Mary H H Ensom, BS(Pharm), PharmD, FASHP, FCCP, FCSHP, FCAHS, Clinical Pharmacy Specialist, Diane Décarie, BSc, Research Consultant
Levetiracetam is used as adjunctive therapy for various types of seizures and other conditions.1–4 Suspensions of levetiracetam prepared in Ora-Sweet sweetening agent or Ora-Plus suspending agent and stored in plastic bottles were stable for 91 days,5 but the drug’s stability when prepared in other commercially available vehicles and stored in glass bottles and plastic syringes is unknown. The current study was performed to ascertain whether levetiracetam suspensions are physically and chemically stable in newer, dye-free vehicles (Oral Mix and Oral Mix SF6) after storage in amber glass and plastic bottles (4°C and 25°C) and amber plastic syringes (25°C) for up to 95 days.
Stock levetiracetam suspensions (50 mg/mL) were prepared by crushing commercially available levetiracetam 500-mg tablets (Pharmascience, Montréal, Quebec; lot 480290, expiry December 2015) in Oral Mix and Oral Mix SF (Medisca Pharmaceutique Inc, Montréal, Quebec; lot 1102/A, expiry September 2015, and lot 57600/A, expiry December 2015, respectively). Each suspension was divided among 6 separate amber glass bottles (Richards Distribution, Richmond, British Columbia), 6 amber plastic (polyethylene terephthalate [PET]) bottles (Richards Distribution), and twenty-five 3-mL amber plastic (polypropylene) oral syringes (PreciseDose Dispenser System, Medisca Pharmaceutique Inc, lot 46959/C). For each suspension, 3 glass and 3 PET bottles were kept at 25°C, and 3 glass and 3 PET bottles at 4°C. All of the syringes were kept at 25°C.
On days 0, 7, 14, 21, 28, 42, 63, 77, and 95, all samples were examined for changes in colour, odour, taste, and ease of resuspension. On each study day, one 3-mL aliquot from each bottle and the contents of 3 syringes from each group were collected to determine pH (model 800 pH meter, VWR International, Mississauga, Ontario). Immediately thereafter, a 1.5-mL aliquot from each bottle or syringe was transferred to threaded, tight-seal cryogenic polypropylene vials (VWR International; lot 1095990) and stored for up to 100 days at −85°C until analysis by a validated, stability-indicating high-performance liquid chromatography (HPLC) – ultraviolet detection method.
Stock solutions (50 mg/mL) were prepared from levetiracetam powder (Sigma-Aldrich, Oakville, Ontario; lot 012M4711V) in HPLC-grade water (Fisher Scientific, Whitby, Ontario; lot 145147). The internal standard was prepared from lamotrigine powder (Sigma-Aldrich; lot 119K1127) diluted to 1 mg/mL in dimethyl sulfoxide (Sigma-Aldrich; lot MKBP3479V).
The HPLC instrumentation (Waters Alliance System model 2690, Waters Corporation, Mississauga, Ontario) consisted of a delivery pump, automated 200-μL injector, XTerra MC C18 4.6 × 100 mm column (Waters Corporation; lot 0273403014015), XTerra 3.9 × 20 mm guard column (Waters Corporation; lot 02277341271), and ultraviolet detector (model 2487, Waters Alliance System) set at 212 nm. The gradient mobile phase consisted of 16%–36%–16% methanol (Fisher Scientific; lot 136336) and 84%–64%–84% 10 mmol/L ammonium formate buffer (Sigma-Aldrich; lot BCBL4456V) at pH 4.0 and 25°C. All solvents were HPLC-grade and filtered before use. The gradient flow rate was 0.5 mL/min at time 0, was gradually increased to 0.8 mL/min at 2.5 min, and remained at 0.8 mL/min thereafter.
Levetiracetam standard solutions, each containing 0.005 mg/mL of lamotrigine, were prepared in HPLC-grade water to final concentrations of 0.180, 0.220, 0.240, 0.260, 0.280, and 0.320 mg/mL. All standards were passed through 0.45-μm microfilters (Acrodisc, Waters Corporation; lot 21829235) to prevent injection of impurities onto the column. The range of the 6-point calibration curve (0.180 to 0.320 mg/mL) encompassed the diluted test concentration of levetiracetam (0.250 mg/mL). Acceptable coefficients of variation for precision were defined as less than 10% and acceptable values for accuracy as greater than 90%.
Forced degradation of levetiracetam was achieved as follows. Suspensions of levetiracetam 1.0 mg/mL in Oral Mix and Oral Mix SF were prepared (without internal standard) from levetiracetam 500-mg tablets. Aliquots were mixed (v:v) with 0.1N hydrochloric acid, 0.1N sodium hydroxide, 3% hydrogen peroxide, or water, then vortex-mixed and incubated overnight at 60°C. Samples were cooled to 25°C, and their concentrations adjusted to 0.250 mg/mL with HPLC-grade water. The samples were then filtered and injected onto the column. The assay and degradation methods were developed in the authors’ laboratory on the basis of previous work.5,7
Regression analysis showed linearity, with coefficient of determination (r2) of 0.0995 or above (n = 4). Intraday and interday coefficients of variation were within acceptable limits (i.e., < 10%): 0.60% and 1.27%, respectively, for the 0.180 mg/mL solution; 1.05% and 1.64%, respectively, for the 0.200 mg/mL solution; 0.44% and 0.59%, respectively, for the 0.250 mg/mL solution; and 1.20% and 1.25%, respectively, for the 0.300 mg/mL solution. Intraday and interday accuracy values (± standard deviation) were also within acceptable limits (i.e., > 90%): 99.53% ± 0.24% and 98.92% ± 0.78%, respectively, for the 0.180 mg/mL solution; 99.43% ± 0.27% and 98.39% ± 1.20%, respectively, for the 0.200 mg/mL solution; 99.83% ± 0.20% and 99.54% ± 0.41%, respectively, for the 0.250 mg/mL solution; and 99.31% ± 0.54% and 98.75% ± 0.61%, respectively, for the 0.300 mg/mL solution.
Retention times were 2.7 min for lamotrigine and 5.7 min for levetiracetam. No interfering peaks were generated by forced degradation of levetiracetam. In general, the levetiracetam peaks decreased with forced degradation, and non-interfering degradation peaks were observed at 2.5 min, 4.6 min, 5.9 min, and 6.7 min.
No notable formation of precipitate or changes in the acidic (lemony) taste or odour occurred. The milky vanilla-coloured suspensions were easily resuspended throughout the study period. The mean pH remained stable, ranging from 4.48 to 4.50 for the Oral Mix suspensions and from 4.57 to 4.58 for the Oral Mix SF suspensions.
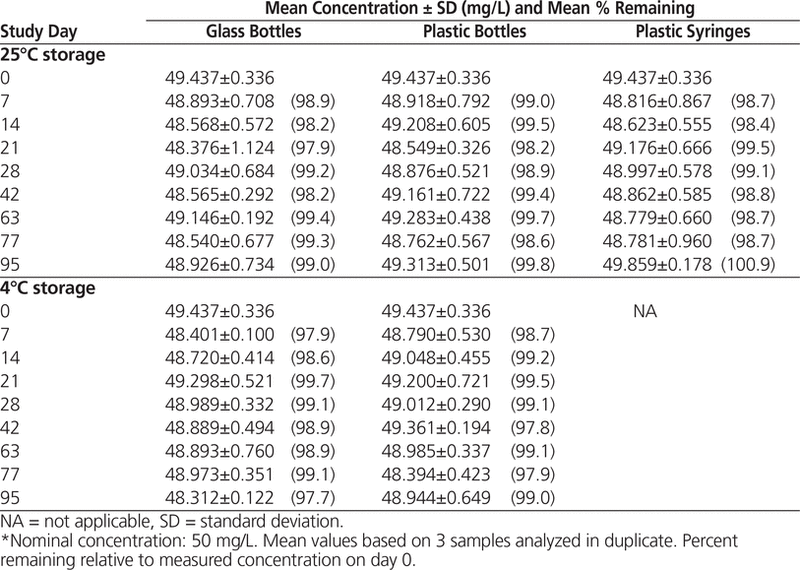
HPLC analysis showed that all suspensions maintained at least 96.9% of the original concentration for 95 days (Tables 1 and 2).
Table 1. Concentration of Levetiracetam in Oral Mix Suspension Vehicle During 95 Days of Storage in Glass Bottles, Plastic Bottles, and Plastic Syringes at 25°C and in Glass and Plastic Bottles at 4°C*
Table 2. Concentration of Levetiracetam in Oral Mix SF Suspension Vehicle During 95 Days of Storage in Glass Bottles, Plastic Bottles, and Plastic Syringes at 25°C and in Glass and Plastic Bottles at 4°C*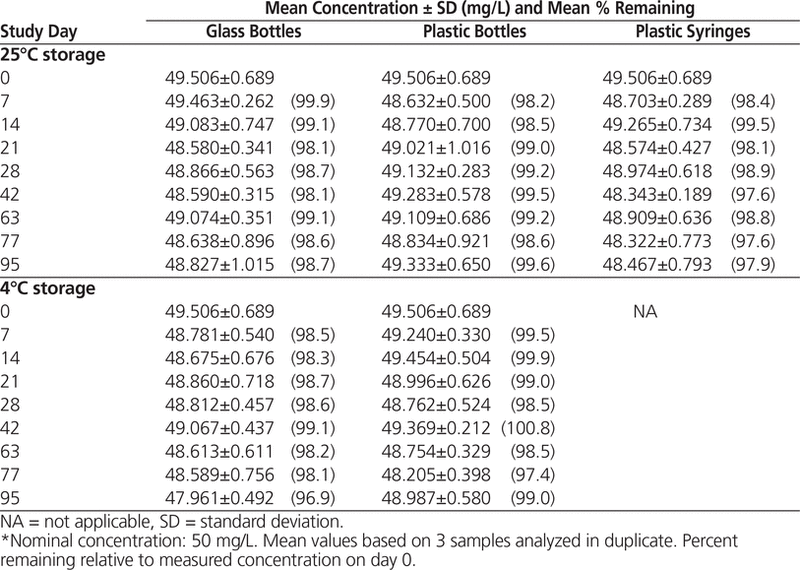
In conclusion, according to serial qualitative assessment of physical properties, determination of pH, and HPLC analysis, levetiracetam suspensions (50 mg/mL) prepared in Oral Mix and Oral Mix SF suspension vehicles and stored in amber glass bottles, plastic bottles, or oral plastic syringes at 25°C or in amber glass or plastic bottles at 4°C can be expected to remain stable for up to 95 days.
1. Levetiracetam. In: Lexicomp Online [database on Internet]. Lexi-Comp Inc; 2014 [cited 2015 Jul 22]. Available from: http://online.lexi.com. Subscription required to access content.
2. Perry MS, Benatar M. Efficacy and tolerability of levetiracetam in children younger than 4 years: a retrospective review. Epilepsia. 2007;48(6):1123–7.
3. Awaad Y, Michon AM, Minarik S. Use of levetiracetam to treat tics in children and adolescents with Tourette syndrome. Mov Disord. 2005;20(6):714–8.
4. Ettinger AB, Argoff CE. Use of antiepileptic drugs for nonepileptic conditions: psychiatric disorders and chronic pain. Neurotherapeutics. 2007;4(1):75–83.
5. Ensom MHH, Decarie D, Rudolph S. Stability of levetiracetam in extemporaneously compounded suspensions. Can J Hosp Pharm. 2011;64(3):207–11.
6. Medisca’s oral bases. Montréal (Québec): Medisca Pharmaceutique Inc; 2013 [cited 2015 Feb 18]. Available from: https://www.medisca.ca/NDC_SPECS/MCA/2512/EN/Downloads/Oral%20Bases%20Sell%20Sheet%20Flyer.PDF
7. Matar KM, Nicholls PJ, Bawazir SA, al-Hassan MI, Tekle A. A rapid liquid chromatographic method for the determination of lamotrigine in plasma. J Pharm Biomed Anal. 1998;17(3):525–31.
Mary Ensom is also a Professor in the Faculty of Pharmaceutical Sciences and Distinguished University Scholar, The University of British Columbia, Vancouver, British Columbia. She is the Editor of the CJHP. ( Return to Text )
Funding: This study was funded by an unrestricted educational grant from Medisca Pharmaceutique Inc.
Competing interests: Other than grant support, no competing interests were declared.
Canadian Journal of Hospital Pharmacy, VOLUME 68, NUMBER 4, July-August 2015