Cheryl A Sadowski, Catherine Lyder, Nesé YukselABSTRACT
Background:
Clinicians often face challenges in deciding how to treat osteoporosis in patients with chronic kidney disease. As background to offering guidance to health care providers, it is important to understand their practices and beliefs.
Objectives:
To describe the practices and beliefs of pharmacists regarding use of bisphosphonates for patients with osteoporosis and chronic kidney disease.
Methods:
A cross-sectional survey of pharmacists working in hospitals and related health care settings was conducted. A 34-item online questionnaire was developed consisting of 4 sections: demographic characteristics, practices, beliefs, and comfort level with making decisions about osteoporosis treatment. An e-mail invitation was sent to members of the Canadian Society of Hospital Pharmacists (n = 2499) in November 2012.
Results:
A total of 367 pharmacists completed the survey. Most of the respondents were women (258 [70%]), had more than 10 years in practice (213 [58%]), and were providing care to 1 or more osteoporosis patients per week (212 [58%]). Over one-third (150 [41%]) stated that they would use a bisphosphonate for patients with creatinine clearance (CrCl) of 15–30 mL/min, but more than half (207 [56%]) stated that they would avoid a bisphosphonate (and recommend another medication) for patients with CrCl below 15 mL/min. Forty-eight percent (176/363) agreed that oral bisphosphonates could be used for patients with renal failure (defined as CrCl < 30 mL/min), so long as dosage adjustments are made. More than half (206/363 [57%]) believed that the adverse effects of oral bisphosphonates increase for patients with renal failure. Respondents expressed a low level of comfort in assessing and initiating osteoporosis treatment for patients with renal failure.
Conclusions:
Pharmacists had varying beliefs about managing osteoporosis in patients with chronic kidney disease. This study highlights the need for practice tools and targeted education addressing the use of bisphosphonates for these patients.
KEYWORDS: osteoporosis, renal insufficiency, pharmacists, bisphosphonates, beliefs
RÉSUMÉ
Contexte :
Les cliniciens sont souvent confrontés à des défis lorsqu’ils doivent choisir un traitement contre l’ostéoporose chez les patients atteints d’insuffisance rénale chronique. Afin d’être en mesure de guider les fournisseurs de soins de santé, il importe de comprendre leurs pratiques et leurs croyances.
Objectif :
Offrir un portrait des pratiques et croyances des pharmaciens en ce qui a trait à l’emploi des bisphosphonates chez les insuffisants rénaux chroniques atteints d’ostéoporose.
Méthodes :
Une enquête transversale a été réalisée auprès de pharmaciens exerçant en établissements de santé. Un questionnaire en ligne a été élaboré. Il contenait 34 éléments répartis en 4 sections, soit : les caractéristiques démographiques; les pratiques; les croyances; et le degré d’aisance en ce qui a trait au choix d’un traitement contre l’ostéoporose. Un courriel d’invitation a été envoyé aux membres de la Société canadienne des pharmaciens d’hôpitaux (n = 2499) en novembre 2012.
Résultats :
Au total, 367 pharmaciens ont participé au sondage. La plupart des répondants étaient des femmes (258 [70 %]), possédaient plus de 10 années de pratique (213 [58 %]) et fournissaient hebdomadairement des soins à au moins un patient ostéoporotique (212 [58 %]). Plus d’un tiers (150 [41 %]) ont indiqué qu’ils emploieraient un bisphosphonate chez les patients affichant une clairance de la créatinine (ClCr) entre 15 et 30 mL/min, mais plus de la moitié (207 [56 %]) ont affirmé qu’ils n’en utiliseraient pas (et qu’ils recommandaient un autre médicament) chez les patients présentant une ClCr en deçà de 15 mL/min. Quarante-huit pour cent (176/363) ont affirmé que les bisphosphonates oraux pouvaient être employés pour les patients présentant une insuffisance rénale (définie comme une ClCr < 30 mL/min), pourvu que l’on procède à des ajustements posologiques. Plus de la moitié (206/363 [57 %]) croyaient que les effets indésirables des bisphosphonates oraux sont plus importants chez les patients souffrant d’insuffisance rénale. Les répondants ont indiqué être peu à l’aise lorsque vient le temps d’évaluer et d’amorcer un traitement contre l’ostéoporose chez les insuffisants rénaux.
Conclusions :
Les pharmaciens adhéraient à diverses croyances en ce qui touche la prise en charge de l’ostéoporose chez les insuffisants rénaux. La présente étude met en évidence le besoin d’outils pour la pratique ainsi que d’enseignement ciblé portant sur l’emploi des bisphosphonates chez les patients atteints d’insuffisance rénale.
MOTS CLÉS: ostéoporose, insuffisance rénale, pharmaciens, bisphosphonates, croyances
The consequence of osteoporosis, the occurrence of fragility fractures, is a public health concern. Fractures affect the health care system and society at large through costs of acute care, demand for rehabilitation, and loss of productivity.1 Older adults are particularly affected by the loss of function, which may lead to a loss of independence.1 In addition, older adults have excess mortality following fractures, particularly if they are frail.2
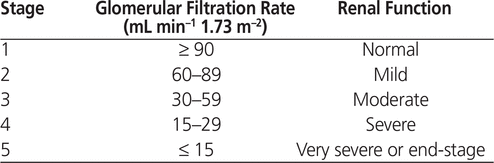
Both renal function and bone mass decline with age, and evidence indicates that many people with osteoporosis also have age-related chronic kidney disease (CKD).3 The Kidney Disease Outcomes and Quality Initiative guidelines from the US National Kidney Foundation classify CKD as glomerular filtration rate less than 60 mL/min (Table 1).4 The incidences of osteoporosis and age-related CKD are expected to increase.5 Although prevalence differs from one country to another, in part because of differences in socioeconomic status and life expectancy, up to 24% of people in their 70s may have CKD.6
Table 1. Stages of Chronic Kidney Disease, as Defined by the Kidney Disease Outcomes and Quality Initiative Guidelines of the National Kidney Foundation4
A number of pharmacologic options are available for treating osteoporosis, all of which have good evidence for vertebral fracture reduction of 30% to 70%, depending on the agent. Several agents have evidence for reduction of both nonvertebral and hip fracture as well.5 However, there is limited and conflicting information regarding the treatment of osteoporosis in patients with CKD.
Bisphosphonates are the first-line options for preventing osteoporosis-related fractures.5 They are efficacious in numerous populations, including elderly people.7 Oral bisphosphonates are poorly absorbed, with absorption of less than 1% of the dose administered.8 An estimated 50% of the absorbed dose is sequestered in bone, while the remaining drug is eliminated, unchanged, by filtration and secretion in the kidneys.8 The manufacturers’ product monographs for bisphosphonates warn against using these drugs for patients with reduced creatinine clearance (CrCl), specifically less than 35 mL/min for alendronate and zoledronic acid and less than 30 mL/min for risedronate.9–11 The main reason for this contraindication is the paucity of data about using bisphosphonates for this population, as many phase III trials have excluded patients with poor renal function.12 Because of the age-related decline in renal function, it is not unusual for elderly patients to have CrCl below these cut-offs.
In patients with CKD, bisphosphonates may therefore accumulate, theoretically leading to an increase in adverse effects. Safety concerns related to long-term use of bisphosphonates include osteonecrosis of the jaw and atypical fractures.13 At this time, the evidence for increased risk of long-term adverse events with bisphosphonates in patients with renal failure is unclear.3
The challenge of managing osteoporosis in patients with CKD arises frequently for clinicians. Fractures occur more commonly and have worse outcomes in individuals with CKD.14 Unfortunately, available osteoporosis guidelines do not provide direction in caring for this population.5 Health care professionals may observe conflicting approaches in practice, especially as the available data suggest that bisphosphonates are often used for patients with renal function below that recommended by the manufacturer, with no adverse outcomes.15 An understanding of the current practices and beliefs of health care professionals could be used to direct the development of practice tools and knowledge translation initiatives. The purpose of this study was to describe the current practices and beliefs of pharmacists regarding the use of bisphosphonates for patients with osteoporosis and renal insufficiency.
This study involved a cross-sectional survey of pharmacists working in hospitals and related health care settings across Canada. Participants were selected from the list of members of the Canadian Society of Hospital Pharmacists (CSHP) who had agreed to share their contact information for practice research. Pharmacists were included if they were practising in a hospital or health care setting (e.g., long term care, primary care) and were willing to take an online survey. Pharmacy students and interns were excluded. The study was approved by the University of Alberta Research Ethics Board.
An e-mail invitation to participate in the web-based survey was sent directly to CSHP members who met the inclusion criteria. The online survey was available to members over a 6-week period in November and December 2012, with e-mail reminders sent each week to nonresponders. As an incentive to participate in the study, respondents were eligible for a draw for 1 of 2 prizes (iPod Touch). The survey was administered by the Information Services and Technology department at the University of Alberta.
A 34-item, self-administered online questionnaire was developed by the study team, consisting mainly of quantitative components (closed-ended and Likert scale questions). All survey questions were generated by the investigators because literature searches yielded no similar survey instruments. The questionnaire had 4 domains: demographic characteristics, general practice in the treatment of osteoporosis in patients with various levels of renal function, beliefs about the safety and efficacy of bisphosphonates in renal failure (defined as CrCl < 30 mL/min), and comfort level with the treatment of osteoporosis and learning needs. Because bisphosphonates are the most widely prescribed group of medications used to treat osteoporosis, and because guidelines recommend these drugs as first-line therapy, it was decided to focus on safety concerns with bisphosphonates.
Beliefs about the use of osteoporosis medications were assessed with a 4-point Likert scale, where 1 = strongly disagree and 4 = strongly agree. A “do not know” response option was also available. Levels of comfort and interest were assessed on a 5-point Likert scale, from 1 = low to 5 = high. Osteoporosis medications were defined as any prescription drug used to treat osteoporosis, not including calcium or vitamin D.
The questionnaire was initially reviewed for content validity by a sample of experts (n = 5). Pilot testing for face validity and comprehensibility was completed through cognitive semistructured interviews with 10 pharmacists (a convenience sample recruited from a variety of hospitals and related health care settings). The questionnaire was further revised to ensure clarity of questions. To determine test–retest reliability, the survey was sent a second time to 40 pharmacists who agreed to be contacted 2 weeks after initial survey completion. Nine of these pharmacists completed the survey a second time, and the test–retest reliability coefficient was 1 (p < 0.01).
About 2600 pharmacists were members of CSHP at the time of the study, and most had agreed to be contacted for research purposes. Assuming a 95% confidence interval with a 5% margin of error and a proportional variable of interest, 341 respondents were needed. According to previous experience, survey response rates for pharmacists are often low (i.e., < 20%)16; therefore, the survey was sent to all CSHP members who met the inclusion criteria (n = 2499).
Summary statistics were used to characterize the cohort. Univariate and multivariate logistic regression using the purposeful selection method of Hosmer and Lemeshow was used to examine associations between demographic variables and pharmacists’ beliefs.17 Univariate regression models were initially completed, and variables with p < 0.2 in the univariate analysis were then entered in the multivariate analysis. Variables that were insignificant (p > 0.05) were dropped from the analysis. No adjustments were decided upon a priori, but the confounding effects of insignificant variables were checked before they were removed from the final main effect model by calculating the percentage change in beta coefficients; if an insignificant variable produced a greater than 15% change in the beta coefficient, then it was considered to be a confounder and was retained in the final main effect model. Collinearity among independent variables was checked for the variance inflation factor before the univariate analysis was performed, with values of 10 or higher indicating collinearity. All “do not know” responses were excluded from the multivariate analysis. All statistical analyses were performed using PASW Statistics for Windows, version 18.0 (SPSS Inc, Chicago, Illinois).
Invitations were sent by e-mail to 2499 pharmacists, of whom 367 completed the survey (response rate 15%). Table 2 highlights the respondents’ characteristics. Respondents were mostly women (258 [70%]), the majority had more than 10 years in practice (213 [58%]), and most were working in an inpatient unit at a hospital (236 [64%]). The most common practice areas were general practice (99 [27%]), internal medicine specialty (49 [13%]), and geriatrics (41 [11%]). More than half of respondents (212 [58%]) provided care to 1 or more patients with osteoporosis per week, whereas less than one-third (109 [30%]) reported providing care to 1 or more patients with both osteoporosis and renal insufficiency per week.
Table 2. Demographic and Practice-Related Characteristics of Survey Respondents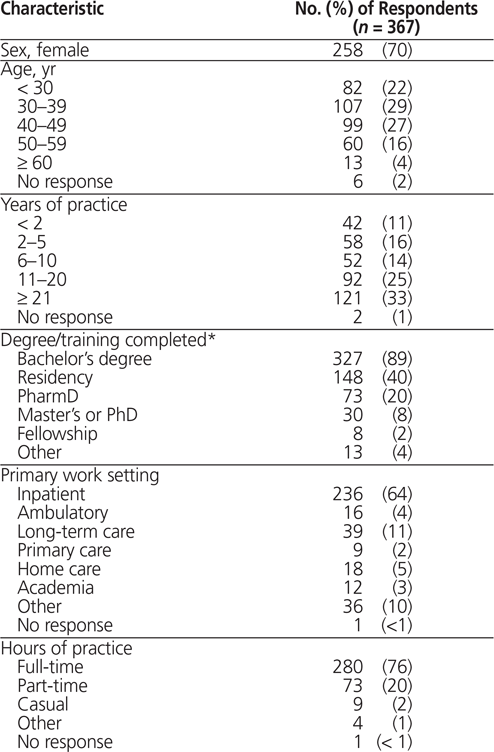
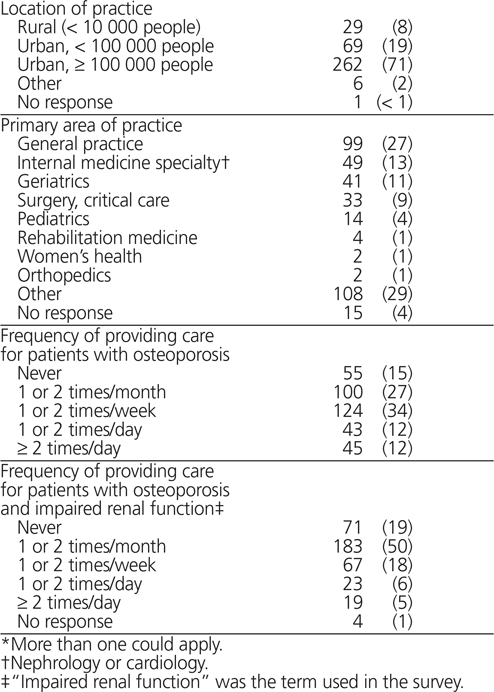
Table 3 provides an overview of respondents’ practices for patients with osteoporosis and various levels of renal function. Most respondents (335 [91%]) indicated that they would consider using a bisphosphonate for patients with CrCl between 30 and 60 mL/min; about one-fifth (70 [19%]) of respondents would adjust the dose in these patients. For patients with CrCl of 15 to 30 mL/min, responses were more varied, with 33% (122) using a bisphosphonate and adjusting the dose and 44% (160) avoiding bisphosphonates and using another osteoporosis medication. For patients with CrCl less than 15 mL/min, more than half of respondents (207 [56%]) reporting avoiding bisphos phonates and using another osteoporosis medication, with 17% (64) not using any osteoporosis medication for these patients.
Table 3. Pharmacists’ Practices for Patients with Osteoporosis and High Risk of Fracture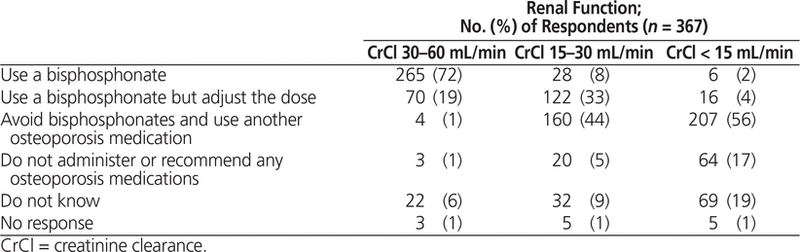
Respondents’ level of agreement with statements regarding bisphosphonates is summarized in Table 4. Respondents agreed or strongly agreed that oral bisphosphonates can be used in patients with renal failure as long as dosage adjustments are made (176/363 [48%]). Many disagreed or strongly disagreed that bisphosphonates are not as effective in preventing fractures in patients with renal failure (197/365 [54%]); however, most believed there was an increase in general adverse effects in this population. Most respondents believed that long-term use of oral bisphosphonates increases the risk of osteonecrosis of the jaw (255/364 [70%]) and atypical fractures (279/363 [77%]); however, fewer respondents thought that these risks were increased in patients with renal failure (91/365 [25%] and 101/364 [28%], respectively), with about half choosing the “do not know” option for each risk. Fewer than half of respondents believed that IV bisphosphonates are associated with nephrotoxicity (163/363 [45%]), whereas just over half (204/364 [56%]) disagreed or strongly disagreed that oral bisphosphonates are associated with nephrotoxicity.
Table 4. Pharmacists’ Beliefs about Efficacy and Adverse Effects of Bisphosphonates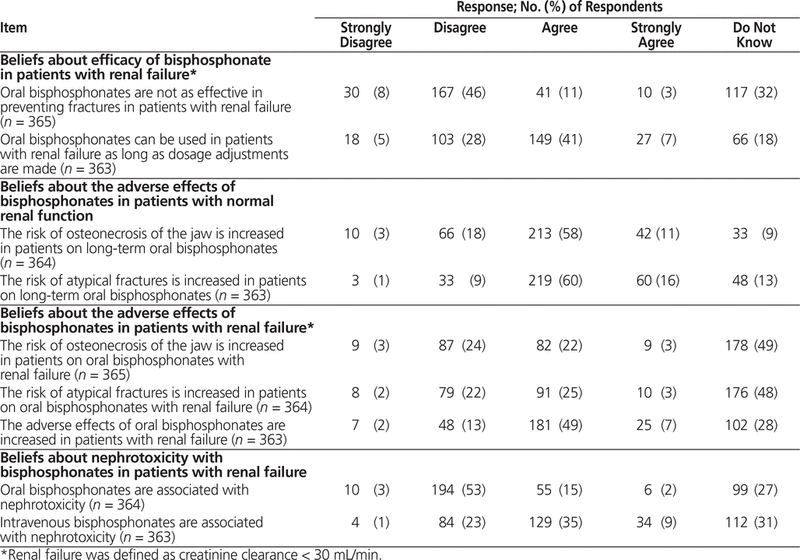
Overall, respondents expressed a low level of comfort in screening (mean ± standard deviation 2.47 ± 1.10 [maximum score 5]), assessing (2.46 ± 1.07), and monitoring therapy (2.49 ± 1.03) for patients with osteoporosis and renal failure. Similar results were obtained for comfort level in initiating therapy (2.40 ± 1.01) and understanding the effectiveness (2.53 ± 1.04) and safety (2.48 ± 1.03) of bisphosphonates. Respondents expressed a high level of interest in learning more about assessing (3.49 ± 1.19), monitoring (3.76 ± 1.12), and safety of bisphosphonates (4.03 ± 1.04) and other osteoporosis medications (4.01 ± 1.03) in patients with osteoporosis and renal failure.
In the multivariate logistic regression analysis, the only variable associated with beliefs about osteoporosis in patients with renal failure was area of practice (Table 5). Pharmacists who worked in general practice (e.g., family practice or internal medicine), as opposed to a specialty practice, were nearly 3 times more likely to agree that there was an increased risk of atypical fractures with bisphosphonates in patients with renal failure (compared with patients with normal renal function) (adjusted odds ratio [OR] 2.85, 95% confidence interval [CI] 2.35–3.35, p = 0.036).
Table 5. Factors Significantly Associated with Practices and Beliefs in Caring for Patients with Osteoporosis and Chronic Kidney Disease by Multivariate Analysis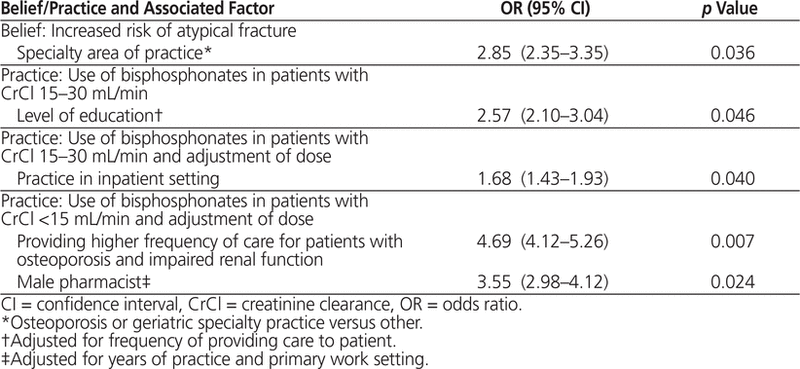
Pharmacists with advanced training were more likely to use a bisphosphonate for patients with CrCl of 15 to 30 mL/min (adjusted OR 2.57, 95% CI 2.10–3.04, p = 0.046). Pharmacists who worked in inpatient settings were also more likely to use a bisphosphonate, with adjustment of the dose (adjusted OR 1.68, 95% CI 1.43–1.93, p = 0.040). Pharmacists who provided a higher frequency of care to patients with both osteoporosis and renal failure and male pharmacists were more likely to use a bisphosphonate in patients with CrCl below 15 mL/min, with adjustment of the dose (adjusted OR 4.69, 95% CI 4.12–5.25, p = 0.007; adjusted OR 3.55, 95% CI 2.98–4.12, p = 0.024, respectively).
Pharmacists had varying practices and beliefs regarding bisphosphonate use in patients with osteoporosis and severe CKD. Despite frequent clinical encounters, respondents expressed a low level of comfort in assessing and managing these patients, but had a high level of interest in learning more about this topic.
These findings emphasize the challenges that health care professionals face in caring for patients with osteoporosis and severe CKD. Hospital pharmacists are in a prime position to influence outcomes within this population. They are viewed as drug therapy experts18 and are leaders in medication safety.19 Hospital pharmacists collaborate with other health care professionals and play an important role in the management of patients with osteoporosis, which includes screening for patients at high risk of fracture and recommending therapy.20–22 The responses collected in this survey highlight the need to adapt current best practice guidelines to provide clearer evidence-based direction for the care of this population, as well as knowledge translation initiatives such as decision support tools and targeted active education programs.23 Given that pharmacists are often consulted by other health care professionals for recommendations in renal dosing of drugs, it is imperative that they have the necessary tools to support them in their practice.
This survey focused on the use of bisphosphonates, because these agents are first-line therapy for fracture prevention.5 These medications have been on the Canadian market for decades and have clearly labelled contraindications and precautions related to renal function. CrCl was used in this study as an indicator of renal function, as it is the standard used by Health Canada and the US Food and Drug Administration for official product monographs and labelling. The drug monographs indicate a CrCl cut-off of 30 or 35 mL/min for most bisphosphonates, with no dosage adjustments required if CrCl is above this level.15 The majority of respondents to the current survey stated that they would consider using a bisphosphonate for patients with CrCl between 30 and 60 mL/min; surprisingly, however, nearly a fifth stated that they would also adjust the dose. For patients with CrCl of 15 to 30 mL/min, respondents were split between “using a bisphosphonate while adjusting the dose” and “avoiding a bisphosphonate and using another osteoporosis medication”, which highlights the lack of clear guidelines regarding the use of bisphosphonates for patients with CrCl in this range.
Although not conclusive, a few studies have shown that oral bisphosphonates can be safely used for patients with CrCl less than 30 mL/min without compromising efficacy. In a pooled analysis of 9 published clinical trials of risedronate, Miller and others24 found that about 7% of participants enrolled in the trials had CrCl less than 30 mL/min. Risedronate was not associated with an increased risk of adverse events in this subgroup of patients, and vertebral fracture reduction was consistent regardless of renal function. The authors concluded that risedronate for up to 3 years was safe and efficacious in patients with CrCl as low as 15 mL/min.24 In the Fracture Intervention Trial, nearly 10% of patients had CrCl below the manufacturer’s cut-off for alendronate of 35 mL/min, and a secondary analysis showed that fracture reduction with this drug was similar for women with reduced renal function and women with normal renal function.25 A small case–control study showed no difference in bone mineral density changes and no increase in adverse effects with the use of alendronate in elderly men with CrCl below 35 mL/min, relative to patients with normal renal function.26 Several chart reviews of bisphosphonate use in elderly patients have shown that many patients with severe CKD and CrCl below the manufacturers’ recommended cut-off (ranging from 17% to 50% of the patients in the studies) continued to receive bisphosphonates without adverse outcomes.27,28
Respondents to this survey were notably hesitant to treat osteoporosis in patients with CrCl below 30 mL/min. Indeed, treatment of patients with CrCl below 30 mL/min requires caution, as these patients may have bone turnover disorders (e.g., osteomalacia) and are susceptible to hypocalcemia if exposed to a bisphosphonate.29 However, there is some evidence that even patients in CKD stages 4 and 5 may benefit from bisphosphonate therapy.30 Some clinicians have advocated dosage adjustments for these patients,31 including recommendations to reduce the oral bisphosphonate dose by half.32 However, there is little evidence to support such recommendations. Unfortunately, long-term pharmacokinetic studies looking at the outcomes of bisphosphonate dosage adjustment in CKD are not available,33 which may be due in part to potential liability associated with studying a contraindicated use, the complexity of this patient population, and the existence of other, more urgent health priorities for patients with CKD.
Further information about the use of the RANK-ligand inhibitor denosumab in patients with renal failure has been published since this survey was developed. Denosumab is not cleared by the kidneys and has been shown to prevent fractures in patients with renal failure.34 Pharmacokinetic studies have shown no need for dosage adjustment35; however, there is still a potential concern about hypocalcemia in patients with severe CKD.36
There is limited information about drug dosing or drug use in elderly patients with CKD, as most CKD studies have excluded patients above 70 years of age.37 The pattern of excluding elderly or frail patients from research studies extends beyond studies of bone health or kidney disease.38 As a result, few clinical practice guidelines are available to provide direction on those older than 70 or 75 years of age, despite this age group being at high risk for osteoporosis and fractures.39 Of particular concern are those in the “oldest old” age group (age 80 years or older), as the risks and benefits of drug therapy for bone health and the impact of renal insufficiency are uncertain.40 In addition, studies involving older adults focus more on the risks than the benefits, which may lead to undertreatment of a vulnerable population.41 Fortunately, a few studies have considered older women up to age 100, although these studies have excluded patients with CKD and other significant medical illnesses.42 Boonen and others42 demonstrated that vertebral fractures could be reduced with risedronate, combined with calcium and vitamin D, over a 3-year period, with side effects similar to the placebo arms. The primary end point was at 3 years, with the risedronate 5 mg group showing a 44% reduction in fractures relative to control (hazard ratio [HR] 0.56, 95% CI 0.39–0.81, p < 0.003). They also analyzed the data at 1 year and found an 81% reduction in fractures (HR 0.19, 95% CI 0.09–0.40, p < 0.001). This suggests that use of bisphosphonate in older patients can have benefit within 1 year and continuing for years to come. However, these trials provided no evidence on how to treat frail seniors, those with a chronic medical condition such as CDK, or older men.
The safety of long-term use of bisphosphonates has been questioned because of emerging reports of osteonecrosis of the jaw and atypical fractures.43 The risk of fracture from osteoporosis is far greater than the overall risk of osteonecrosis of the jaw or atypical fractures; both would be considered very rare events with bisphosphonates.43,44 The greatest association with osteonecrosis of the jaw has involved high-dose IV bisphosphonates in patients with cancer and patients undergoing major dental work.13 A number of specialists are now recommending “drug holidays” for patients at low to moderate risk of fracture after 3 to 5 years of use.13,43 The majority of respondents to this survey agreed that there was an increased risk of osteonecrosis of the jaw and atypical fractures in patients on long-term bisphosphonate therapy. In contrast, nearly half of the respondents chose the “do not know” option on questions about these risks being increased in patients with severe CKD as compared to patients with normal renal function. This result may reflect uncertainty about these long-term risks in this population.3
This study had several limitations. Even though respondents represented a national sample of CSHP members, it may be difficult to generalize the results to all front-line hospital pharmacists. Membership in CSHP is optional, with about 44% of hospital pharmacists across Canada being members (Robyn Rockwell, CSHP Membership Administrator: personal communication, July 2014). There were far fewer respondents from Quebec, as CSHP membership is lower in that province than in other provinces, and the survey was not available in French. In addition, the sample included more pharmacists with advanced training than may typically be seen for front-line staff. The response rate was low but was targeted a priori to meet the desired sample size, based on previously published survey response rates in pharmacy practice research.16 In addition, except for a slightly higher proportion of female respondents in the current survey (70% versus 60%), the sample characteristics were similar to those of the Canadian pharmacist workforce in terms of age ranges and hours of practice.45 Because osteoporosis is more common among women than men, the beliefs of female respondents may have differed from those of their male counterparts, which may in turn have influenced the overall results. Further research is required to determine the influence of health care professionals’ personal and professional characteristics on their attitudes and practices. Other limitations were based on the survey design, such as self-reporting bias and social desirability, which may have influenced how pharmacists responded to questions about a controversial topic such as bisphosphonate use in patients with CKD.
This survey has identified pharmacists’ practices and beliefs related to the management of osteoporosis in patients with CKD. This information is important to capture, as pharmacists are often consulted about dosage adjustment in patients with CKD. This study highlights the need for evidence-based knowledge translation initiatives addressing fracture prevention in patients with osteoporosis and CKD, such as practice tools and targeted educational programs, to support pharmacists in their practice. Future research should look at the perspectives of other health care professionals with regard to the treatment of osteoporosis in this population.
1. Cauley JA. Public health impact of osteoporosis. J Gerontol. 2013;68(10): 1243–51.
2. Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20(10):1633–50.

3. Miller PD. The kidney and bisphosphonates. Bone. 2011;49(1):77–81.

4. National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–201.

5. Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al.; Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182(17):1864–73.


6. Tonelli M, Riella M. Chronic kidney disease and the aging population. Am J Hypertens. 2014;27(3):287–90.

7. Dunn RL, Bird ML, Conway SE, Stratton MA. Use of bisphosphonates in older adults: how long is long enough? Consult Pharm. 2013;28(1):39–57.

8. Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18(2):75–85.

9. Fosamax [product monograph]. Kirkland (QC): Merck Canada Inc; 2013 Oct 21 [cited 2015 Mar 2]. Available from: www.merck.ca/assets/en/pdf/products/FOSAMAX-PM_E.pdf
10. Aclasta [product monograph]. Dorval (QC): Novartis Pharmaceuticals Canada Inc; 2005 Jun 30 [cited 2015 Mar 2]. Available from: https://www.novartis.ca/en/our-products/pharmaceuticals
11. Actonel and Actonel DR [product monograph]. Toronto (ON): Warner Chilcott Canada Co; 2011 Jul 15 [cited 2015 Mar 2]. Available from: www.sanofi.ca/products/en/actonel.pdf
12. Cortet B. Osteoporosis medications and renal failure. Joint Bone Spine. 2011;78(3):228–9.

13. McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126(1):13–20.
14. Salam SN, Eastell R, Khwaja A. Fragility fractures and osteoporosis in CKD: pathophysiology and diagnostic methods. Am J Kidney Dis. 2014;63(6): 1049–59.

15. Sadowski CA, Spencer T, Yuksel N. Use of oral bisphosphonates by older adults with fractures and impaired renal function. Can J Hosp Pharm. 2011;64(1):36–41.

16. Siyam T, Yuksel N. Beliefs about bioidentical hormone therapy: a cross-sectional survey of pharmacists. Maturitas. 2013;74(2):196–202.
17. Hosmer DW Jr, Lemeshow S, Sturdivant RX, editors. Model-building strategies and methods for logistic regression. Applied logistic regression. 3rd ed. John Wiley and Sons, Inc; 2013.
18. Moffett BS, Mott AR, Nelson DP, Gurwitch KD. Medication dosing and renal insufficiency in a pediatric cardiac intensive care unit: impact of pharmacist consultation. Pediatr Cardiol. 2008;29(4):744–8.
19. Young D. Pharmacist travels medication safety path. Am J Health Syst Pharm. 2007;64(9):912, 916.

20. MacLaughlin EJ. Improving osteoporosis screening, risk assessment, diagnosis, and treatment initiation: role of the health-system pharmacist in closing the gap. Am J Health Syst Pharm. 2010:67(7 Suppl 3):S4–8.

21. Banakh I. PRO-OSTEO Project (improving osteoporosis management in the acute hospital setting): a pilot single-centre study. Arch Osteoporos. 2011;6: 157–65.
22. Heilmann RMF, Friesleben CR, Billups SJ. Impact of a pharmacist-directed intervention in postmenopausal women after fracture. Am J Health Syst Pharm. 2012;69(6):504–9.

23. Straus SE, Tetroe J, Graham ID. Knowledge translation: what it is and what it isn’t. In: Straus SE, Tetroe J, Graham ID, editors. Knowledge translation in health care: moving from evidence to practice. 2nd ed. Oxford (UK): Wiley Blackwell; 2013. p. 3–13
24. Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20(12):2105–15.

25. Jamal SA, Bauer DC, Ensrud KE, Cauley JA, Hochberg M, Ishani A, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res. 2007;22(4):503–8.

26. Hansen KE, Hofmann RM, Drake RK, Argall TR, Bier HA, Grigg KT, et al. An exploratory analysis of alendronate in older men with low glomerular filtration rate. J Aging Pharmacother. 2006;13(2):21–33.
27. Lewiecki E, Rudolph L. Oral bisphosphonate for treatment of osteoporosis in elderly patients with impaired renal function [abstract]. J Bone Miner Res. 2002;17 Suppl 1:SU335.
28. Bernett GB, Feldman S, Martin H, Smith BC, Raineri BD. An opportunity for medication risk reduction, healthcare provider collaboration, and improved patient care: a retrospective analysis of osteoporosis management. JAMA. 2003;4(6):329–36.
29. Liu WC, Yen JF, Lang CL, Yan MT, Lu KC. Bisphophonates in CKD patients with low bone mineral density. Sci World J. 2013;2013:837573.
30. Toussaint ND, Elder GJ, Kerr PG. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4(1):221–33.
31. Miller PD. Treatment of osteoporosis in chronic kidney disease and end-stage renal disease. Curr Osteoporos Rep. 2005;3(1):5–12

32. Ozmen B. Use of bisphosphonates in chronic kidney disease. World J Nephrol Urol. 2012;1(1):1–7.
33. Courtney AE, Maxwell AP. Chronic kidney disease and bisphosphonate treatment: are prescribing guidelines unnecessarily restrictive? Postgrad Med J. 2009;85(1004):327–30.

34. Jamal SA, Ljunggren O, Stehman-Breen C, Cummings SR, McClung MR, Goemaere S, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res. 2011;26(8):1829–35.

35. Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res. 2012;27(7):1471–9.


36. Dave V, Chiang CY, Booth J, Mount PF. Hypocalcemia post denosumab in patients with chronic kidney disease stage 4–5. Am J Neprol. 2015;41(2):129–37.
37. Fassett RG. Current and emerging treatment options for the elderly patient with chronic kidney disease. Clin Interv Aging. 2014;9:191–9.


38. Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. J Gen Intern Med. 2011; 26(7):783–90.


39. Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24.

40. Colon-Emeric CS. Recent advances: osteoporosis in the “oldest old”. Curr Osteoporos Rep. 2013;11(4):270–5.

41. Gosch M, Jeske M, Kammerlander C, Roth T. Osteoporosis and polypharmacy. Z Gerontol Geriatr. 2012;45(6):450–4.

42. Boonen S, McClung MR, Eastell R, El-Hajj Fuleihan G, Barton IP, Delmas P. Safety and efficacy of risedronate in reducing fracture risk in osteoporotic women aged 80 and older: implications for the use of antiresorptive agents in the old and oldest old. J Am Geriatr Soc. 2004;52(11):1832–9.

43. Brown JP, Morin S, Leslie W, Papaioannou A, Cheung AM, Davison KS, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician. 2014;60(4):324–33.

44. Saleh A, Hegde VV, Potty AG, Lane JM. Bisphosphonate therapy and atypical fractures. Orthop Clin North Am. 2013;44(2):137–51.

45. Pharmacist workforce, 2012—provincial/territorial highlights. Ottawa (ON): Canadian Institute for Health Information; 2013 [cited 2015 Jun 19]. Available from:https://secure.cihi.ca/free_products/PharmacistWorkforce2012HighlightsEN.pdf
Competing interests: Cheryl A Sadowski has received a grant from Pfizer Canada Inc for an unrelated research project. Catherine Lyder has provided continuing education on behalf of MDBriefcase and HealthPRO. Nesé Yuksel has provided continuing education on behalf of Warner Chilcott and has received honoraria for participation in advisory boards and consult meetings for Teva Pharmaceuticals and Pfizer Canada Inc.
Funding: This project was funded by a grant from the CSHP Research and Education Foundation (now known as the CSHP Foundation).
The authors would like to thank the Information Services and Technology department at the University of Alberta for administering the online version of the survey and Tasneem Siyam for providing statistical support for the project. The authors would also like to thank the administrative staff of the Canadian Society of Hospital Pharmacists for assisting with this study, as well as all the pharmacists who participated in the survey.
Canadian Journal of Hospital Pharmacy, VOLUME 69, NUMBER 1, January-February 2016