Medical Inc, San Clemente, California) (n = 6), PhaSeal (Becton, Dickinson and Company, Franklin Lakes, New Jersey) (n = 3), Chemo Dispensing Pin (B Braun Medical Inc, Bethlehem, Pennsylvania) (n = 1), unspecified (n = 2). Greater proportions of these hospitals removed the outer packaging after receipt (29/49 [59%]) and cleaned the vials after receipt (28/49 [57%]). Among the 49 respondents providing information about working practices, 7 (14%) used a CSTD, removed outer packaging, and cleaned vials after receipt.
A total of 584 samples were collected between February and September 2014 (303 from pharmacy areas and 281 from patient care areas). The median number of sites per hospital with at least one positive sample for any drug was 7 (range 1–11). All participating hospitals had at least 1 positive sample for at least 1 of the 3 antineoplastic drugs evaluated (cyclophosphamide, ifosfamide, or methotrexate).
Overall, 50% (294/584) of the samples were positive for cyclophosphamide, 21% (125/584) were positive for ifosfamide, and 9% (54/584) were positive for methotrexate (Table 2). For 6 sampling sites—the storage shelf or bin in the pharmacy, the front grille inside the hood, the floor in front of the hood, the armrest, a counter in the patient’s room, and a counter used for priming—at least half of the samples were positive for cyclophosphamide.
Table 2. Proportion of Samples Testing Positive* for Antineoplastic Drugs in Pharmacy and Patient Care Areas in 51 Canadian Hospitals (Sampling in 2014)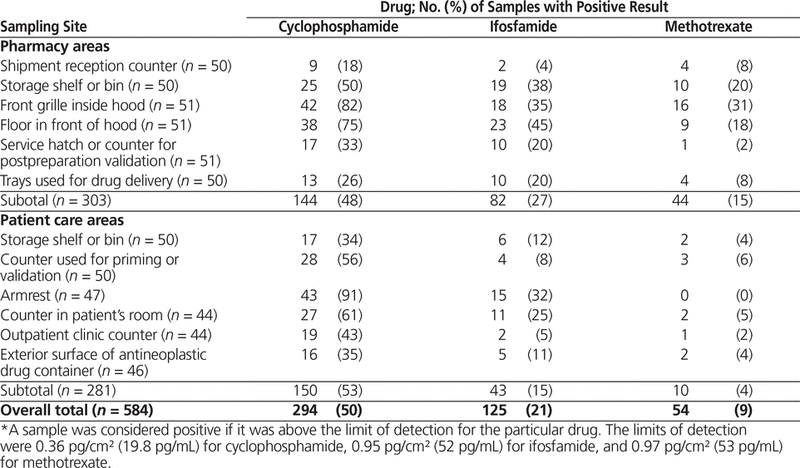
The overall 75th percentiles for drug concentration were 10.8 pg/cm2 for cyclophosphamide, 1.59 pg/cm2 for ifosfamide, and below the limit of detection for methotrexate (Table 3).
Table 3. Surface Contamination with Antineoplastic Drugs in Pharmacy and Patient Care Areas in 51 Canadian Hospitals (Sampling in 2014)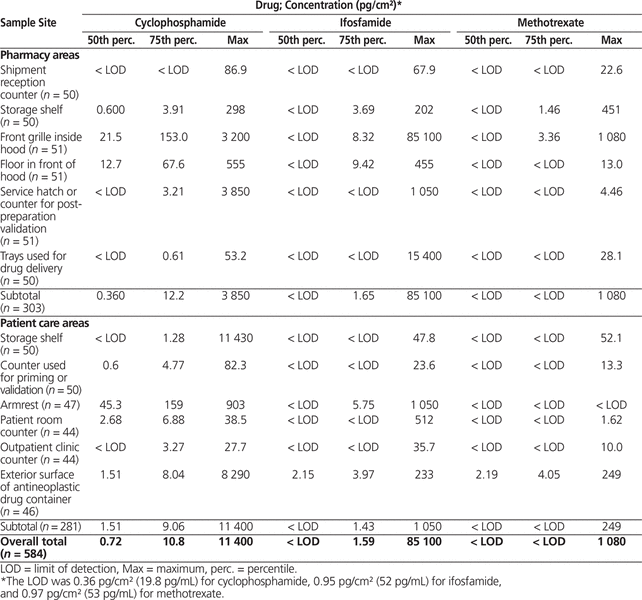
Because participants in this study included centres located outside Quebec, the level of surface contamination was compared according to geographic location. Surface contamination with cyclophosphamide was similar in pharmacy areas in hospitals inside and outside Quebec (Figure 1). However, contamination with cyclophosphamide was higher in patient care areas from Quebec hospitals, which resulted in higher overall contamination (Figure 1). The most contaminated sites were the same for all Canadian centres.
|
|
||
|
Figure 1. Level of cyclophosphamide surface contamination in pharmacy and patient care areas in Canadian hospitals. A: Proportion of cyclophosphamide-positive samples. B: 75th percentile of cyclophosphamide concentration. A sample was considered positive if it was above the limit of detection for cyclophosphamide, which was 0.36 pg/cm2 (19.8 pg/mL). |
||
Three similar studies were conducted in Quebec in 2008–2010, 2012, and 2013, with 25, 33, and 36 participating hospitals, respectively.10–12 Nineteen hospitals participated in all 4 studies. There was no difference in surface contamination between the 19 centres that participated in all 4 studies and the 51 centres that participated in the 2014 study (Figure 2). The proportion of positive samples remained constant over the years (Figure 2A), but the 75th percentile of cyclophosphamide surface concentration declined and reached a steady state in 2012 (Figure 2B).
|
|
||
|
Figure 2. Surface contamination with antineoplastic drugs for the 19 “common centres” that participated in all 4 studies (the current study [2014] and 3 earlier studies [2008–2010, 2012, and 2013]10–12) and for all centres that participated in each individual study (n = 51 in the current study and n = 25, 33, and 36, respectively, in the 3 earlier studies). A: Proportion of positive samples (pharmacy and patient care areas combined). B: 75th percentile of surface concentration. A sample was considered positive if it was above the limit of detection, which was 0.36 pg/cm2 (19.8 pg/mL) for cyclophosphamide, 0.95 pg/cm2 (52 pg/mL) for ifosfamide, and 0.97 pg/cm2 (53 pg/mL) for methotrexate. |
||
It is relevant to mention that the limits of detection declined over the years, which may have limited comparisons among the 4 studies. To test this possibility, the proportion of positive samples was recalculated using the value for limit of detection that was in effect in the 2008–2010 study, which was higher than the current limit of detection. With this change, the proportion of positive samples still remained constant over the years (data not shown). Thus, we are confident that the trend as reported here is accurate and was not caused by a change in the limits of detection.
Over the years, the most frequent cyclophosphamide-positive sampling sites in the pharmacy were the front grille of the hood and the floor in front of the hood (Figure 3A). In patient care areas, the most frequent cyclophosphamide-positive sampling site was the armrest (Figure 3B).
|
|
||
|
Figure 3. Proportion of cyclophosphamide-positive samples per sampling site for the 19 centres that participated in all 4 studies (the current study [2014] and 3 prior studies [2008–2010, 2012, 2013]10–12). A: Pharmacy areas. B: Patient care areas. A sample was considered positive if it was above the limit of detection for cyclophosphamide: 0.36 pg/cm2 (19.8 pg/mL) in the current study (2014), 1.8 pg/cm2 (100 pg/mL) in 2012 and 2013,11,12 and 1.5 pg/cm2 (80 pg/mL) in 2008–2010.10 |
||
The potential link between certain working practices and surface concentration with cyclophosphamide in the pharmacy was investigated. Overall, the 75th percentile for cyclophosphamide concentration was lower for centres that used a CSTD, removed the outer packaging of vials after receipt, and/or cleaned vials after receipt, but this difference was not significant (Tables 4–6).
Table 4. Effect of Using Closed-System Transfer Devices (CSTDs) on Contamination of Selected Sampling Sites in the Pharmacy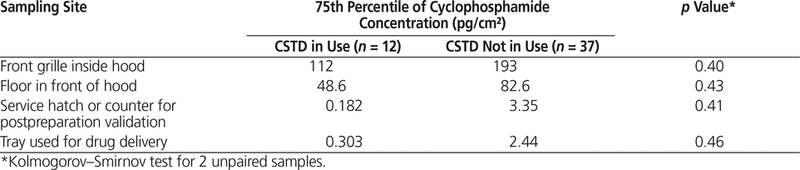
Table 5. Effect of Removing Outer Vial Packaging on Contamination of Selected Sampling Sites in the Pharmacy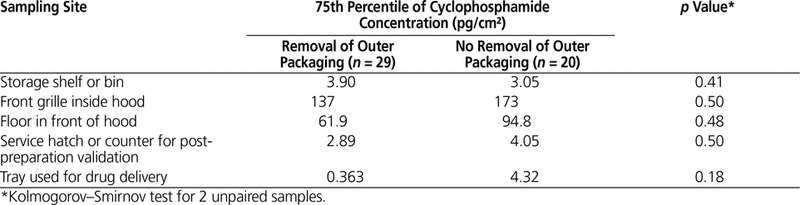
Table 6. Effect of Cleaning Vials on Contamination of Selected Sampling Sites in the Pharmacy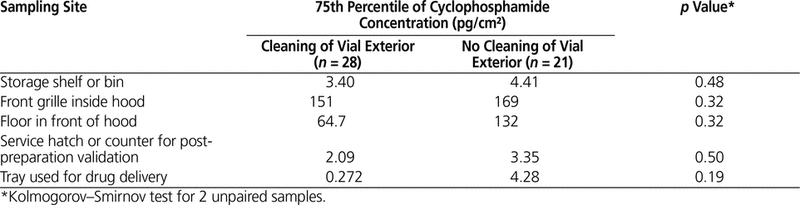
Overall, among samples obtained from 51 Canadian hospitals participating in this study, 50% were positive for cyclophosphamide, 21% were positive for ifosfamide, and 9% were positive for methotrexate. The 75th percentile values for surface concentration were 10.8 pg/cm2 for cyclophosphamide, 1.59 pg/cm2 for ifosfamide, and below the limit of detection for methotrexate.
Relative to 3 other multicentre studies conducted within Quebec in 2008–2010, 2012, and 2013, respectively,10–12 the proportion of positive samples in the current study remained constant. Nonetheless, the surface concentration of antineoplastic drugs measured has been decreasing over the years and seems to have reached a plateau in 2012.
This reduction in surface contamination can probably be explained by an increase in awareness of the importance of safe-handling practices, following the adoption of improved local procedures8,9 and the publication of international guidelines.6,7 It will be interesting to find out, over the coming years, whether the new guideline for compounding hazardous drugs of the Ordre des pharmaciens du Québec9 will have an effect on surface contamination.
Over the 4 studies, the most frequent cyclophosphamide-positive sample sites were the front grille of the hood, the floor in front of the hood, and the armrest of the chair used during administration of antineoplastic drugs. Similar sites were found to be highly contaminated in another recent large study involving 30 US hospitals. In that study, Sessink and others16 found that 97% of samples from the front grille of the hood and 82% of those from the floor in front of the hood were positive for cyclophosphamide.
These frequently contaminated sites correspond to sites where large quantities of antineoplastic drugs are manipulated. In a Canadian study, Hon and others17 identified the steps of drug preparation and drug administration as critical aspects of the medication-use system. In addition to sites where large quantities of antineoplastic drugs are manipulated, these authors found contamination on everyday objects such as pencils and door handles.17 Indeed, skin absorption and inhalation are frequent sources of contamination, but hand-to-mouth contact also leads to occupational exposure.3 In another Canadian study, Hon and others18 evaluated the contamination of hands of health care workers. They found that workers in the patient care unit who were not directly involved in drug administration, such as dieticians, ward aides, oncologists, and volunteers, had the highest rate of hand contamination, with 28.6% of this group testing positive for contamination.18
In addition to emphasizing the importance of adequate working practices and personal protective equipment, the presence of contamination highlights the usefulness of suitable cleaning methods. The use of alcohol to clean a surface can often spread the contamination, rather than eliminating it.19 The use of detergent and water is better for cleaning a surface, but to totally eliminate all traces of contaminants, decontamination should be done with a combination of sodium hypochlorite and sodium thiosulfate.20
Overall, surface contamination in the current study was similar in pharmacy and patient care areas. However, contamination of patient care areas with cyclophosphamide was higher for Quebec hospitals than for hospitals from other provinces. Although the data collected for this study were insufficient to explain this difference, it would be interesting to determine whether different practices for activities such as cleaning, drug preparation, and drug administration are used in patient care units in Quebec and the other provinces. The difference in response rates between Quebec and the rest of Canada limits this comparison, and participating hospitals may not be representative of all Canadian hospitals.
Subanalyses were performed to evaluate the effect of 3 working practices: use of a CSTD, removal of packaging, and cleaning of vials after initial receipt. No statistically significant differences were found between hospitals that did and did not follow these practices. In the 2013 study,12 the concentration of contaminants was lower on the front grille of the hood at centres that used CSTDs, removed the outer packaging, and cleaned vials after receipt. Even though numerous studies have shown that CSTDs can reduce contamination, their use does not completely eliminate contamination. For instance, Sessink and others16 found a median of 1.69 ng/cm2 (or 1690 pg/cm2) of cyclophosphamide on the front grille of the hood before CSTDs were in use and 0.39 ng/cm2 (or 390 pg/cm2) after CSTDs were implemented. In the current study, the median concentration of cyclophosphamide on the front grille of the hood was 43 pg/cm2 at hospitals not using a CSTD (n = 12) and 15 pg/cm2 at hospitals that did use such devices (n = 37). As such, hospitals in this study that did not use CSTDs had a 26-fold lower concentration of cyclophosphamide than hospitals in other studies that did use CSTDs (15 pg/cm2 versus 390 pg/cm2). The very low surface contamination observed in the current multicentre study, despite the fact that few participating hospitals (12/49) reported the use of CSTDs, indicates that many strategies can contribute to a low level of surface contamination.
It is recommended that vials be unpacked and cleaned after initial receipt.8 Although we did not find a significant difference between hospitals that did and did not follow this practice, this low-cost solution can probably help to reduce contamination, given that the exterior surface of vials is often contaminated during the manufacturing process.21
As long as no health-based safe limit of exposure is known, workers’ exposure to antineoplastic and other hazardous drugs should be kept as low as reasonably achievable. We suggest the use of local (country- or region-specific) and attainable goals and recommend that centres strive to attain a level of contamination lower than these targets. For each drug, the value of the most recent global 75th percentile (derived from all centres that participated in the current study) should be used as a manageable target for Canadian hospitals. It is hoped that these target values will continue to be reduced over the years to come.
How can hospitals reduce their level of contamination? Environmental sampling can help to identify problem areas, which can then be cleaned thoroughly with a combination of sodium hypochlorite and sodium thiosulfate. Such cleaning should be done regularly, and a log should be signed by the workers responsible, especially for areas where large quantities of antineoplastic agents are manipulated (e.g., the hood in the pharmacy) and areas that are touched frequently (e.g., armrests). The source of the contamination may be the exterior of vials, so cleaning vials when they are received and cleaning the container for the compounded product once prepared can also limit contamination. Materials that cannot be cleaned properly should be substituted with materials that can be cleaned, if possible.
Finally, it is important to continue raising the awareness of all workers who are potentially exposed to antineoplastic drugs, to ensure that they use proper working practices that will limit the spread of contamination and that they adequately protect themselves with personal protective equipment.8
This study was the largest multicentre study of hazardous-drug contamination to date in Canadian hospitals and provides results for both pharmacy and patient care areas. To limit technical bias and to ensure a consistent sampling method across all centres, the investigators supplied a video demonstrating the correct sampling technique. As much as possible, sampling was performed at the end of the day, to generate values that were representative of a working day and also representative of the potential professional exposure to these drugs among health care workers. However, all sampling at each institution was performed on a single day, and different results might have been obtained on a different day. Many different analytical techniques are available, so caution should be used when comparing these results with the results of other studies. The limits of detection were comparable to those used by other investigators. The global recovery rate from surfaces was adequate; however, the recovery from linoleum (floors) was lower, so those results might be underestimations. The cost of the analysis may have prevented some hospitals from participating.
Relative to 3 other multicentre studies conducted in Quebec over the past several years, the proportion of positive samples remained constant. Nonetheless, the 75th percentile surface concentration of antineoplastic drugs has been decreasing over time and seems to have reached a plateau. As long as no health-based limit of exposure is known, local (country- or region-specific) and attainable goals for surface contamination with hazardous drugs should be set annually.
1. Medical surveillance for healthcare workers exposed to hazardous drugs. Cincinnati (OH): Department of Health and Human Services (US), Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2012 [cited 2013 Oct 15]. Available from: www.cdc.gov/niosh/docs/wp-solutions/2013-103/pdfs/2013-103.pdf
2. Dranitsaris G, Johnston M, Poirier S, Schueller T, Milliken D, Green E, et al. Are health care providers who work with cancer drugs at an increased risk for toxic events? A systematic review and meta-analysis of the literature. J Oncol Pharm Pract. 2005;11(2):69–78.
3. Connor TH, Lawson CC, Polovich M, McDiarmid MA. Reproductive health risks associated with occupational exposures to antineoplastic drugs in health care settings: a review of the evidence. J Occup Environ Med. 2014;56(9):901–10.
4. Preventing occupational exposure to antineoplastic and other hazardous drugs in healthcare settings. Publ No. 2004-165. Cincinnati (OH): Department of Health and Human Services (US), Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2004 [cited 2014 Oct 24]. Available from: www.cdc.gov/niosh/docs/2004-165/
5. Connor TH, MacKenzie BA, DeBord DG, Trout DB, O’Callaghan JP. NIOSH list of antineoplastic and other hazardous drugs in healthcare settings, 2014. Cincinnati (OH): Department of Health and Human Services (US), Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2014 [cited 2014 Oct 24]. Available from: www.cdc.gov/niosh/docs/2014-138/pdfs/2014-138.pdf
6. American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health Syst Pharm. 2006;63(12):1172–93.
7. <800> Hazardous drugs—handling in healthcare settings [briefing]. Rockville (MD): U.S. Pharmacopeial Convention; 2014 [cited 2014 Oct 24]. Available from: www.usp.org/sites/default/files/usp_pdf/EN/m7808.pdf
8. Prevention guide—safe handling of hazardous drugs. Montréal (QC): Association paritaire pour la santé et la sécurité du travail du secteur des affaires sociales; 2008 [cited 2014 Oct 24]. Available from: www.asstsas.qc.ca/publications/publications-specialisees/guides-de-prevention/prevention-guide-safe-handling-of-hazardous-drugs.html
9. Norme 2014.02 : Préparation de produits stériles dangereux en pharmacie. Montréal (QC): Ordre des pharmaciens du Québec; 2014 [cited 2014 Mar 20]. Available from: www.opq.org/fr-CA/publications/normes-de-pratiqueet-lignes-directrices/
10. Bussières JF, Tanguay C, Touzin K, Langlois É, Lefebvre M. Environmental contamination with hazardous drugs in Quebec hospitals. Can J Hosp Pharm. 2012;65(6):428–35.
11. Merger D, Tanguay C, Langlois E, Lefebvre M, Bussières JF. Multicenter study of environmental contamination with antineoplastic drugs in 33 Canadian hospitals. Int Arch Occup Environ Health. 2014;87(3):307–13.
12. Berruyer M, Tanguay C, Caron NJ, Lefebvre M, Bussières JF. Multicenter study of environmental contamination with antineoplastic drugs in 36 Canadian hospitals: a 2013 follow-up study. J Occup Environ Hyg. 2015;12(2):87–94.
13. Larson RR, Khazaeli MB, Dillon HK. Monitoring method for surface contamination caused by selected antineoplastic agents. Am J Health Syst Pharm. 2002;59(3):270–7.
14. Article 5: calculation of mean values. In: Commission directive 2009/90/CE of 31 July 2009 laying down, pursuant to Directive 2000/60/EC of the European Parliament and of the Council, technical specifications for chemical analysis and monitoring of water status. Official Journal of the European Union. Brussels (Belgium): European Union; 2009 [cited 2014 Oct 24]. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:201:0036:0038:EN:PDF
15. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51.
16. Sessink PJ, Trahan J, Coyne W. Reduction in surface contamination with cyclophosphamide in 30 US hospital pharmacies following implementation of a closed-system drug transfer device. Hosp Pharm. 2013;48(3):204–12.
17. Hon CY, Teschke K, Chu W, Demers P, Venners S. Antineoplastic drug contamination of surfaces throughout the hospital medication system in Canadian hospitals. J Occup Environ Hyg. 2013;10(7):374–83.
18. Hon CY, Teschke K, Demers PA, Venners S. Antineoplastic drug contamination on the hands of employees working throughout the hospital medication system. Ann Occup Hyg. 2014;58(6):761–70.
19. Chu WC, Hon CY, Danyluk Q, Chua PP, Astrakianakis G. Pilot assessment of the antineoplastic drug contamination levels in British Columbian hospitals pre- and post-cleaning. J Oncol Pharm Pract. 2012;18(1):46–51.
20. Touzin K, Bussières JF, Langlois E, Lefebvre M, Métra A. Pilot study comparing the efficacy of two cleaning techniques in reducing environmental contamination with cyclophosphamide. Ann Occup Hyg. 2010;54(3):351–9.
21. Schierl R, Herwig A, Pfaller A, Groebmair S, Fischer E. Surface contamination of antineoplastic drug vials: comparison of unprotected and protected vials. Am J Health Syst Pharm. 2010;67(6):428–9.
The authors would like to thank the 51 health care centres that participated in the 2014 study.
Competing interests: None declared.
Funding: No funding was received for this study.
Canadian Journal of Hospital Pharmacy, VOLUME 68, NUMBER 4, July-August 2015