
Mary H H Ensom, PharmD, FASHP, FCCP, FCSHP, Clinical Pharmacy Specialist, Diane Décarie, BSc, Research Consultant
Dexamethasone suspensions extemporaneously prepared from dexamethasone for injection are stable for 91 days.1,2 However, because some pharmacies prefer to use tablets rather than the injectable formulation, this study was undertaken to evaluate the stability of dexamethasone from tablets in suspensions (1 mg/mL) prepared with commercially available dye-free vehicles after storage at 25°C and 4°C in various types of container for up to 91 days.
Stock suspensions of dexamethasone 1 mg/mL were prepared by crushing commercially available dexamethasone tablets (4 mg; Pharmascience Inc, Montréal, Quebec; lot 480851, expiry February 2018) and resuspending the powder in 400-mL volumes of each of Oral Mix and Oral Mix SF (Medisca Inc, Plattsburg, New York; lot I074/A, expiry February 2015, and lot I071/A, expiry January 2015, respectively). Two replicates of each suspension were aliquoted into 4 amber glass bottles (Richards Distribution, Richmond, British Columbia), 4 amber polyethylene terephthalate prescription bottles (Richards Distribution), and twelve 5-mL amber oral polypropylene syringes (PreciseDose Dispenser System, Medisca Pharmaceutique Inc, Montréal, Quebec; lot 46959/C). Two glass and 2 plastic bottles of each suspension and all syringes were kept at 25°C, and 2 glass and 2 plastic bottles of each suspension were kept at 4°C.
All samples were examined for colour, odour, taste, and ease of resuspension on days 0, 7, 14, 28, 49, 70, and 91, after which a 2.5-mL aliquot from each bottle and the contents of 2 syringes from each group were collected to determine pH (pH meter model 800, VWR International, Mississauga, Ontario). A 1.5-mL aliquot from each bottle or syringe was transferred to a threaded, tight-seal cryogenic polypropylene vial (VWR International; lot 1095990) and frozen (−85°C) until analysis by a validated, stability-indicating high-performance liquid chromatography (HPLC) – ultraviolet detection method developed in the authors’ laboratory on the basis of previous work.1,2
On each analysis day, stock suspensions of dexamethasone were prepared in Oral Mix from dexamethasone 4-mg tablets to construct a standard curve. Naproxen powder (Sigma-Aldrich, Oakville, Ontario; lot SLBH7957V), diluted in HPLC-grade methanol (Fisher Scientific, Whitby, Ontario; lot 152928) to 0.200 mg/mL, served as the internal standard. Dexamethasone standard solutions (containing internal standard 0.020 mg/mL) were prepared in a mixture of 55% HPLC-grade water (Fisher Scientific; lot 151592), 30% HPLC-grade methanol, and 15% HPLC-grade acetonitrile (Fisher Scientific; lot 150174) to final concentrations of 0.050, 0.060, 0.080, 0.120, and 0.150 mg/mL, with quality control concentrations of 0.050, 0.070, 0.100, and 0.130 mg/mL. All standards were passed through a 0.45-µm microfilter (Acrodisc, Waters Corporation, Mississauga, Ontario; lot 21844792). Standard curves were generated by least-squares regression of the peak area ratio of dexamethasone to internal standard and the concentration of each dexamethasone standard.
The HPLC instrumentation (Waters Alliance System model 2690, Waters Corporation) consisted of a delivery pump, automated 200-µL injector, Atlantis dC18 4.6 × 150 mm column (Waters Corporation; lot 014443514914028), Atlantis dC18 3.9 × 20 mm guard column (Waters Corporation; lot 013931339), and ultraviolet detector set at 238 nm. The mobile phase consisted of 76% methanol and 24% 10 mmol/L ammonium formate buffer (Sigma-Aldrich; lot BCBL4456V) at pH 4.0 and 25°C. The flow rate was 1.0 mL/min.
On analysis days, samples were thawed and vortex-mixed. A 0.2-mL aliquot from each thawed sample was diluted with 1.8 mL of HPLC-grade methanol and centrifuged at 5200 rpm for 5 min. Supernatant (100 µL) was diluted to a final nominal concentration of 0.100 mg/mL in HPLC-grade methanol containing 0.0200 mg/mL internal standard. Each sample (10 µL) was filtered before injection onto the column.
Accelerated degradation was achieved as follows. Suspensions of dexamethasone 2.0 mg/mL in Oral Mix and Oral Mix SF were prepared, mixed (v/v) with water, 2N sodium hydroxide (NaOH), 2N hydrochloric acid (HCl), or 30% hydrogen peroxide (H2O2), then vortex-mixed and incubated for 4–18 h at 90°C. Samples were cooled to 25°C, diluted in acetonitrile, and centrifuged; concentration was adjusted to 0.100 mg/mL with water–methanol–acetonitrile; and samples were filtered and injected onto the column.
Regression analysis demonstrated linearity, with coefficient of determination (r2) of at least 0.994 (n = 4). Intraday and interday coefficients of variation for quality control samples were within acceptable limits (< 10%): 1.71% and 1.68%, respectively, for the 0.050 mg/mL solution; 0.73% and 1.01%, respectively, for the 0.070 mg/mL solution; 0.90% and 1.31%, respectively, for the 0.100 mg/mL solution; and 0.88% and 1.01%, respectively, for the 0.130 mg/mL solution. Mean intraday and interday accuracy values (± standard deviation) were also within acceptable limits (> 90%): 98.41% ± 0.84% and 93.53% ± 0.2.63%, respectively, for the 0.050 mg/mL solution; 98.21% ± 0.85% and 97.30% ± 3.31%, respectively, for the 0.070 mg/mL solution; 98.91% ± 0.71% and 98.48% ± 1.20%, respectively, for the 0.100 mg/mL solution; and 99.39% ± 0.0.67% and 99.34% ± 1.38%, respectively, for the 0.130 mg/mL solution.
Retention times were 3.48 min for dexamethasone and 4.01 min for the internal standard. No interfering peaks were generated by forced degradation of dexamethasone with heat, NaOH, HCl, or H2O2. With forced degradation, dexamethasone decreased by 10.2% to 85.6% in Oral Mix and by 42.1% to 85.5% in Oral Mix SF; minor, non-interfering peaks were observed at 1.76, 1.99, 2.06, 2.30, 2.69, 3.07, 4.56, 4.74, and 4.89 min. Time 0 samples stored for 91 days at −85°C showed no degradation products and contained the expected concentration of dexamethasone, which indicated that freezing did not affect stability.
The milky white suspensions were easily resuspended throughout the study, with no notable changes in colour, odour, or taste. The mean pH remained stable (range 4.39–4.44 in Oral Mix and 4.48–4.51 in Oral Mix SF). HPLC analysis showed that all suspensions maintained more than 90% of original concentration for up to 91 days (Tables 1 and 2).
Table 1. Concentration of Dexamethasone (from Tablets) in Oral Mix Suspension Vehicle During 91 Days of Storage in Glass Bottles, Plastic Bottles, and Plastic Syringes at 25°C and in Glass and Plastic Bottles at 4°C*
Table 2. Concentration of Dexamethasone (from Tablets) in Oral Mix SF Suspension Vehicle During 91 Days of Storage in Glass Bottles, Plastic Bottles, and Plastic Syringes at 25°C and in Glass and Plastic Bottles at 4°C*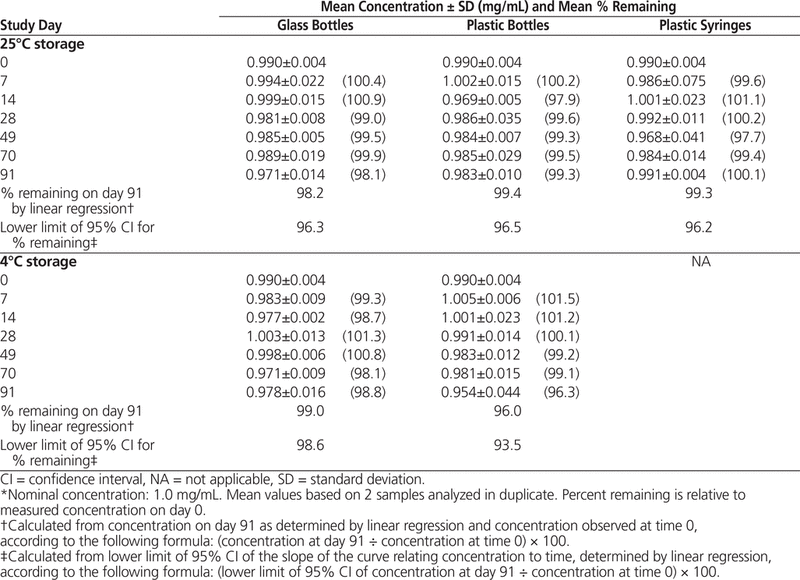
In conclusion, dexamethasone suspensions (1 mg/mL) in Oral Mix and Oral Mix SF remained stable for up to 91 days when stored in glass and plastic bottles (25°C or 4°C) or syringes (25°C).
1. Chou JWL, Décarie D, Dumont RJ, Ensom MHH. Stability of dexamethasone in extemporaneously prepared oral suspensions. Can J Hosp Pharm. 2001;54(2):96–101.
2. Ensom MHH, Décarie D. Stability of extemporaneously compounded dexamethasone in glass and plastic bottles and plastic syringes. Can J Hosp Pharm. 2014;67(4):274–9.

Competing interests: Other than grant support, no competing interests were declared.
Mary Ensom is also a Professor, Faculty of Pharmaceutical Sciences, and Distinguished University Scholar, The University of British Columbia, Vancouver, British Columbia. She is also the Editor of the CJHP. ( Return to Text )
Funding: This study was funded by an unrestricted educational grant from Medisca Pharmaceutique Inc.
Canadian Journal of Hospital Pharmacy, VOLUME 69, NUMBER 1, January-February 2016