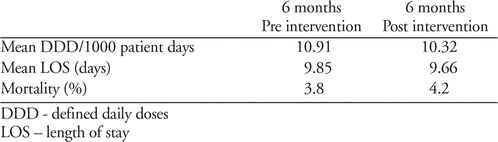CSHP Professional Practice Conference 2016: Poster Abstracts / Conférence sur la pratique professionnelle 2016 de la SCPH : Résumés des affiches
Sunday, January 31, 2016 • Dimanche 31 janvier 2016
Facilitated Poster Sessions: Discussion of original research, award-winning projects, and pharmacy practice projects
Séance animée de présentations par affiches : Discussions sur des projets de recherche originale, des projets primés et des projets dans le domaine de la pratique pharmaceutique
Clinical
-
Short-versus Standard-Term Dual Antiplatelet Therapy after Percutaneous Coronary Intervention with Drug-Eluting Stent Implantation: A Meta-Analysis
-
Dosing of Amiodarone for Post-Operative Atrial Fibrillation in Cardiac Surgery Patients
-
Evaluation of Novel Protocol for Treatment of Alcohol Withdrawal Syndrome in Psychiatric Inpatient Populations
-
Analysis of the Adherence to Seizure Prophylaxis Guidelines in Neurosurgery Patients
-
Interaction between Psychotropic Medications and Alcohol: Perception in Patients Attending a Psychiatric Day Hospital Program
Infectious Diseases
-
Development and Evaluation of a Screening Tool for Bacteremia in Neonates
-
Evaluation of Oseltamivir Dosing Regimens in Renal Dysfunction: 45 is the New 30
-
Epidemiology of Six Types of Bacteria, Hospital Resistance Rates and Associated Antibiotic Drug Consumption Rates in a Teaching Hospital
-
Implementation of a “Virtual” Vancomycin Clinic
-
Guideline Adherence Audit for Treatment of Pediatric Outpatient Urinary Tract Infection
Infectious Diseases/Stewardship
-
Developing and Evaluating an Educational Intervention to Guide in the Implementation of Antimicrobial Stewardship Programs in Community Hospitals
-
Clinicians’ Perspectives of the Enablers of and Barriers to Antibiotic De-Escalation
-
Impact of Hospital Wide Implementation of an Antimicrobial Stewardship Program on the Incidence of Hospital Acquired Clostridium difficile Infection
-
A Retrospective Review of Treatment Appropriateness and Outcomes of Enterobacteriaceae Bacteremias at a Large Academic Medical Centre: Opportunities and Implications for Antimicrobial Stewardship
Pharmacy Practice/Education
-
Medication Safety Culture Indicator Matrix: Going Beyond the Numbers and Using Incident Reports to Assess Medication Safety Culture
-
Does Peer-to-Peer Mentoring Make a Difference in Therapeutic Knowledge Acquisition for Student Learning?
-
Pharmacy Discharge Services: A Pilot Project Involving Pharmacist and Pharmacy Technicians at a Pediatric Hospital
-
Pharmaceutical Care for Children: A Survey of Pharmacy Students’ Preparedness and Confidence
-
Novel Models of Precepting: The Alberta Experience
Monday, February 1, 2016 • Lundi 1er février 2016
-
Structure and Function of Teams in the Pediatric Intensive Care Unit: A Social Network Analysis
-
A Failure Mode Effect Analysis Pre-Post Implementation of an Electronic Medication Administration Record
-
Étude intérimaire de satisfaction du personnel soignant dans l’implantation d’un formulaire électronique d’administration des doses de médicaments dans le cadre d’un projet pilote en chirurgie pédiatrique
-
Describing the Benefits of Residency Training through Key Performance Indicators
-
Engaging Leadership to Expand Experiential Capacity in Institutional Settings
-
Standardization of Criteria Used by Pharmacists to Prioritize New Patients for Comprehensive Pharmaceutical Care
-
Evaluation of Standardization of Pharmacist Involvement in Best Possible Medication Histories and Medication Reconciliation
-
Safe Insulin Pen Labelling Practices in a Community Teaching Hospital: A Post-Implementation Evaluation
-
Cost and Waste Impact of Implementing Insulin Pen Devices in a Community Teaching Hospital
-
Incidence des effets indésirables médicamenteux dans un centre hospitalier universitaire mère-enfant de 1989 à 2014 : la loi de Vanessa permettrat-elle d’augmenter le taux de déclaration?
-
Developing and Implementing a Competency Based Program for Pharmacy Technician Students
-
A 10-Year Retrospective Study of the Medication Incidents and Accidents Associated with Drug Dose Documentation in a Teaching Hospital
-
Hospital Pharmacy in Canada Report: 30th Anniversary
-
Impact of Comprehensive Medication Reviews Completed by Pharmacy Students in a Complex Continuing Care Program: A Pilot Project
-
Safety Alerts as Drivers for the Pharmaceutical Opinion Program: A Pilot Study to Reduce Potential Hospitalizations Due to Preventable Drug-Drug Interactions
-
Medication Incidents Involving Drug Tapering
-
Medication Incidents Involving Insulin: A Multi-Incident Analysis
-
Development of a Framework for Podcast Creation to Supplement Pharmacy Students Learning
-
Complexity and Vulnerability of Multi-Medication Compliance Aids
-
Economic Evaluation of Adding a Drug to the Hospital’s Hazardous Drug List
-
Impact of a Geriatric-Hospitalist-Orthopaedic Co-Management Program on Closing the Post-Fracture Care Gap
-
The Opinions of Hospital Pharmacists in Canada Regarding Marihuana for Medical Purposes
-
Safety Implications of the Dose Change Alert in Smart Pumps on the Administration of High-Alert Medications
-
Implantation d’un logiciel de gestion de la conformité en établissement de santé
-
Reconstruction de l’hôpital Saint-Michel en Haïti : perspectives après cinq missions pharmaceutiques
-
Pilot Study of Biological Monitoring of Four Antineoplastic Drugs among Canadian Healthcare Workers – see page 56
-
Feasibility of Pharmacist-Run Group Diabetes Education Sessions for Patients on a Stroke Rehab Unit
-
Integrating Smartphone Communication Strategy and Technology into Clinical Practice: A Mixed Methods Research Study
-
Headline News: A Simulation Game for Hospital Pharmacy Leaders
-
Medication Incident Analysis Knowledge Mobilization Tool: Medication Safety Expertise at Your Fingertips
-
Comparison of Information Available in the Medication Profile of an Electronic Health Record and the Inpatient Best Possible Medication History
-
A Comparative Study of the Conformity of the Documentation of Drug Doses Administered Pre and Post Implementation of an Electronic Medication Record
-
Drug Shortages in Health Care Institutions: Perspectives in 2014–2015
-
Comparaison de la perception des pharmaciens québécois et français vis-à-vis des données sur les rôles et retombées des activités pharmaceutiques
-
Réactions des étudiants en pharmacie à la mise en place d’un code de bonnes pratiques pharmaceutiques en ligne et dans les réseaux sociaux
-
Chambre des erreurs : une simulation afin de sensibiliser le personnel soignant aux risques du circuit du médicament
Tuesday, February 2, 2016 • Mardi 2 février 2016
-
Evaluation of the Sterility of Single-Use Vials Undergoing Multiple Access Following Application of a Closed System Transfer Device
-
Evaluation of a Critical Incident: Simulating Hydromorphone Concentrations Using Population-Based Pharmacokinetic Parameters
-
Rhabdomyolysis during Treatment with Thrice-Weekly Daptomycin
-
Clinical and Economic Outcomes of Outpatient Parenteral Antimicrobial Therapy: Experience at a Community Hospital
-
Varicella Zoster Virus Meningitis Possibly Associated with Zoster Vaccination
-
Compliance to a Febrile Neutropenia Protocol for Leukemia Patients Based on Antimicrobial Stewardship Principles and Human Factors Engineering
-
Once Daily Aminoglycoside Pharmacokinetics and Optimal Dosing in the Burn Population: A Prospective Study
-
Multicenter Study of Environmental Contamination with Antineoplastic Drugs in 47 Canadian Hospitals
-
Investigation of the Face and Content Validity, and Perceived Usefulness of the Pharmacy Residency Competency Based Assessment Tools
-
Development and Validation of a Screening Tool for Bacteremia in Acute Burn Injury Patients
-
A Study to Determine the Pharmacological Management of Delirium in the Cardiovascular Intensive Care Unit
-
Design and Implementation of Pharmacy Services for the Toronto 2015 Pan Am and Parapan Am Games
-
Ciprofloxacin-Induced Stevens-Johnson Syndrome Treated with Cyclosporine: A Case Report
-
Enjeux relatifs à l’exercice de la pharmacie : perception des résidents en pharmacie
-
Stability of 1.0 and 2.5 mg/mL Bortezomib Solution in Vials and Syringes Following Reconstitution with 0.9% Sodium Chloride at 4°C and Room Temperature (23°C)
-
Bridging the Gap from the Classroom to the Institutional Practice Site: Evaluation of an Online Transition Module for Pharmacy Students
-
Comparison of Pharmacy Students’ and Pharmacists’ Activities Using a Clinical Pharmacist Workload Measurement Tool
-
Evaluation of Standardization of Pharmacist Attendance at Rounds
-
Fatigue, Anxiety and Irritability with Apixaban
-
Evaluation of a Communities of Practice Program for Clinical Pharmacists
-
A Canadian Survey of High-Dose Extended-Interval Gentamicin and Tobramycin in Pediatric Inpatients
-
Strategies to Support Pharmacy Students’ Progress in Experiential Learning: A Literature Review
-
Evaluation of Meropenem Usage Patterns in the Paediatric Intensive Care Unit and Cardiac Critical Care Unit
-
Adjunctive Ethanol-Lock Therapy in Paediatric Patients with Catheter-Related Bloodstream Infection
-
Utilisation des données relatives aux rôles et retombées de l’activité pharmaceutique : étude pilote de panels d’experts
-
Pharmacokinetics of Oral Ciprofloxacin at Steady State in Continuous Cycling Peritoneal Dialysis
-
Evaluating the Short-Term Sustainability of Benzodiazepine Receptor Agonist Discontinuation Following Discharge from a General Internal Medicine Program: A Prospective Observational Study
-
Antimicrobials Defined Daily Doses and Days of Therapy in a Mother-Child Teaching Hospital from 2010–2011 to 2014–2015
-
Development of a Standard Assessment Tool for Field-Based Pharmacy Training
-
Attitudes and Beliefs towards Smoking Cessation Medications amongst Canadian Armed Forces Personnel
-
Characterizing the Role of Infectious Diseases Consultant Pharmacists and Antimicrobial Stewardship Pharmacists: A Survey of Canadian Tertiary Care Academic Hospitals
-
Evaluation of Oseltamivir Dosing Recommendations for Elderly Patients
-
Defining the Roles of an Experiential Education Facilitator: A Pilot Project
-
Non-Traditional Learner-Preceptor Models: Development of Preceptor Guidebooks
-
Standardization of Patient Education by Pharmacists
-
Implementation of an Insulin Pen Trial in an Acute Medical and Surgical Patient Population
-
Inappropriate Administration of Oral Valganciclovir to Infants with Congenital Cytomegalovirus Infection
-
Two Cases of New Onset Refractory Status Epilepticus: Prolonged Barbiturate and Ketamine Therapy
The texts of poster abstracts are published exactly as submitted by the authors and have not undergone any copyediting by the Canadian Journal of Hospital Pharmacy. / Le Journal canadien de la pharmacie hospitalière n’a pas soumis le texte des résumés des affiches à une révision linguistique et les publie ici tels que remis par les auteurs.
CSHP Professional Practice Conference 2016: Poster Abstracts / Conférence sur la pratique professionnelle 2016 de la SCPH : Résumés des affiches
Short-versus Standard-Term Dual Antiplatelet Therapy after Percutaneous Coronary Intervention with Drug-Eluting Stent Implantation: A Meta-Analysis
J Basaraba1,2, A Barry3,4
1Mazankowski Alberta Heart Institute, Pharmacy Services, Alberta Health Services, Edmonton, AB
2Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, AB
3Chilliwack General Hospital, Lower Mainland Pharmacy Services, Chilliwack, BC
4Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC
ABSTRACT
Background:
Twelve months of dual antiplatelet therapy (DAPT) is recommended after percutaneous coronary intervention with drug-eluting stent implantation (PCI-DES). However, certain clinical scenarios may require premature discontinuation of therapy (e.g., urgent surgical procedures, major bleeding).
Objective:
To investigate clinically relevant efficacy and safety outcomes associated with a shorter duration of DAPT, compared to 12 months, after PCI-DES.
Methods:
A systematic search of Medline and Embase (inception to April 2015) was conducted. Included were randomized controlled trials that compared 6 months (or less) to 12 months of DAPT in patients who underwent PCI-DES. DAPT was defined as acetylsalicylic acid 75–200 mg daily and clopidogrel 75 mg daily. Outcomes of interest included death, myocardial infarction (MI), definite or probable stent thrombosis, major bleeding, and any bleeding. An odds ratio (OR) and 95% confidence interval (CI) were calculated for each outcome using a Mantel-Haenszel random effects model (Review Manager, version 5.3).
Results:
Five studies (4 open-label, 1 double-blind) were included constituting 12,078 patients. Three studies investigated 6 months of DAPT, and 2 studies investigated 3 months. Outcomes were reported at 12 months in 4 trials and 15 months in 1 trial. Efficacy outcomes were not statistically significantly different between groups including death (OR 0.85, 95% CI 0.61–1.18), MI (1.14, 0.85–1.55), and stent thrombosis (1.20, 0.67–2.15). There was no significant difference in major bleeding (0.61, 0.35–1.03); however, any bleeding was reduced by a relative 41% with shorter-term DAPT (0.59, 0.44–0.79).
Conclusion:
Shorter duration (3–6 months) of DAPT was not associated with a higher risk of death, MI, or stent thrombosis compared to 12 months. Furthermore, it was associated with a lower rate of overall bleeding, but not major bleeding. Though limited by low event rates and study methodology, these data support that DAPT could be safely discontinued before 12 months if required.
Dosing of Amiodarone for Post-Operative Atrial Fibrillation in Cardiac Surgery Patients
T Lau1, E Wang2, J Ye2, C Chu3, D Chua2
1Vancouver General Hospital, Vancouver, BC
2St. Paul’s Hospital, Vancouver, BC
3Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC
ABSTRACT
Background:
Amiodarone is used for the management of post-operative atrial fibrillation (POAF) after cardiac surgery. There is significant heterogeneity in the dosing of amiodarone for POAF in the literature and a lack of guideline recommendations. The optimal dosing strategy of amiodarone for the prevention or treatment of POAF is unknown.
Objective:
To determine the optimal dosing strategy of amiodarone to prevent or treat POAF.
Methods:
We conducted a cross-sectional, retrospective chart review of adults receiving amiodarone for POAF after cardiac surgery from February 2013 to July 2014. The primary outcomes were to compare the dose of amiodarone in those who develop POAF versus those who remain in normal sinus rhythm (NSR) for POAF prevention, and to compare the dose of amiodarone in those who convert to NSR versus those who remain in POAF at discharge for POAF treatment.
Results:
Two hundred patients were included with mean age of 68.9 years. A total of 51.5% underwent coronary artery bypass grafting with an average post-operative length of stay of 11.1 days. The majority received amiodarone for treatment versus prevention of POAF (67% vs. 33%). A total of 166 (83%) patients developed POAF, with a mean time to POAF of 2.1±1.2 post-operative days, and 144 (87%) of these patients converted to NSR before discharge. There was no statistically significant difference in either dose of amiodarone for POAF prevention in those who develop POAF versus those who remain in NSR (5897±4223mg vs. 5061±3519mg, p=0.38) or dose of amiodarone for POAF treatment in those who are discharged in POAF versus those who convert to NSR (5775±3118mg vs. 5067±3462mg, p=0.43).
Conclusion:
The rate of POAF is high in patients receiving amiodarone for prevention or treatment. Receiving approximately 5000mg (oral equivalent) of amiodarone.
Evaluation of Novel Protocol for Treatment of Alcohol Withdrawal Syndrome in Psychiatric Inpatient Populations
M Butterfield1,2, L Thorne-Humphrey2, N Herschenhous1,2
1Department of Psychiatry, Dalhousie University, Halifax, NS
2Nova Scotia Health Authority – Zone One, Halifax, NS
ABSTRACT
Background:
In collaboration with a multidisciplinary group we developed a novel evidence based treatment algorithm with the goal of more effectively managing alcohol withdrawal (AW) in individuals admitted to acute psychiatric units. Due to the symptom driven nature of the commonly employed CIWA-Ar protocol patients suffering from psychotic, mood, and/or anxiety disorders may receive inappropriate treatment due to some of the counfounding comorbid symptoms seen in both AW and these psychiatric illnesses.
Objectives:
To improve clincal outcomes while maintaining or improving safety in psychaitric inpatints suffering from AW by employing a novel standardized treatment protocol.
Methods:
Using a retrospective cohort study design, we compared outcomes between patients admitted to psychiatric inpatient units who required treatment or were at high risk for AW before and after the implementation of this novel treatment algorithm. Our primary outcomes were mean duration of benzodiazepine treatment and cumulative dose administered. Our secondary outcomes were harm associated with AW related complications and benzodiazepine toxicity.
Results:
Preliminary data show that there are significant differences in our primary outcomes. Total benzodiazepine dose in diazepam equivalents administered to individuals treated for AW prior to the implementation of the algorithm was 42.7mg while individuals treated after the implementation of the algorthm recieved 21.6mg (p<0.01). Mean duration of benzodiazepine treatment time prior to the protocol implementation was 40.2h vs. 9.9h after protocol implementation (p<0.01). Total time on any AW protocol was 174.4h before protocol implementation vs. 38.7h after protocol implementation (p<0.01). No reports of AW seizures or benzodiazepine toxicity were reported in either group. (n= 107).
Conclusions:
Our preliminary data suggests that the implementation of a standardized protocol for the treatment of AW for individuals admitted to psychiatric inpatient units can result in a clinically meaningful reduction in benzodiazepine use without compromising efficacy or safety.
Analysis of the Adherence to Seizure Prophylaxis Guidelines in Neurosurgery Patients
X Liao1,3, A Chiu1, K Li1, D Beechinor1,2
1Trillium Health Partners: Mississauga Hospital, Mississauga, ON
2Trillium Health Partners: Credit Valley Hospital, Mississauga, ON
3Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
In 2000, the American Academy of Neurology guidelines recommended that long-term seizure prophylaxis should not be routine in patients with newly diagnosed brain tumours and that if anti-epileptic drugs (AEDs) are used, they should be tapered and discontinued after 7 days post-operatively. Recent data shows that most newly diagnosed brain tumour patients continue to receive prophylactic AED.
Objective:
This study aimed to examine the seizure prophylaxis practices at a regional neurosurgery centre.
Methods:
A retrospective chart review was performed in adult patients with newly diagnosed brain tumour(s) who underwent neurosurgery between January 2010 and December 2014. Patients who had a seizure history or used AEDs prior to admission were excluded. The percent (%) of patients who received primary seizure prophylaxis was calculated. Secondary outcomes included: in patients who received prophylaxis, the percent (%) of patients whose AED was appropriately discontinued; drug cost savings if these patients had not received prophylaxis; and patient characteristics (in terms of number, size, and location of tumours) most strongly correlated with seizure prophylaxis using a logistic regression model.
Results:
Six hundred and ninety (690) patient charts were screened and 235 patients were excluded, most commonly due to seizure on admission. Overall, 28% (N=129) of 455 included patients over 5 years received seizure prophylaxis. Phenytoin was the most common prophylactic agent. In 61% of patients who received prophylaxis, it was unknown whether the AED was continued in the long-term. The drug cost savings to the organization over 5 years if these patients had not received seizure prophylaxis was $4287.52. The patient characteristics most strongly correlated with receiving seizure prophylaxis were increasing tumour size and decreasing number of tumours.
Conclusion:
One in 3 patients received seizure prophylaxis. Increased awareness of the organization’s practice patterns may help prevent unnecessary long-term AED use in the future.
Interaction between Psychotropic Medications and Alcohol: Perception in Patients Attending a Psychiatric Day Hospital Program
C Cheng, F Mithoowani, M Lee
North York General Hospital, Toronto, ON
ABSTRACT
Background:
Interaction between alcohol and certain medications can lead to adverse consequences. Individuals with mental health disorders are particularly at risk due to the potential pharmacokinetic and pharmacodynamic interactions between psychotropic medications and alcohol. It is unknown what education these patients received from their healthcare providers and how such interactions are managed.
Objectives:
To examine whether individuals with mental health disorders are aware of the alcohol-drug interactions and how the information is used.
Methods:
A paper survey was developed to explore mental health patients’ perception of the alcohol-drug interaction. The survey included questions in three domains: (1) knowledge of the drug-alcohol interaction, (2) consumption of alcohol while taking psychotropic medications, and (3) source of advice regarding the drug-alcohol interaction. Attendants of the Psychiatric Day Hospital Program at North York General Hospital, Toronto, Ontario were invited to participate. Informed consent was obtained from all participants.
Results:
One hundred surveys were completed between July 2014 and February 2015. Thirty-six responders reported consuming alcohol at least once a week. Seventy-five participants had been counselled by a healthcare provider regarding the alcohol-drug interaction, with 49 of them indicating that they followed the advice provided. Half of the responders denied using alcohol while taking psychotropic medications, while 20 reported worsening of their psychiatric conditions and/or requiring hospitalization when alcohol and psychotropic medications were used concurrently. Forty-two participants recalled receiving advice from their pharmacists regarding the interaction. The majority of the responders considered physicians to be the best healthcare professional to provide such information.
Conclusions:
Although the majority of participants were provided strategies to avoid negative consequences from the alcohol-drug interaction, one-fifth reported an adverse outcome. This indicates that patients do not necessarily follow the advice from their healthcare providers. Future studies should explore why this gap exists and how to minimize it.
Development and Evaluation of a Screening Tool for Bacteremia in Neonates
M Cormier1, SAN Walker1,2,3,4, M Elligsen1, J Choudhury1, SE Walker1,2, A Rolnitsky5,6, C Findlater6, D Iaboni6
1Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
3Division of Infectious Diseases, Sunnybrook Health Sciences Centre, Toronto, ON
4Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, ON
5Faculty of Medicine, The Hebrew University of Jerusalem, Jerusalem, Israel
6Women and Babies Program, Sunnybrook Health Sciences Centre, Toronto, ON
ABSTRACT
Background:
Clinical and laboratory parameters can aid in the detection of bacteremia before clinical deterioration occurs, however current prediction models have poor diagnostic capabilities.
Objectives:
To develop a screening tool for bacteremia in neonates using common laboratory and clinical parameters and determine its predictive value to assist in the early identification of late onset bacteremia (LOB) (>72h following birth).
Methods:
A retrospective chart review of neonates admitted to a level II–III neonatal intensive care unit (NICU) between March 1, 2012 and January 14, 2015 and a prospective evaluation of neonates admitted to the same NICU between January 15, 2015 and March 30, 2015 were completed. LOB patients were matched to non-infected controls on several demographic parameters. A Pearson’s Correlation matrix was completed to identify independent variables that were significantly associated with infection (p<0.05, univariate analysis). Significant parameters were analyzed using iterative binary logistic regression to identify the simplest statistically significant model (p<0.05). The optimal probability cut-off for LOB was determined using a Receiver Operating Characteristic curve and the predictive value of the model was assessed.
Results:
Thirty-one of 61 patients with a complete data set had LOB (51%). The best binary logistic regression model was (p<0.05): Ln[Odds LOB] = − 25.459 + 0.752(MaxGlucose) + 0.119(MaxHeart Rate (bpm)) + 0.108(Bands) + 0.071(MaxNeutrophils)] with a threshold probability categorizing LOB of >41.5%. The sensitivity, specificity and accuracy were 90%, 80% and 85%, respectively, with a false positive rate of 20% and a false negative rate of 9.7%. At the study LOB prevalence of 51%, the positive predictive value, negative predictive value and negative post-test probability were 82%, 89% and 11%, respectively.
Conclusion:
The model developed exhibited superior overall predictive value compared to currently published neonatal bacteremia screening tools. Validation of the tool in a historic data set will be completed.
Evaluation of Oseltamivir Dosing Regimens in Renal Dysfunction: 45 is the New 30
JO Lee, SAN Walker, SE Walker
Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON
Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
In 2014 the Tamiflu® product monograph (PM) was revised to markedly decrease oseltamivir doses for patients with creatinine clearance less than 60 and 30 mL/min.
Objective:
To evaluate the performance of the old and new recommended product monograph dosing regimens based on review of previously published literature and development of a relationship between creatinine clearance and the half-life of oseltamivir carboxylate, the active metabolite of oseltamivir.
Methods:
Articles that reported creatinine clearance (CrCl) and pharmacokinetics parameters (volume, clearance, half-life or elimination rate constant (K) and Tmax), or permitted calculation of the pharmacokinetic parameters for oseltamivir carboxylate (OC) were reviewed. The mean and standard deviation of each parameter was collected and a relationship between creatinine clearance and K developed. A multiple oral dosing simulation was created to obtain a steady-state concentration-time profile for dose regimens at varying creatinine clearance values.
Results:
9 papers describing OC kinetics in 513 adult patients produced the relationship K= 0.007 + 0.001CrCl allowing simulation of concentration–time profiles with an average volume of 186 L for both treatment and prevention. The older PM recommended dosing produced concentrations which doubled the peak concentration for patients with CrCl between 30 and 60 and 10 and 30mL/min relative to patients with 100mL/min. The 2014 PM recommended dosing produced peak concentrations which were about 75% of the peak concentration for patients with CrCl of 100 mL/min. Based on the simulation, to achieve concentrations similar to patients with 100 mL/min, patients receiving treatment with a CrCl between 30–60mL/min should receive 45mg BID and patients with CrCl of 10–30mL/min should receive 45mg once daily. For prevention, patients should have the dosing interval doubled.
Conclusion:
The 2014 PM recommended dosing seems to under-dose patients with reduced renal function. Concentrations can be corrected by administration of 45 mg rather than 30 mg.
Epidemiology of Six Types of Bacteria, Hospital Resistance Rates and Associated Antibiotic Drug Consumption Rates in a Teaching Hospital
C Cotteret1, H Roy1, D Lebel1, J Turcot2, P Ovetchkine3, JF Bussières1,4
1Pharmacy Practice Research Unit, Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Central Laboratory, CHU Sainte-Justine, Montréal, QC
3Pediatric Department, CHU Sainte-Justine, Montréal, QC
4Faculty of Pharmacy, Université de Montréal, Montréal, QC
ABSTRACT
Background:
Bacterial drug resistance is associated with antimicrobial misuse. Such resistances can increase length of stay, drug costs, and other co-morbidities. Antimicrobial stewardship program should monitor drug resistances and consumption.
Objectives:
To describe the epidemiology of hospital resistance rates with regards in antibiotic drug consumption in a teaching hospital.
Methods:
This cross-sectional descriptive study was conducted in a 500-bed teaching hospital. Pediatric microbiology data (specimens, type of samples, resistance) from April 1st, 2010 to March 31st, 2015 were extracted from the central laboratory database. Antimicrobial drug consumption data were extracted from GesphaRx® software and reported according to defined daily dose (DDD)/1000 patient-days (PD) and days of therapy (DOT)/1000 PD. Six bacteria were targeted: Enteroccocus faecium, Staphylococcus aureus, Pseudomonas aeruginosa, Acinotobacter baumannii, Klebsiella pneumoniae and Escherichia coli. We conducted further data analysis to describe potential associations between resistance and drugs consumption.
Results:
A total of 189 types of specimens collected from 10,259 patients were available during the five-year period. The 6 targeted bacteria represented 0.4%, 2.6%, 2.9%, 0.1%, 5.2% and 45.8% of all isolates, respectively. The aggregated resistance rate was 41.5%, 17.45%, 21.73%, data unavailable, 18.04% and 5.55%, respectively. For the five-year period, the antibiotic DDD/1000 PD was 554, 601, 569, 535 and 556, respectively. The antibiotic DOT/1000 PD was 928, 987, 918, 868, and 866. Further data analysis revealed a decrease of susceptibility of E.coli to fluoroquinolones in the intensive care unit associated with an increase of fluoroquinolones consumption. A similar trend was noticed for susceptibility of E.coli to piperacillin-tazobactam combination and K. pneumoniae with third and fourth generation cephalosporins.
Conclusions:
The drug resistance to six key bacteria remained stable from 2010 to 2015 as well as DOT/1000 PD in a teaching hospital. Surveillance of these markers suggests that optimal drug use can contribute to limit the increase of bacterial resistance.
Implementation of a “Virtual” Vancomycin Clinic
K Gupta, S Elsayed, GW Thompson, SM Hosseini-Moghaddam, M Silverman, AM Bombassaro
London Health Sciences Centre, London, ON
ABSTRACT
Background:
Bed management pressures frequently necessitate the transfer of stable patients to the outpatient setting. This poses challenges for intravenous vancomycin monitoring in patients receiving prolonged treatment courses.
Description:
The Infectious Diseases (ID) service implemented a “virtual” vancomycin clinic as a quality improvement initiative to optimize outpatient monitoring. The goal is to ensure that blood work is scheduled and collected appropriately relative to dose administration and promptly interpreted.
Action:
The clinic is “virtual” because it operates from the hospital in real time while the patient remains in the community. Prior to discharge, ID patients requiring intravenous vancomycin are assessed regarding their ability to attend a medical laboratory and counselled about the importance and timing of serum concentrations. A plan is developed regarding the location and day of monitoring. Approximately 24 hours after discharge the dose and blood work timing is verbally confirmed with the patient. Lab results are reviewed by ID and dose changes conveyed to the community care provider. Results and subsequent monitoring are discussed with the patient.
Evaluation:
Fifteen patients (17 episodes) have been followed over 8 months. One patient was noncompliant and a second was switched to an alternative antibiotic resulting in 13 monitored patients (15 episodes). Individual episodes were monitored for a range of 6 to 59 days. All serum concentrations were interpretable and resulted in 4 dose changes. One patient experienced a toxic trough concentration and nephrotoxicity precipitated by newly initiated medications. Results were available within 24 hours of collection in all 12 episodes in which blood work was performed at a hospital laboratory.
Implications:
The “virtual” vancomycin clinic provides the opportunity for patients to be actively involved in the management of their therapy. ID consultants receive timely and interpretable serum concentrations leading to a subjective assessment of improved quality of outpatient intravenous vancomycin care.
Guideline Adherence Audit for Treatment of Pediatric Outpatient Urinary Tract Infection
M MacInnis1,3, S MacPhee1,2, E Fitzpatrick1,2, K Hurley1,2, E Burns1,2, R Lee1,3
1IWK Health Centre, Halifax, NS
2Department of Emergency Medicine, Dalhousie University, Halifax, NS
3College of Pharmacy, Dalhousie University, Halifax, NS
ABSTRACT
Background:
Our institution’s Pediatric Emergency Department (ED) estimates approximately 2000 patients per year present with a urinary tract infection (UTI). ED physicians perceive that UTI treatment shows the greatest variation in antimicrobial prescribing. Several clinical and safety organizations recognize that antimicrobial stewardship is an important intervention in optimizing patient safety, reducing microbial resistance, and improving patient outcomes.
Objective:
To obtain data regarding empiric antimicrobial selection for pediatric patients presenting with signs and symptoms of a UTI.
Methods:
We conducted a randomized retrospective chart review of 100 patients aged 2 months–16 years presenting to the ED from 1st April 2013 to 31st March 2014 from a convenience sample. Demographic data, choice of empiric antimicrobials and culture and sensitivity results were abstracted from patients’ charts; obtained using a collection tool developed by the clinical investigators based on current Canadian guidelines for antimicrobial selection.
Results:
There were 85 females and 20 males in the sample. Forty three percent (46) of patients were febrile in the ED. Patients presented with a previous history of urinary tract infections 5.7% (6) of the time, and antimicrobial use within the previous 3 months was documented in 2.9% (3) of cases. Urinalysis showed 78.1% (82) had white blood cells, 75.2% (79) had microscopic hematuria, 53.3% (56) had bacteriuria and 38.1% (40) had nitrites. Of 105 discharge prescriptions written, 47.6% (50) aligned with the 6 regimens that were predetermined by the clinical investigators to be in accordance with guideline adherence. Urine culture results obtained after patient discharge revealed 83% (71/86) of positive bacterial cultures were E. coli.
Conclusions:
The results of this audit confirm a broad variation in empiric prescribing selection by our ED physicians. This examination of practice is an important first step in providing feedback to the prescribers with the aim of improving empiric antimicrobial selection for treatment of UTIs.
Developing and Evaluating an Educational Intervention to Guide in the Implementation of Antimicrobial Stewardship Programs in Community Hospitals
L Dresser1,2, M Steinberg1, K Duplisea3, S Nelson1, M So1,2, C Bell1,4, A Morris1,4
1University Health Network/Mount Sinai Hospital Antimicrobial Stewardship Program, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
3Toronto East General Hospital, Toronto, ON
4Department of Medicine, University of Toronto, Toronto, ON
ABSTRACT
Background:
Antimicrobial stewardship is a multi-disciplinary programmatic initiative to optimize antimicrobial therapy. Antimicrobial Stewardship Programs (ASP) are an Accreditation Canada (AcC) Required Organizational Practice (ROP). There was a perceived need in community hospitals for educational support.
Description:
We developed and evaluated an educational intervention designed to provide pharmacists in community hospitals with the knowledge and skills required to implement an ASP.
Action:
This was a multi-phased project involving 13 self-identified sites. A needs survey identified learning priorities. Eleven educational modules were developed and delivered over 6 months. Each module included a narrated PowerPoint lecture, recommended readings, case-based scenarios and a live webinar discussion. All sites agreed to submit baseline and post-intervention data which included antibiotic consumption; cost, defined daily doses (DDD) or days of therapy (DOT), with length of stay (LOS) and mortality as balancing measures.
Evaluation:
A post-intervention satisfaction survey was distributed (response rate 59% ([n=17/29]). A large majority of respondents found the presentations useful in improving their infectious diseases knowledge (94%), in utilizing ASP principles to optimize antimicrobial prescribing (90%) and in helping their site meet AcC’s ASP ROP (94%). Data requested for 1-year pre- and 1-year post-intervention served to gauge the impact of the ASP activities and ensured the site’s ability to demonstrate metrics of an ASP. All sites encountered significant challenges in obtaining this data. We present 12 months of data from 7 sites (Table 1).
Table 1
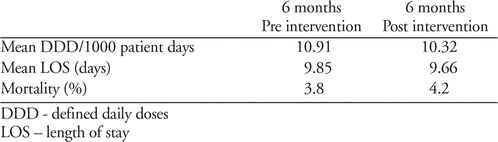
Implications:
Community hospital pharmacists without formal ID training tasked with developing and implementing an ASP can benefit from an educational intervention. Our program can serve as a foundation for future ASP education programs.
Clinicians’ Perspectives of the Enablers of and Barriers to Antibiotic De-Escalation
M Gauthier, LR Taggart, R Haj
St. Michael’s Hospital, Toronto, ON
ABSTRACT
Background:
Antibiotic de-escalation decreases unwarranted antimicrobial exposure and could reduce the emergence of resistant pathogens. As bacterial resistance rates continue to rise, interventions aiming to maximize de-escalation of antibacterial therapy are encouraged.
Objectives:
We aimed to describe the enablers of and barriers to antibiotic de-escalation and to measure the frequency of de-escalation following initiation of broad-spectrum antibiotics in a tertiary-care hospital on units without involvement of the antimicrobial stewardship program.
Methods:
Semi-structured interviews were conducted with medical residents, nurse practitioners, pharmacists and staff physicians to identify enablers of and barriers to de-escalation. Two clinical pharmacists independently conducted thematic analysis and agreement on themes was reached by consensus. Concurrently, a two-month prospective chart audit of new orders of piperacillin-tazobactam or any carbapenem for non-critically ill hospitalized patients determined the frequency of de-escalation at 72 hours of therapy. To triangulate the themes identified from the interviews, the clinical pharmacist was contacted if broad-spectrum antimicrobials were continued past 72 hours and barriers to de-escalation were discussed.
Results:
Seventeen health professionals agreed to participate in the interviews. The enablers and barriers identified from the interviews were consistent with those from the prospective chart audit. Enablers of de-escalation included pharmacist recommendation to de-escalate therapy and prescriber willingness to discuss de-escalation. Barriers to de-escalation included higher patient acuity, immunosuppression, the fact that de-escalation is not prioritized, the absence of alerts for updated culture results and the lack of readily-accessible resources promoting de-escalation strategies. Based on a chart review of 66 orders of piperacillintazobactam or any carbapenem, the frequency of de-escalation at 72 hours of therapy was 50%.
Conclusion:
Clinicians identified enablers and barriers, which can guide the design of interventions to optimize the frequency of antibiotic de-escalation. The frequency of de-escalation 72 hours following the initiation of piperacillin-tazobactam or any carbapenem was found to be 50%.
Impact of Hospital Wide Implementation of an Antimicrobial Stewardship Program on the Incidence of Hospital Acquired Clostridium difficile Infection
X Wang1, SAN Walker1,2,3,4, JA Leis4,5
1Department of Pharmacy, Sunnybrook Heath Sciences Centre, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
3Division of Infectious Diseases, Sunnybrook Health Sciences Centre, Toronto, ON
4Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, ON
5Faculty of Medicine, University of Toronto, Toronto, ON
ABSTRACT
Background:
Antimicrobial stewardship programs (ASP) have been implemented worldwide to decrease inappropriate antibiotic use, risk of resistant organisms and C. difficile infection (CDI). Although there has been some attempt to evaluate the impact of ASPs on CDI, the evidence is limited to before and after study designs, while methodologically superior studies are lacking.
Objective:
The objective of this study was to examine the impact of hospital wide implementation of ASP on the incidence of hospital acquired-CDI (HA-CDI).
Methods:
An interrupted time series study was conducted at Sunnybrook Health Sciences Centre (SHCS) using prospectively collected monthly HA-CDI and community acquired-CDI (CA-CDI) incidence based on Society of Healthcare Epidemiology of America case definitions and institutional antimicrobial utilization from the SHSC-ASP electronic database. The study time frame was divided into 63 monthly intervals from September 2008 to November 2013. A segmented autoregressive integrated moving average model was used to compare the pre-ASP and post-ASP periods in the time series using the pre-ASP period as the reference. CA-CDI was examined using the same method, to reduce bias attributable to secular trends.
Results:
Compared to the pre-ASP period, a cumulative reduction in HA-CDI incidence of 41 per 1000 patients (relative risk reduction 27%; 95% CI 10 (7%) −71(47%); p=0.006) was observed at the end of the 37 month post-ASP period (study month 63). The reduction in cumulative HA-CDI incidence did not plateau and was sustained throughout the post-ASP period. Conversely, CA-CDI remained unchanged from the pre- to post-ASP period (p =0.43).
Conclusion:
Our study demonstrated a sustained decrease in cumulative HA-CDI incidence following ASP implementation. While other factors related to Infection Prevention and Control may have contributed to this effect, these findings provide high level evidence to support the beneficial effect of ASPs on institutional incidence of CDI.
A Retrospective Review of Treatment Appropriateness and Outcomes of Enterobacteriaceae Bacteremias at a Large Academic Medical Centre: Opportunities and Implications for Antimicrobial Stewardship
J Yung1, A Campigotto2, L Taggart3, E Leung3
1University of Toronto, Toronto, ON
2Microbiology, St. Michael’s Hospital, Toronto, ON
3Infectious Diseases/Antimicrobial Stewardship, St. Michael’s Hospital, Toronto, ON
ABSTRACT
Background:
Infections caused by AmpC carrying enterobacteriaceae can be problematic. Organismal ß-lactamase production can be inducible upon exposure to ß-lactams and lead to clinical treatment failure. Choice of antimicrobial therapy is important, as delay in appropriate empiric therapy is associated with mortality, while avoiding unneeded broad-spectrum antimicrobials is an important component of antimicrobial stewardship. There is little data to support the current practice of using carbapenems as first-line agents to treat AmpC enterobacteriaceae, and a recent study showed cefepime to be as effective as carbapenems.
Objective:
We aimed to study treatment appropriateness and outcomes for serious enterobacteriaceae infections, and explore carbapenem-sparing options.
Methods:
Microbiology reports identified patients with bacteremia caused by AmpC carrying enterobacteriaceae during a 1 year period at a large academic medical centre. Appropriateness of therapy was assessed by 2 independent reviewers, for both empiric and definitive regimens. Clinical outcomes included all-cause mortality, hospital length of stay, and microbiological cure.
Results:
Only 13% of enterobacteriacae bacteremias presented with derepression of the AmpC gene upon first culture. Initial empiric therapy was inappropriate (agent not active based on organism sensitivity) in 16% of patients. Most patients (58%) were started on empiric piperacillin-tazobactam (P/T), while few (6%) received an empiric carbapenem. Definitive therapy was often a fluoroquinolone (FQNs) (29%), a carbapenem (25%) or P/T (25%). The remainder of patients received either sulfamethoxazole-trimethoprim (SMX-TMP) or ceftriaxone. Only one patient had persistent bacteremia; other clinical outcomes in patients deemed to have an “appropriate agent” for definitive therapy were not significantly different among agents.
Conclusion:
Most patients received appropriate empiric antimicrobial therapy. Although some patients received definitive therapy with a carbapenem, many received therapy with a non-carbapenem agent with similar outcomes. We suggest that non-ß-lactams, especially SMX-TMP, should be explored as carbapenem-sparing treatment options for AmpC carrying enterobacteriaceae.
Medication Safety Culture Indicator Matrix: Going Beyond the Numbers and Using Incident Reports to Assess Medication Safety Culture
C Poon, A Kawano, L Yoo, C Ho
Institute for Safe Medication Practices Canada, Toronto, ON
ABSTRACT
Background:
A positive patient safety culture is an integral component of a safer healthcare system. Conventional cultural measurement tools, such as surveys and questionnaires, are largely limited by their inability to capture the complex human interaction.
Objective:
The purpose of this initiative was to create an analytic tool leveraging medication incidents as a data source to assess safety culture.
Methods:
Two independent analysts conducted a qualitative analysis examining 200 medication incidents from two incident reporting databases. Themes that were suggestive of a positive safety culture were identified and subsequently led to the development of an analytic tool. The tool was consolidated and validated by obtaining input from an inter-professional patient safety expert panel.
Results:
The analytic tool is a 3×4 matrix called the Medication Safety Culture Indicator Matrix (MedSCIM). MedSCIM uses qualitative analysis methods to assess a medication incident on two dimensions: (1) completeness of documentation, and (2) maturity of the incident report with regards to the perspective on patient safety. A low grade on the maturity score is termed “blame-and-shame,” where the clinician at fault was victimized and held responsible for the error. The highest maturity score is “generative,” where the incident reporter adopts a systems-based approach for identification of vulnerable safety gaps in the healthcare system and implements an action plan.
Conclusion:
MedSCIM is an innovative tool that uses medication incidents to evaluate medication safety culture in healthcare settings. An ISMP Canada educational workshop with interactive components that enabled application of MedSCIM was developed. In order to advance patient safety, more resources must be available to better understand and measure patient safety culture. MedSCIM offers a novel approach to understand safety culture through the lens of medication incident reporting and analysis.
Does Peer-to-Peer Mentoring Make a Difference in Therapeutic Knowledge Acquisition for Student Learning?
TB Huang, C Ho, E Paw Cho Sing, M Vinh, A Lee
Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
Aiming to better prepare students for Advanced Pharmacy Practice Experience rotations and the licensing examination, we implemented a peer-to-peer mentoring model to analyze the learning impact and the utility of student-led pharmacotherapy sessions (SLPS).
Description:
The SLPS concept strengthens the confidence and preparedness of students for transition from classroom to practice. This peer-to-peer mentoring model was implemented as a result of student identified need to reinforce pharmacotherapy concepts.
Action:
Fourth-year pharmacy students delivered SLPS on Cardiology, Infectious Diseases, and Patient Self-Care online and live to both third- and fourth-year students. Retrospective self-assessment surveys were administered after each SLPS. Five knowledge domains (etiology, clinical presentation, pharmacotherapy, monitoring/follow-up, and overall knowledge) were assessed. A paired t-test was utilized to evaluate the survey data. Thematic analysis was applied to the qualitative comments on the survey.
Evaluation:
Eighty-one students (59% third-years; 41% fourth-years) responded to the survey. Prior to the SLPS, 46.8% of students rated their knowledge ‘Average’ (3 of 5), while 33.1% rated their knowledge ‘Above Average’ (4 of 5) in all knowledge domains. After the SLPS, 53.6% of students rated their knowledge to be ‘Above Average’ (4 of 5), while 27.5% rated their knowledge ‘Excellent’ (5 of 5) in all knowledge domains. Statistically significant increase (p≤0.01) was shown in all five knowledge domains post-SLPS for both third- and fourth-year students. Students found cases, drug charts and therapeutic overview most useful. Suggestions for improvement included increased interactions, counselling points, and experiential examples.
Implications:
The peer-to-peer mentoring model was well received by students. Students perceived an increased level of therapeutic knowledge after the SLPS. Future SLPS should include more interactive activities, case development support, and student association involvement. Wide adoption of this peer-mentoring model in pharmacy education is contingent on research to elucidate its utility and design.
Pharmacy Discharge Services: A Pilot Project Involving Pharmacist and Pharmacy Technicians at a Pediatric Hospital
R Vaillancourt, J McDonald, A Homsma, A Pouliot
Children’s Hospital of Eastern Ontario, Ottawa, ON
ABSTRACT
Background:
It has been estimated that 30% of all hospital discharges are delayed for non-medical reasons including poor organization of post-discharge health, poor knowledge of social circumstances, and poor communication between the hospital and community pharmacy. Studies have shown that patients are at greater risk for medication errors during transitions of care. A pharmacy discharge planning service has the potential to decrease length of stay, readmission rates due to medication errors, and inappropriate prescribing.
Description:
The pharmacy department, at our hospital, implemented a pilot project to offer pharmacy discharge services on two units from July 13, 2015 to September 11, 2015. The objectives were to: 1) improve patient flow by reducing medication-related discharge delays, and 2) improve patient safety through education and medication reconciliation at discharge. Patients eligible for the service were “at risk” for: 1) low medication literacy; 2) discharge delays; 3) financial barriers, and 4) non-adherence.
Action:
The medication reconciliation technician’s role was to identify patients eligible for the service. Interventions by the discharge pharmacist included education, illustrated medication calendars, suggesting medication changes to physicians, completing application forms for drug coverage, preparing discharge prescriptions, communicating with the community pharmacy, requesting social work consultation, and providing compassionate drugs.
Evaluation:
A total of 308 patients were admitted during the pilot phase. Fifty patients were referred to the pharmacy discharge service, the most common reason for referring patients was “complex medication regimens” (36.7%). A total of 73 interventions were made, with the most common being the preparation of discharge prescriptions with communication with the community pharmacy to ensure drug availability (n=32).
Implications:
Pharmacy technicians and pharmacists play an important role in discharge planning with nearly 1 pharmacist intervention for every 4 patients admitted. Future evaluation will focus on readmission rates, patient and physician satisfaction, drug adherence and patient health literacy.
Pharmaceutical Care for Children: A Survey of Pharmacy Students’ Preparedness and Confidence
J Otal1, M Duffett2
1The Hospital for Sick Children, Toronto, ON
2McMaster Children’s Hospital and McMaster University, Hamilton, ON
ABSTRACT
Background:
Approximately half of the children in Canada receive at least one prescription medication each year. Pharmacy graduates must be prepared to confidently provide care to these children.
Objective:
To assess the perceived preparedness and confidence of pharmacy students to provide safe and effective pharmaceutical care to children.
Methods:
We conducted a self-administered online survey of students in their final 2 years of pharmacy schools in Canada. The questionnaire focused on: 1) perceived preparedness to provide pharmaceutical care to children; 2) confidence in performing pharmacist duties related to pediatric prescriptions; 3) pediatric exposure in formal education; 4) demographics. We used linear regression to examine the relationship between demographics and self-reported preparedness
Results:
We received 379 useable responses from 8 of the 10 pharmacy schools in Canada. 10.0% of students had completed an elective course in pediatrics and 11.7% had completed a clinical placement in pediatric care. Overall, 32% of respondents reported being prepared (rating of 5 or higher on a scale from 1 = not prepared to 7 = very prepared) to provide safe and effective pharmaceutical care to children. Students’ preparedness for all pediatric topics was significantly less than the same topic in adults. 61.5% felt confident in assessing the dose for a child’s prescription, and 57.1% felt confident in searching for, and interpreting, literature to guide safe and effective pediatric therapy. Using linear regression, the factors associated with increased self-reported preparedness were the completion of an elective pediatric course, increased experience with pediatric patients on clinical placements, and university attended.
Conclusion(s):
A majority of pharmacy students do not feel prepared to provide pharmaceutical care to children upon graduation. Those with more pediatric exposure during their education felt more prepared. How to ensure graduates are prepared to meet practice standards in providing care to children remains an important challenge.
Novel Models of Precepting: The Alberta Experience
M MacDonald1,2, A Thompson2, J Ton2, T Mysak3
1Pharmacy Services, Alberta Health Services, Calgary, AB
2Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, AB
3Pharmacy Services, Alberta Health Services, Edmonton, AB
ABSTRACT
Background:
To advance the practice of precepting, and meet expanding capacity needs in institutional settings, there is interest in novel models of precepting. These models expand from the traditional model of one learner precepted by one preceptor.
Objectives:
The study’s primary objective was to quantify use of peer-assisted learning (PAL), near-peer teaching (NPT) and co-precepting (CoP) novel models. Advantages, challenges and strategies to address them, and supports needed for implementation were also explored.
Methods:
All institutional pharmacists in Alberta were invited via a global email list to complete an anonymous online survey (July 29 - August 21, 2015) using Qualtrics Survey Software. Responses were summarized using descriptive statistics and content analysis of open ended responses.
Results:
The survey was completed by 126 pharmacists (12% response rate). The majority represented staff pharmacists (79%) with acute care practices (57%) in an urban setting (72%). CoP was the highest utilized model (71%) followed by PAL (31%) and NPT (16%). Learners supporting each other and promotion of team work were the most agreed upon PAL and NPT advantages. Sharing precepting responsibility and providing different perspectives for assessing student performance were the top CoP advantages. Common challenges selected for PAL and NPT were space/technology/computer access limitations, and differences in both learner’s levels and competency. CoP challenges were ensuring communication continuity between preceptors and the differences in preceptors’ expectations and subsequent student assessment. The top 4 modalities for supporting a preceptor to try a new model were online educational sessions (58%), website resources (50%), 1:1 mentorship support (49%), and live educational sessions (47%).
Conclusion:
Three novel models of precepting are being used to varying degrees in institutional practice in Alberta. The survey results provide insight for promotion, adoption, and implementation of models to advance the practice of precepting and increase placement capacity.
Structure and Function of Teams in the Pediatric Intensive Care Unit: A Social Network Analysis
J Wong1,2, M Duffett3
1Department of Pharmacy, Hamilton Health Sciences, Hamilton, ON
2Department of Pharmacy, The Hospital for Sick Children, Toronto, ON
3Department of Pediatrics and Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, ON
ABSTRACT
Background:
Healthcare team dynamics help to shape the delivery and quality of care. In focusing on the relationships among team members, social network analysis is an approach to better understanding team structure and function.
Objective:
To describe team structure and function within a pediatric intensive care unit (PICU).
Methods:
In this self-administered online survey, we asked all PICU staff to select up to 5 of their most influential colleagues in 3 contexts: information seeking (colleagues that advise on patient care challenges), social influence (colleagues that influence their clinical practice), and social support (colleagues that help with work-related personal problems). We used anonymized data and Gephi to generate network diagrams for each context. Regression was used to analyze relationships between participant’s characteristics and influence in the unit.
Results:
98 (86.7%) of 113 staff participated. Amongst the 3 networks, there were no weakly connected groups. Few individuals reported no links to any colleague: none for information seeking, 2 (2.2%) for social influence, and 7 (7.8%) for social support. The number of links among colleagues was greatest for the information seeking network (density=0.048), followed by social influence (density=0.041) and social support (density = 0.031). 5 individuals (2 intensivists, 2 RNs and 1 RT), 3 of whom had formal leadership roles, were amongst the 10 most influential team members in all 3 networks. Using regression, a formal leadership role was associated with higher influence in all 3 networks. The role of profession, part-time status, and years of experience varied in influence amongst the networks.
Conclusions:
In this healthcare team, there was a core group of individuals who were among the most relied upon in different contexts and few individuals who were weakly connected. These relationship patterns can be used to inform the implementation of practice changes and for focusing interventions to enhance team functioning.
A Failure Mode Effect Analysis Pre-Post Implementation of an Electronic Medication Administration Record
A Rousseau1, D Lebel1, G Mercier2, JF Bussières1,3
1Pharmacy Practice Research Unit, Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Electronic Health Record Team, CHU Sainte-Justine, Montréal, QC
3Faculty of Pharmacy, Université de Montréal, Montréal, QC
ABSTRACT
Background:
Failure mode effect analysis can be use in the drug use process to assess risks. The documentation of drug doses on printed medication administration record (MAR) and electronic MAR (eMAR) is associated with medication errors.
Objectives:
To conduct a failure mode effect analysis pre-post implementation of an eMAR.
Methods:
This is a prospective descriptive study conducted in a 500-bed mother-child teaching hospital. An Ishikawa diagram of failure modes associated with the documentation of drug doses was developed by the research team composed of 4 members. It contained 26 failures modes grouped in six categories. Occurence rating, detection rating and severity rating were adapted from published data. An expert panel composed of a group of 3 pharmacists and 4 nurses exposed to both MAR and eMAR met once during 90 minutes to assess the first 2 ratings. Severity was scored a posteriori by the research team. Pre-post individual and average criticality indexes (CI) per group and convergence (e.g. when risk reduction ratio of pre implementation CI/post implementation CI per group were in the same direction) were calculated.
Results:
Average CI were reduced from 3084 pre-implementation to 1905 post-implementation (1.6 times reduction). Average CI for pharmacists were higher pre-implementation than nurses (4572 vs. 2180).They were similar post-implementation (1910 vs. 1913). Risk reduction ratio were convergent between pharmacists and nurses for 62% (16/26) of the failure modes. The most important perceived risk reduction for nurses were related to the following modes: missing double-check documentation (ratio of 9.1), missing traceability documentation (5.8), missing double-check action (3.4), wrong patient identity (3.3), missing drug administration information (2.1) and missing drug dose administered (2.0).
Conclusions:
eMAR was theoretically associated with a 1.6 time risk reduction by a panel of seven experts. Risks associated with MAR was perceived higher by pharmacists than nurses.
Étude intérimaire de satisfaction du personnel soignant dans l’implantation d’un formulaire électronique d’administration des doses de médicaments dans le cadre d’un projet pilote en chirurgie pédiatrique
G Mercier1, T Dulermez2, A Rousseau2, D Lebel2, JF Bussières2,3
1Direction du dossier Clinique Informatisé, CHU Sainte-Justine, Montréal, QC
2Unité de recherche en pratique pharmaceutique, Département de pharmacie, CHU Sainte-Justine, Montréal, QC
3Faculté de pharmacie, Université de Montréal, Montréal, QC
ABSTRACT
Contexte :
L’introduction de nouvelles technologies en santé représente un défi important.
Objectifs :
Évaluer la satisfaction du personnel infirmier dans l’implantation d’un formulaire électronique d’administration des doses de médicaments (FADME) dans le cadre d’un projet pilote en chirurgie pédiatrique.
Méthodologie :
Il s’agit d’une étude descriptive transversale menée dans un établissement universitaire de 500 lits. Nous avons établi un questionnaire pré-implantation et un questionnaire post-implantation comportant rsepectivement 10 et 17 questions, dont 9 communes aux 2 phases. Une échelle Likert à six choix a été utilisée. Les questionnaires ont été remis au personnel infirmier sur les trois quarts de travail en pré-implantation (10 au 25 novembre 2014) et post-implantation (18 mai au 12 aout 2015).
Résultats :
Nous avons obtenu un taux de participation de 96% en pré (79/82) et de 96% en post (52/54). Des 9 questions communes, on a noté une amélioration associée au FADME pour trois questions, soit pour l’obligation de retranscrire trop fréquemment une entrée manuelle (72% c. 48%), la présence de risques à la retranscription (73% c. 48%), la charge de travail associée à la révision à chaque quart de travail (54% c. 27%). On a noté une détérioration pour la perception de la sécurité (79% c. 50%), la facilité d’effectuer des ajouts manuels (84% c. 57%) et la satisfaction globale (70% c. 55%). On n’a pas noté de différence pour la documentation (96% c 95%) et le processus de double vérification (77% c. 80%). Les risques et l’insatisfaction notées ne sont pas étonnants en phase d’implantation lors d’une évaluation intérimaire. Seulement 21% accepteraient de revenir à une documentation papier. Au total, 15 avantages et 11 inconvénients ont été recensés.
Conclusion :
L’implantation d’un FADME est un changement important qui comporte des avantages et des inconvénients à prendre en considération afin de l’optimiser.
Describing the Benefits of Residency Training through Key Performance Indicators
T Mysak, S Neilson
Pharmacy Services, Alberta Health Services, Edmonton, AB
ABSTRACT
Background:
If pharmacists apply for authorization, full scope of practice in Alberta may include Additional Prescribing Authority (APA) or administration of medications by injection. Pharmacists who complete an Accredited Canadian Pharmacy Residency (ACPR) program receive training that requires demonstration of competency in provision of patient care and education of other health care professionals. Within our organization, pharmacists are encouraged to work to their full scope, precept pharmacists in training, and contribute to teaching and scholarly activities. We wished to measure the relationship between completing a residency and the skills and participation of the pharmacy workforce to better articulate the benefits of hiring pharmacy residents.
Description:
Pharmacy Services maintains a centralized database of employed pharmacists and monitors progress to strategic targets for the scope and ability with which they practice. The goal of this project was to use Key Performance Indicator (KPI) data to characterize the relationship between completing a residency and obtaining expanded pharmacist scope designations or participating in teaching, scholarly and precepting activities.
Action:
The completion of an ACPR was determined, entered into the database and results for several KPI were compared between ACPR and non-ACPR pharmacists.
Evaluation:
The percentage of pharmacists who completed an ACPR was approximately equivalent (18.4% vs. 18.6%) between frontline pharmacists and leadership. More clinically deployed pharmacists with the ACPR designation obtained additional prescribing authorization than those without ACPR (39.5% vs. 21.8%). ACPR pharmacists were more likely to precept a pharmacy resident or senior undergraduate pharmacy students than junior pharmacy students. A higher proportion of frontline ACPR pharmacists teach at the University of Alberta compared to non-ACPR pharmacists (16.7% vs. 4.0%).
Implications:
Pharmacists who completed an ACPR had higher participation rates than their colleagues for key metrics, further supporting the impact residency trained pharmacists provide in our organization.
Engaging Leadership to Expand Experiential Capacity in Institutional Settings
T Mysak1, M MacDonald1,2, A Thompson2
1Pharmacy Services, Alberta Health Services, Edmonton, AB
2Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, AB
ABSTRACT
Background:
The proposed introduction of an entry-to-practice PharmD program will significantly increase placement requirements. In Alberta, there is a provincial approach to delivery of pharmacy services, which provided opportunity to develop and implement a coordinated approach to engaging preceptors and leadership.
Description:
A standing committee was created, consisting of experiential education faculty as well as senior leadership from Alberta Health Services (AHS) provincial pharmacy practice. This committee provides oversight and guidance related to delivery of experiential education. Three strategic committee priorities were identified: engaging pharmacists and leadership to promote a culture of precepting, determining and building capacity for placements, and developing /supporting preceptors.
Action:
Specific committee projects included harmonizing an annual Call for Offer (CFO) for placements across the province, setting zone capacity targets, facilitating setting of zone specific SMART goals to promote preceptorship, initiating preceptor communities of support utilizing bimonthly networking meetings, coordinating communication to management and pharmacists, preceptor development and regular engagement of leadership at zone pharmacy management meetings.
Evaluation:
Feedback was received from provincial leadership through a survey and focused questions at zone pharmacy management meetings. Responses were aggregated and summarized. There was general support to continue the harmonize CFO process. Top factors influencing site capacity were staffing levels, time commitment and willingness of pharmacists to precept and the ability to schedule consistent preceptors with the student. Top strategies to address increasing capacity included supporting pharmacist precepting skills, trialing novel models of precepting (e.g. peer-assisted learning), and promoting the benefits of precepting to pharmacists.
Implications:
The harmonized yearly CFO process will be maintained going forward. Setting strategic priorities and regular communication between faculty, practice leadership and zone leadership is required to achieve common goals. The standing committee will focus future efforts on supporting preceptors, in particular with regards to embracing new models of precepting to build capacity.
Standardization of Criteria Used by Pharmacists to Prioritize New Patients for Comprehensive Pharmaceutical Care
L Wong1,2, NF Dewhurst1,2, E Tom1, J Proceviat1, C Chant1,2
1St. Michael’s Hospital, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
Prioritization of new patients for comprehensive pharmaceutical care is necessary given time constraints and limited resources.
Description:
Standard criteria for patient prioritization are needed to allow pharmacists to apply a consistent approach and ensure comprehensive pharmaceutical care is provided in a timely manner.
Action:
Criteria used to prioritize new patients were identified through a survey administered to all inpatient clinical pharmacists. To corroborate survey findings, select pharmacists were interviewed alongside a patient chart audit on days with higher anticipated workload. Usual practice is to complete a brief initial review of all new patients in order to prioritize them for comprehensive pharmaceutical care. Pharmacists were interviewed to identify information sources and criteria typically used to assign priority during the initial review of all patients. Pharmacists then demonstrated their prioritization process by explaining the rationale behind the rank assigned to each new patient for the audit. Criteria identified were categorized into patient and medication factors, and were ranked according to frequency of use.
Evaluation:
Thirty-four (88%) pharmacists responded to the survey, and 13 pharmacists from 3 medical (28 patients) and 5 surgical (33 patients) units were interviewed and audited. Information sources most commonly used during initial review of all patients were: 1) patient chart and current admission medication profile, 2) home medication list/best possible medication history/medication reconciliation, 3) medication issue flagged by central pharmacist, 4) patient rounds, and 5) consult/referral from healthcare team. Patient factors ranked highest across the survey, pharmacist interview, and chart audit consistently were: 1) acuity of illness, 2) high-risk transfer from another unit (e.g. ICU), and 3) age≥65 years. Medication factors ranked highest were: 1) high-risk medications, 2) therapeutic drug monitoring, and 3) antibiotics.
Implications:
Results were used to update practice policy that provides guidance on the thought process for prioritizing new patients for comprehensive pharmaceutical care.
Evaluation of Standardization of Pharmacist Involvement in Best Possible Medication Histories and Medication Reconciliation
NF Dewhurst1,2, J Proceviat1, P Gillespie2, E Tom1, C Chant1,2
1St. Michael’s Hospital, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
Evidence supports the involvement of pharmacists in the medication reconciliation (MR) process to reduce the frequency of medication errors. To ensure a consistent approach and balance with other pharmacist duties, a standardized policy for best possible medication history (BPMH) and MR was developed. Patients taking “high risk” medications were deemed most likely to experience an adverse drug event and thus labeled “high priority” for BPMH and MR by pharmacists.
Description:
Following implementation of a standardized BPMH and MR policy for pharmacists, evaluation of adherence and response was required.
Action:
An audit was performed to evaluate the number of BPMHs completed by pharmacists on 2 medical and 2 surgical nursing units. Patient and medication related characteristics were collected and analyzed to quantify the proportion of patients fitting characteristics and subsequently having a BPMH form completed by a pharmacist. Quantitative data was compared to that collected prior to implementation of the prioritization criteria. Pharmacists were interviewed to collect data regarding response to the policy and its effect on their practice.
Evaluation:
The overall number of pharmacist initiated, completed and verified BMPHs has increased from 66% to 71% post-policy implementation. Furthermore, pharmacists are completing BPMHs 34% more often on high risk patients than other healthcare providers, an improvement from 15% pre-policy. According to interviews, on average, 1 hour per day is spent on BPMH and MR, with 50% of that time being spent on high-priority patients. According to feedback, 74% of pharmacists agree that the new policy helps to prioritize patients, and 81% are able to assess all high priority patients on their unit.
Implications:
The new policy has helped to prioritize patients when pharmacists are not able to see all patients for BPMH and MR.
Safe Insulin Pen Labelling Practices in a Community Teaching Hospital: A Post-implementation Evaluation
M Shi, N Broad, J Choy
North York General Hospital, Toronto, ON
ABSTRACT
Background:
The Institute for Safe Medication Practices recommended the use of insulin pens for the safe administration of subcutaneous insulin for inpatients. Concerns of unintentional sharing of insulin pens among patients have been raised in the literature. Therefore, an audit was conducted 13 months after the implementation of insulin pens to evaluate if they were appropriately labelled as a surrogate indicator for correct administration practices.
Description and Action:
Insulin pens were introduced between May and July, 2014. Education promoting “One Pen, One Patient” and proper insulin pen labelling was provided via in-service, posters, and web-based training. Nurses were educated to retrieve insulin pens from floor stock and label them with the patient’s identifier. Thirteen months after the introduction of insulin pens, a physical audit was performed on the nursing units over 5 consecutive days and repeated 30 days later to ensure that the results were consistent. All insulin pens in patient-specific medications bins were evaluated visually, and unlabeled pens were discarded. The patient’s nurse was then notified, and education regarding correct labelling practices was provided.
Evaluation:
A total of 175 pens in the inpatient setting were evaluated and 92.6% of pens were found to be labelled.
Table 1. Results from both audits

Implications:
The results of this evaluation suggest that 13 months after implementation of insulin pens, compliance with proper labelling is high even without continuous education and reinforcement. Future research is required to ascertain if proper labelling correlates with correct administration practices.
Cost and Waste Impact of Implementing Insulin Pen Devices in a Community Teaching Hospital
M Shi, A Devereaux, S Nauth, J Choy
North York General Hospital, Toronto, ON
ABSTRACT
Background:
Insulin pen devices were introduced to replace vials for the administration of subcutaneous insulin for inpatients in a community teaching hospital. The cost and waste impact of the conversion was evaluated.
Description and Action:
Conversion from 3 mL insulin vials to pens began on May 5, 2014. Pharmacy purchasing data over a 9-month period between July 1, 2014 and March 31, 2015 was analyzed and compared to the data in the same interval over the previous year to avoid seasonal variations. Insulin pens used in the inpatient setting were returned to the pharmacy for usage auditing. Usage was evaluated as volume remaining and categorized as follows: unused, slightly used (0–20%), moderately used (20–80%), and close to empty (>80% used).
Evaluation:
Acquisition costs prior to conversion were $7,836.80 for 4,260 mL of short- and rapid-acting insulins and $14,561.22 for 4,415 mL of combination, intermediate-, and long-acting insulins. After the conversion, acquisition costs were $12,633.54 for 5,655 mL of short-and rapid-acting insulins (61.2% increase in cost, 32.7% increase in volume) and $18,461.93 for 3,950 mL of combination, intermediate-, and long-acting insulins (26.8% increase in cost, 10.5% decrease in volume). A total of 3,632 pens were collected from returns and the majority were slightly used (58.5%), while 28.2% were moderately used and 12.8% were close to empty. Few pens were returned unused (0.4%). More short- and rapid-acting insulin pens were returned slightly used (66.2%) than intermediate- and long-acting insulin pens (46.8%).
Implications:
Results suggest conversion to insulin pens from 3 mL vials resulted in increased acquisition costs with a higher increase in usage of short- and rapid-acting insulin products. This may potentially be due to a decrease in opportunity of vial sharing when insulin is administered on an ad hoc basis such as with sliding scale orders or one-time orders.
Incidence des effets indésirables médicamenteux dans un centre hospitalier universitaire mère-enfant de 1989 à 2014 : la loi de Vanessa permettra-t-elle d’augmenter le taux de déclaration?
M Harry1, D Lebel1, A Comtois2, JF Bussières1,3
1Unité de recherche en pratique pharmaceutique, Département de pharmacie, CHU Sainte-Justine, Montréal, QC
2Service des archives médicales, CHU Sainte-Justine, Montréal, QC
3Faculté de pharmacie, Université de Montréal, Montréal, QC
ABSTRACT
Contexte :
Les études concernant l’incidence des effets indésirables médicamenteux (EIM) en pédiatrie sont peu nombreuses malgré le risque accru pour cette population de développer des EIM graves.
Objectifs :
L’objectif principal de notre étude est d’évaluer l’incidence des EIM codés entre 1989 et 2015. L’objectif secondaire est de déterminer les classes thérapeutiques causant les EIM en fonction de l’âge ainsi que la proportion d’EIM déclarés auprès de Santé Canada.
Méthodologie :
Il s’agit d’une étude descriptive rétrospective des EIM dans un centre hospitalier universitaire mère-enfant. Ont été inclus tous les EIM survenus chez des patients âgés de 18 ans et moins sur la période du 1/4/2010 au 18/10/2014. Les résultats issus d’une précédente étude, réalisée sur la période 1989–2010, ont été utilisés pour fins de comparaison historique. Les moyennes ont été comparées par un test t de Student.
Résultats :
Entre 2010 et 2014, on a dénombré en moyenne 1,18 ± 0,08 EIM codés par jour contre 0,62 ± 0,01 EIM entre 1989 et 2010 (p < 0,001). L’incidence moyenne journalière des EIM en pédiatrie était de 2,1% ±0,7% en 2010–2014 contre 1,2% ± 0,4% entre 1989 et 2010. Les antibiotiques étaient responsables du tiers des EIM chez les enfants de 0–2 ans et les anticancéreux d’un peu plus de 20% des EIM chez les 3–11 ans. Seulement 39% des EIM codés ont été déclarés à Santé Canada en 2010–2014 contre 37% en 1989–2010.
Conclusion :
Le nombre d’EIM codés a significativement augmenté au cours des dernières années. Avec l’adoption de la loi de Vanessa, la détection et la codification des EIM devrait continuer d’augmenter. Une collaboration accrue entre les archives médicales et le service de pharmacovigilance du département de pharmacie de l’hôpital pourrait aussi être envisagée afin d’accroître le taux de déclaration auprès des autorités.
Developing and Implementing a Competency Based Program for Pharmacy Technician Students
C Nguyen1, A Sengar2
1University of Toronto, Toronto, ON
2Trillium Health Partners, Mississauga, ON
ABSTRACT
Background:
Competency-based programs (CBPs) are emerging in the medical community because they graduate more competent professionals compared to traditional teaching programs. CBPs shift the focus on teaching towards learning outcomes as outcomes are a way to assure teaching methods are effective. This hospital offers a newly redesigned outcome based 4-week placement for college students enrolled in pharmacy technician programs. CBP principles were used with the objective to improve learning outcomes and job satisfaction for students upon graduation.
Description:
There are many different strategies to implement CBPs. This program uses 1-on-1 training, hands on experience, multiple assessments, and peer teaching and learning. Student goals and objectives were designed using Structure of Observed Learning Outcomes (SOLO) taxonomy which provides a systematic way of describing how a student’s performance grows in complexity, when mastering tasks. SOLO taxonomy can be applied across different types of learning outcomes and has been shown to instill deeper learning in students. Evaluation forms designed using a rubric, are now used in place of the college’s evaluation forms.
Action:
Essential components of CBP were determined before redesigning the learning objectives to incorporate effective strategies into the new curriculum. Research was done to determine how to effectively apply SOLO taxonomy to the development of the curriculum goals and objectives.
Evaluation:
Evaluation forms were completed by students to provide feedback on preceptor performance and program content. The majority of feedback received was positive and common themes included an “enriching experience” and “thorough understanding of various concepts”. A total of 57 students have completed the program with a pass rate of 96%.
Implications:
Currently 5 Colleges are associated with the program and the hospital is remunerated for each student placement. As regulated health care professionals, pharmacy technicians can now demonstrate key competencies and improve job satisfaction upon graduation
A 10-Year Retrospective Study of the Medication Incidents and Accidents Associated with Drug Dose Documentation in a Teaching Hospital
A Rousseau1, D Lebel1, G Mercier2, JF Bussières1,3
1Pharmacy Practice Research Unit, Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Electronic Health Record Team, CHU Sainte-Justine, Montréal, QC
3Faculté de pharmacie, Université de Montréal, Montréal, QC
ABSTRACT
Background:
There is a growing need for better documentation of drug doses in patient records and a higher traceability of steps performed by healthcare professionals. Medication administration records (MAR) are used for such purpose.
Objectives:
To describe a 10-year period of the medication incidents and accidents (I/A) associated with drug dose documentation.
Methods:
This is a retrospective study conducted in a 500-bed mother-child teaching hospital. All medication I/A were extracted from GesRisk® from April 1st, 2004 until March 31st, 2015 (dates, patient care areas, descriptions, types, consequences, corrective measures, severity indexes). Medication I/A associated with drug dose documentation were identified if the description included the words profile, sheet or MAR. A random sample of medication I/A were audited to confirm their classification.
Results:
A total 20,598 medication I/A were identified. A quarter were associated with drugs dose documentation (n=5403, 26.2%). Medication documentation I/A were attributed to surgical wards (18%), oncology wards (14%), pharmacy department (12%) and others areas (56%). They were mainly related to drug omissions (33%), error intercepted before drug administration (20%), time/date of administration (17%), dose/rate (13%) and others (17%). Their severity index was, in decreasing order of severity: B-incident (23%), C-accident with no consequence (67%), D-patient tested for consequences (7%) and others (3%). Ten percent (5/50) of randomly selected medication I/A that were classified as not associated with drug dose documentation were considered related to drug dose documentation after analysis. Inversely, 8% (4/50) were included, but were not associated with documentation.
Conclusions:
Twenty-six percent of medication I/A were associated with drug dose documentation. This high proportion demonstrates the importance of the risks associated with manual documentation of drug doses by nurses. A better understanding of the I/A associated with drugs dose documentation can be useful in a patient safety program and when implementing an electronic MAR.
Hospital Pharmacy in Canada Report: 30th Anniversary
JF Bussières1, K Hall2, R Mckerrow2
1Pharmacy Practice Research Unit, Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Consulting pharmacist
ABSTRACT
Background:
Pharmacy practice has considerably changed over the years. Some countries survey hospital pharmacy practice (e.g. USA, Europe). In Canada, an editorial board of hospital pharmacy leaders has surveyed hospitals pharmacists in the last 30 years.
Objectives:
To describe the evolution of the Hospital Pharmacy in Canada Report (HPCR).
Methods:
This is a retrospective descriptive study. Using published reports, we extracted the following key variables: editorial board foundation, board members, reports response rate, number of pages/themes, trends for key topics, diffusion strategies and key issues.
Results:
The editorial board for the HPCR has been founded in 1985 with four members. Throughout the years, 30 hospital pharmacists joined the board and four of these were executive editors. Response rates stayed high with a low 50%(2786/542) in 1985/86 to a high 80%(176/219) in 2011/12. From an initial 18-page length in 1985/86, it reached a record 148-page in 2013/14. At least 20 themes were covered systematically or periodically: demographics, clinical pharmacy, drug distribution, financial resources, human resources including pharmacists and technicians, technologies, workload management, patient safety, CSHP2015, regionalization and centralization, pediatrics, evaluation of pharmacy services, ethics and future trends. The report has been annual from 1985/86 until 1997/98 and then every second year. Over time, pharmacy staffing and expenditures have increased with the decentralization of pharmacists in a large number of clinical patient care programs, combined to a progressive delegation of distribution services to pharmacy technicians with the support of technologies. Diffusion strategies included a paper publication followed by a web publication in 2001/02 and emails to key stakeholders including Hospitals chief executive officers. Regional management pharmacy seminars and bi-annual Canadian management seminars were opportunities for larger diffusion.
Conclusions:
Few healthcare professions can rely on such extensive data about their activity. The HPCR is celebrating his 30th Anniversary in 2015.
Impact of Comprehensive Medication Reviews Completed by Pharmacy Students in a Complex Continuing Care Program: A Pilot Project
H Rodrigues, W Li, L Kelebay, P Nedzka, S Ng
Runnymede Healthcare Centre, Toronto, ON
ABSTRACT
Background:
Adults receiving a large number of medications are at increased risk for an adverse drug event and increased risk of admission to acute care. Adults with complex medical needs often require a complex drug regimen and benefit from regular review of medications to ensure safe medication use. Pharmacists and pharmacy students can play an important role in evaluating the appropriateness of medications and improving medication safety.
Description:
A step-wise, multidisciplinary procedure for completing a comprehensive medication review (CMR) was developed. The number of patients receiving this intervention was limited due to the amount of pharmacist time required to complete the CMR.
Action:
A CMR role was developed for pharmacy students as part of a pilot project to increase the number of complex continuing care patients receiving this intervention. Pharmacy students were oriented to the process and followed a systematic approach to assessing each patient’s medications. All suggestions were reviewed and cosigned by a clinical pharmacist prior to documentation and discussion with the physician.
Evaluation:
A retrospective audit was completed in order to determine the impact of the intervention. Three pharmacy students completed 47 CMRs over a total of 14 weeks resulting in 139 recommendations. Physicians implemented 77% of the recommendations. Of the accepted recommendations, 52% resulted in the discontinuation of a medication, while 40% resulted in the addition of a new order (optimization of an existing medication, new medication, monitoring test or consultation). Pharmacy student, pharmacist and physician satisfaction was found to be positive.
Implications:
The successful integration of pharmacy students into the CMR process for adults in a complex continuing care program may have important implications. It provides pharmacy students an opportunity to practice their skills and actively engage in providing meaningful patient care while helping pharmacists and physicians optimize drug therapy for patients.
Safety Alerts as Drivers for the Pharmaceutical Opinion Program: A Pilot Study to Reduce Potential Hospitalizations Due to Preventable Drug-Drug Interactions
C Ho, R Cheng, A Kawano, B Morphy, L Yoo, M Ng, J Kong, S Liang, S Arjomandpour, A Chen, A Sharma, S Hyland
Institute for Safe Medication Practices Canada, Toronto, ON
ABSTRACT
Background:
Drug-drug interactions (DDIs) represent a potentially serious problem that can result in adverse drug events (ADEs), which account for 2.8% of hospital admissions. Pharmacists are uniquely positioned to prevent ADEs by intervening in DDIs.
Objectives:
This project aims to reduce the occurrence of DDIs associated with potential hospitalizations, offer continuing professional development opportunities while providing a financially sustainable business model via the Pharmaceutical Opinion Program (POP).
Methods:
A Safety Alert regarding 13 evidence-based DDIs was disseminated and reviewed by pharmacists to allow for recognition of the cited DDIs as they were encountered in practice, and to inform communication with prescribers via a pharmaceutical opinion. Quantitative data was collected for six months in the form of the total number of POP claims submitted to the provincial ministry before and after the Safety Alert, while qualitative data was obtained through three focus group sessions.
Results:
At study completion, 35 pharmacies made 67 POP claims involving the 13 DDIs in this project, translating to a theoretical cost avoidance of approximately $73,184 from potentially averted hospitalizations. The difference in the total number of POP claims was not statistically significant (2845 pre-intervention versus 2399 post-intervention; p = 0.204), however the 18 pharmacies with a net increase in POP submissions exceeded the 13 pharmacies with a net decrease. The focus group discussion indicated that the value of the Safety Alert was unequivocal.
Conclusion:
Through disseminating evidence-based DDIs via the Safety Alert, this project offers an innovative strategy to capture and reduce DDIs associated with potential hospitalizations; deliver continuing education to front-line pharmacists; and provide business opportunities through which cognitive services are reimbursed via the POP.
Medication Incidents Involving Drug Tapering
A Chen1, C Ho1,2
1Institute for Safe Medication Practices Canada, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
Prescriptions involving a drug tapering process are often complex in nature, involving multiple, sequential doses of medication(s), extensive directions of use, and complex mathematical calculations. All of these considerations illustrate the inherent vulnerability of drug tapering to errors that may occur at any stage of the medication-use process.
Description:
The objective of this multi-incident analysis was to identify potential systems-based contributing factors and areas of vulnerability towards medication incidents involving drug tapering.
Action:
An analysis of medication incidents involving drug tapering was performed using reports anonymously submitted to a national incident reporting database from 2010 to 2014.
Evaluation:
122 medication incidents were analyzed and categorized into four major themes, all of which are potential contributing factors for drug-tapering incidents: (1) lack of standardized tapering guidelines, (2) inadequate patient counseling, (3) operational limitations, and (4) complexity of prescription. The four major themes were further divided into subthemes, some of which included labeling restrictions, billing restrictions, and multi-medication compliance aids.
Implications:
Errors associated with drug tapering regimens occur on all levels of patient care involving physicians, pharmacists, patients, and caregivers alike. Learning from medication incidents is an imperative step in improving medication-use systems. Pharmacists can mitigate and prevent the likelihood of negative outcomes with the understanding of common themes and contributing factors that may result in drug tapering errors.
Medication Incidents Involving Insulin: A Multi-Incident Analysis
C Kasprzak, C Ho
Institute for Safe Medication Practices Canada, Toronto, ON
ABSTRACT
Background:
Insulin is a life-saving pharmacological therapy used in the management of blood glucose for many diabetic patients. However, insulin has been identified as a high alert medication as it has the potential to cause detrimental patient harm when used in error; particularly an excessive dose can lead to life-threatening seizures and coma via hypoglycemia. Medication incident reporting can be used to enhance understanding of factors that may contribute to insulin-related medication incidents.
Description:
The objective of this multi-incident analysis was to examine insulin-related medication incidents and determine potential system-based improvements which may be customized in pharmacy practice to enhance medication safety.
Action:
Reports of medication incidents involving insulin were extracted from a national incident reporting database between January and December 2014.
Evaluation:
After a review of 226 incidents, 81 were included in this qualitative, multi-incident analysis. The incidents were then analyzed and categorized into main themes and subthemes. The four main themes identified were: (1) product selection (related to unique insulin properties), with prescribing, order entry and dispensing as subthemes; (2) therapeutic regimen change; (3) dosage calculations; and (4) storage requirements. Potential solutions for prevention of insulin-related incidents included the use of standardized pre-printed order forms, incorporation of independent double checks throughout the entire pharmacy workflow, and conducting comprehensive diabetes-focused medication reviews with patients.
Implications:
Medication incidents involving insulin in pharmacy practice are common and have the potential to cause serious patient harm. Findings from this analysis are intended to educate health care professionals on the vulnerabilities in the medication-use process that may contribute to insulin-specific medication incidents and offer recommendations to prevent such events from recurring.
Development of a Framework for Podcast Creation to Supplement Pharmacy Students Learning
M Kani1, C Ho1,2
1Institute for Safe Medication Practices Canada, Toronto, ON
2School of Pharmacy, University of Waterloo, Kitchener, ON
ABSTRACT
Background:
Literature suggests that students perceive podcast as really useful additional resource to supplement their learning rather than as a substitute for the traditional methods of learning. Podcast takes advantage of the ubiquitous devices and networks to allow for “anytime” learning “anywhere”.
Description:
Currently, the use of podcasts for teaching and learning at the School of Pharmacy is non-existent. This project intended to develop a framework for podcast creation to supplement pharmacy students learning.
Action:
A needs assessment with 138 pharmacy students indicated that 100% of respondents have access to podcast-capable devices for listening. Although 64% of students do not currently listen or subscribe to podcasts, 77% are very interested or somewhat interested in pharmacy student-related content podcasts.
Evaluation:
To initiate the creation of podcasts for pharmacy students, a framework was developed that takes into account the necessary steps and elements needed with minimal cost, ease of use, simplicity, and sustainability. This framework engages both faculty and students in knowledge creation for supplemental student learning and incorporates a continuous quality improvement process to ensure the podcast content is evidence-based.
Implications:
Podcasts may offer pharmacy students the opportunity of supplemental learning through the use of a technology that they already carry, depend on, and is part of their social practice.
Complexity and Vulnerability of Multi-Medication Compliance Aids
J Tsang, C Ho
Institute for Safe Medication Practices Canada, Toronto, ON
ABSTRACT
Background:
Traditional processing and dispensing of prescriptions are involved with high-level procedures; compliance packaging introduces further complexity and vulnerability in the pharmacy workflow due to its multi-compartmental design.
Description:
The objective of this project is to gain a better understanding of the potential contributing factors for compliance-pack related medication incidents and attempt to offer recommendations to prevent such events from recurring.
Action:
An analysis of medication incidents related to compliance pack preparation was performed using reports anonymously submitted to a national incident reporting database from June 2012 to May 2013.
Evaluation:
A total of 170 incident reports were analyzed. Two main themes were identified: (1) order entry and (2) packaging process. Major concerns with order entry were associated with hospital discharge order, discontinuation of medication from new prescription order, new or prospective update of prescriptions, and miscalculation. Other concerns in regards to packaging process included labeling, incorrect time of administration, half-tablet medications, improper return-to-stock procedures, dose or medication omissions, and incorrect medication or strength. With no permanent physical barriers between each packing slot, compliance packaging is more prone to a medication being misplaced in another slot during the sealing process. Conducting independent double checks is essential in the pharmacy workflow.
Implications:
Although multi-medication compliance aids may facilitate patient’s adherence and improve treatment outcomes, the complexity of the design and procedures for preparation may potentially lead to an increased risk of errors. Recognizing the vulnerabilities of compliance pack preparation creates opportunities for pharmacists to implement additional safeguards to enhance medication safety.
Economic Evaluation of Adding a Drug to the Hospital’s Hazardous Drug List
N Ma, A Kulchycki, J Hayes, H Carating, SE Walker
Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON
Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
Preparation and administration of hazardous drugs can potentially expose staff, leading to adverse health outcomes. The National Institute for Occupational Safety and Health (NIOSH) publishes a list of hazardous drugs with recommendations for safe handling during preparation and administration. However, several commonly used drugs have been added to the list (e.g. warfarin and phenytoin) for which harm to staff is questionable. As part of the decision to add a drug to a hospital’s hazardous drug list, the incremental cost for packaging and administration as a hazardous product was considered.
Objective:
To determine the incremental cost of unit dose (UD) packaging a drug as a hazardous product based on NIOSH standards.
Methods:
Labor, material and personal protective equipment (PPE) costs for packaging both hazardous and non-hazardous drugs was determined for various methods including blister packaging, Ziploc® bag over wrap, PACMed®, Cadet®, and AutoBag®. Costs were then used to estimate the cost of adding three drugs, clonazepam, phenytoin, and warfarin, to the hospital’s hazardous drug list.
Results:
The cost to package non-hazardous drugs using either the Cadet® or PACMed® ranged from $0.05–0.09/UD. Two primary methods for packaging intact tablets and capsules as hazardous drugs were identified. The first method, manually blister packing followed by overwrapping with a Ziploc® bag, cost $0.77/UD. The second was to package the drug with the Cadet®, followed by an AutoBag® overwrap, which cost $0.54/UD. In the previous fiscal year, 108,193 doses of clonazepam, phenytoin, and warfarin were dispensed. The total cost to package and administer all doses using the Cadet® packaging method was estimated as $58,236.77 annually, representing a $50,151.45 increase from baseline cost. Of this, $14,104.09 was estimated for nursing PPE, $30,233.71 for pharmacy labor and $5,813.63 for packaging materials.
Conclusion:
Packaging and administering hazardous drugs cost about $0.50/UD more than non-hazardous oral dosage forms.
Impact of a Geriatric-Hospitalist-Orthopaedic Co-Management Program on Closing the Post-Fracture Care Gap
W Miao, S Alibhai, B Coleman, C Fan-Lun
Mount Sinai Hospital, Toronto, ON
ABSTRACT
Background:
Over a 6-month period in 2010–2011, Mount Sinai Hospital (MSH) developed and established a new geriatric and hospitalist co-management program to care for geriatric hip fracture patients admitted to the orthopaedics service.
Objectives:
To evaluate the effectiveness of MSH’s geriatric-hospitalistorthopaedic co-management program at increasing osteoporosis treatment among hospitalized older patients post hip fracture and assess whether the program decreases prescribing of medications associated with falls risk.
Methods:
This single-center, retrospective, pre-post intervention study compared the frequency of osteoporosis and falls risk medication prescribing before (March 2009–September 2010) and after (March 2011–December 2012) implementation of the co-management program. Every second hip fracture patient aged ≥ 65 years consecutively admitted to the orthopaedics service between March 2009 and December 2012 was screened for eligibility. A single reviewer extracted data through a structured chart review. The 2010 Osteoporosis Canada Guidelines were used to define appropriate agents and doses for osteoporosis treatment. The Beers Criteria were used to identify fall-risk-increasing drugs (FRIDs). Primary outcomes were analyzed using interrupted time series methods.
Results:
Three hundred forty-two patients were included (pre-intervention, n=139; post-intervention, n=171; transition period, n=32). Primary outcomes: Implementation of the co-management program was associated with an increase in number of patients whose osteoporosis regimen was optimized to meet guideline-recommended doses (RR=1.9, 95% CI: 1.7–2.2, p<0.001). It was also associated with an increase in number of patients newly initiated on anti-resorptive therapy (RR=1.7, 95% CI: 1.3–2.3, p<0.001). Secondary Outcomes: In the pre-intervention period, 7.9% of patients had a decrease in number or dose of FRIDs during admission compared 18.7% of patients in the post-intervention period (p=0.006).
Conclusion:
Implementation of a geriatric-hospitalist-orthopaedic co-management program at MSH was associated with a higher frequency of guideline-concordant osteoporosis treatment and fall-risk medication de-prescribing among older post-hip fracture patients requiring secondary fracture and falls prevention.
The Opinions of Hospital Pharmacists in Canada Regarding Marihuana for Medical Purposes
F Mitchell1, O Gould2, M LeBlanc1, L Manuel1
1Horizon Health Network, Moncton NB
2Mount Allison University, Sackville NB
ABSTRACT
Background:
Health Canada’s most recent Marihuana for Medical Purposes Regulations have changed the way that patients access marihuana and the responsibility to authorize its use has been shifted into the hands of health care prescribers. Pharmacists practicing in hospitals are authorized to place orders for dried marihuana from a licensed producer for in-hospital patient use. As its use increases, hospital pharmacists may find themselves having an increased role in the care of patients using marihuana for medical purposes.
Objectives:
The primary objective of this study was to determine the opinions of hospital pharmacists in Canada regarding marihuana for medical purposes and to assess what factors influence these opinions.
Methods:
This was an online survey distributed to licensed hospital pharmacists in Canada via individual provincial/territorial pharmacy regulatory bodies, pharmacists’ associations, hospital pharmacy directors, the Canadian Society of Hospital Pharmacists, and the Association des pharmaciens des établissements de santé du Québec. Data was collected through FluidSurveys, an online survey tool. Descriptive statistics as well as multivariate logistic regression models were used to analyze study data.
Results:
There were 769 valid responses to the survey. Forty-five percent of pharmacists agreed that marihuana is effective; while 55% agreed that it is safe. Only 17% agreed that they are knowledgeable about marihuana for medical purposes. Factors that influenced pharmacists’ opinions were age, education, area of clinical practice, province of work, and personal experience.
Conclusion:
Many Canadian hospital pharmacists agree that marihuana for medical purposes is safe and effective, yet few consider themselves knowledgeable about it, with approximately 65% of pharmacists reporting no formal training on the topic.
Safety Implications of the Dose Change Alert in Smart Pumps on the Administration of High-Alert Medications
C Goulding1, M Bedard2
1Baxter Corporation, Mississauga, ON
2The Ottawa Hospital, Ottawa, ON
ABSTRACT
Background:
Most intravenous medication errors occur during administration. Smart pumps can reduce the incidence of dose or rate errors using soft and hard limits. But industry standard dose error reduction software misses errors that occur during titration. The dose change alert was developed to detect errors during titration.
Objective:
To evaluate the safety implications of the dose change alert in the SIGMA Spectrum Infusion System on the administration of high alert medications.
Methods:
Observational analysis of continuous quality improvement data from all titratable high-alert drug infusions across 1600 large volume pumps between May 1st and October 31st 2014 (inclusive). Outcomes included dose change alert confirmation and cancellations and drug library compliance.
Results:
Compliance with using the drug library was 96.8%. The percentage of dose change confirmations and cancellations, within the soft limits were 48.1% and 1.9%, respectively. The titration of vasopressors resulted in the highest percentage (75%) of dose change confirmations. The titration of anticoagulants resulted in the highest percentage (12%) of dose change cancellations.
Conclusions:
This study provided insight into the safety implications of the dose change alert on the titration of high alert drugs. The dose change alert allows the hospital to define the percentage dose change limit for individual drugs and plays a large role in potentially preventing medication errors.
Implantation d’un logiciel de gestion de la conformité en établissement de santé
G Rousseaux1, S Atkinson1, D Lebel1, I Piaget1, JF Bussières1,2
1Unité de recherche en pratique pharmaceutique, Département de pharmacie, CHU Sainte-Justine, Montréal, QC
2Faculté de pharmacie, Université de Montréal, Montréal, QC
ABSTRACT
Contexte :
La pharmacie est une profession réglementée par plusieurs centaines de critères de conformité issus du cadre juridique et normatif. Auditer les pratiques et assurer un suivi des écarts auprès de toutes les parties prenantes afin de réduire les risques d’accidents est un défi quotidien.
Objectifs :
L’objectif est de décrire l’implantation d’un logiciel de gestion de la conformité en établissement de santé.
Méthodologie :
Il s’agit d’une étude transversale descriptive menée dans un établissement mère-enfant de 500 lits. Le logiciel Omni-Assistant® (Omnitech Innovations Inc., Canada) a été retenu. L’implantation a été menée selon les étapes suivantes : a) formation, b) création de l’identité des usagers et des locaux, c) activation de la base test, d) exploration des fonctionnalités, e) implantation d’une activité par module, f) production de vidéos pour partage de connaissance, g) rédaction de politiques et procédures. Au fil de l’implantation, les modifications à apporter et les difficultés rencontrées ont été recueillies.
Résultats :
Omni-Assistant® est une application web hébergée à l’extérieur de l’établissement et comporte 14 modules de travail. Sept ont été retenus et cinq implantés au sein de notre établissement, soit les modules M1–optimiseur de tâches, M2–gestion documentaire et centre de formation, M3–locaux et équipements, M4–points de contrôle, M12– inspection et audits. Jusqu’à maintenant, l’outil s’avère prometteur pour centraliser la gestion des écarts pour une majorité des éléments de la pratique pharmaceutique. Toutefois, des modifications doivent être apportées à la base de données, à la navigation en poste fixe et en tablette afin d’en optimiser la navigation et l’utilisation.
Conclusions :
Cette étude décrit l’implantation d’un logiciel de gestion de la qualité et des risques dans un département de pharmacie hospitalier. Ce type d’outil apparait utile et nécessaire à l’identification et la prise en charge efficace des écarts de pratique.
Reconstruction de l’hôpital Saint-Michel en Haïti : perspectives après cinq missions pharmaceutiques
JF Bussières1,2,3, V Bussières3, M Legault3
1Pharmacy Practice Research Unit, Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Faculté de pharmacie, Université de Montréal, Montréal, QC
3Programme intégré de santé en Haïti, CHU Sainte-Justine, Montréal, QC
ABSTRACT
Contexte :
Haïti est un pays en reconstruction et de nombreuses initiatives humanitaires sont encore déployées cinq années après le tremblement de terre. À Jacmel, notre équipe participe à un projet de reconstruction de l’Hôpital Saint-Michel en collaboration avec la Croix-Rouge Canadienne.
Objectif :
Décrire l’état d’avancement des travaux après la tenue de cinq missions pharmaceutiques.
Méthodologie :
Il s’agit d’une étude descriptive rétrospective. À partir des revues documentaires publiées et des rapports de mission rédigés, nous avons fait l’inventaire des constats, des recommandations et des réalisations.
Résultats :
L’Hôpital Saint-Michel est un hôpital départemental de 75 lits. Depuis octobre 2013, nous avons réalisé cinq missions pharmaceutiques pour un total de 55 jours. Quatre constats ont été identifiés, soit la nécessité de réorganiser l’hôpital (n=17 recommandations), le circuit patient (n=9), le circuit du médicament (n=26) et le département de pharmacie (n=63). À ce jour, 27% des recommandations ont été complétées. Les travaux ont notamment permis de structurer et redresser la gestion financière et statistique, d’informatiser la gestion des stocks, de déménager la pharmacie dans un abri temporaire durant les travaux, d’améliorer le circuit du médicament, d’augmenter les revenus et l’inventaire, de former le personnel, de régulariser la chaîne thermique, de créer un comité de pharmacologie pour les médicaments et fournitures de soins, d’évaluer le circuit du médicament et de s’arrimer avec les médecins et infirmières, de créer un site web et de de soutenir l’établissement en dons de médicaments. Le déménagement dans les nouveaux bâtiments est prévu en 2016. Jusqu’à maintenant, un résumé et six articles ont été publiés afin de partager les efforts de reconstruction.
Conclusion :
Cette étude illustre la contribution d’un pharmacien à la reconstruction d’un hôpital départemental en Haïti dans le cadre d’un projet humanitaire canadien.
Feasibility of Pharmacist-Run Group Diabetes Education Sessions for Patients on a Stroke Rehab Unit
R Kumra1, F Schwartz2
1Pharmacy, Toronto Rehabilitation Institute, Toronto, ON
2Patient and Family Education, Toronto Rehabilitation Institute, Toronto, ON
ABSTRACT
Background:
Diabetes education is important post stroke as per stroke best practice guidelines. However patients post stroke can have various deficits that can affect cognition and possibly also impact learning. The feasibility of pharmacist-run group education sessions on a stroke rehab unit was evaluated to determine the feasibility of class-style learning with patients with type 2 diabetes post stroke.
Description:
Needs assessments were completed with 10 diabetes patients on the unit and 6 unit staff. The results were used to develop a diabetes session with consideration of adult education principles. These sessions were held every few weeks and diabetes patients were able to attend the session.
Action:
Patients completed a non-validated diabetes knowledge test before and after going to the diabetes education session. Patients completed an evaluation survey of the session after attending the educational session.
Evaluation:
Between April-September 2015, there were 35 stroke rehab patients with diabetes. The number that attended sessions and signed consent were 7 or 19%. The number of patients that attended the sessions was limited by cognitive barriers of patients, patient language barriers, and patient fatigue. Overall the knowledge test results were improved post diabetes session. The mean test result pre session was 5.3/10 and post session it was 6.8/10. The overall evaluation of the sessions by the patients showed that the sessions met most of their needs and most found the topics useful.
Implications:
Pharmacist-run diabetes sessions in the inpatient rehab setting can be beneficial to patients. The limitations of patient attendance due to cognitive, language barriers should be looked at more closely. Possible considerations should be made to include family members at sessions, and language translation to include more patients.
Integrating Smartphone Communication Strategy and Technology into Clinical Practice: A Mixed Methods Research Study
C Webb1, S Spina1,2, S Young3
1Vancouver Island Health Authority Pharmacy Services, Royal Jubilee Hospital, Victoria, BC
2Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC
3Vancouver Island Health Authority IMIT Client Services, Royal Jubilee Hospital, Victoria, BC
ABSTRACT
Background:
Antiquated paging systems continue to be used by the majority of hospitals in Canada despite the nearly ubiquitous use of personal and/or corporate smartphones by hospital clinicians. There is a lack of literature evaluating the impact of new communication strategies.
Description:
Integrated multi-platform smartphone apps, such as the Vocera® Collaboration Suite (VCS), have the potential to be a reliable, efficient, and secure methods of communication in hospitals.
Action:
We examined the effect of VCS, on communication and the efficiency of communication between hospital clinicians (n=153). Our primary outcome was the difference in page turnaround time before and after the implementation of VCS. We also conducted baseline and post-study surveys. This three-month multidisciplinary prospective observational study involved two tertiary care hospitals, and one community hospital. Hospitalists, obstetricians, intensivists, and pharmacists piloted the VCS smartphone app in the replacement of pagers. Switchboard operators, ICU nurses, and ICU unit clerks had access to the app via a desktop computer based web console.
Evaluation:
Physicians responded to pages from pharmacists faster (5.5 vs 3 min; p < 0.05) when using VCS compared to traditional pagers. Physicians found the app was more efficient, reduced interruptions to patient care, and 81% of physicians wanted to continue using the system. The highest reported drawback of the app was the reduction in phone battery life.
Implications:
This is the first research objectively demonstrating that the speed of communication improves when hospital clinicians use smartphone technology compared to pagers. Smartphone based communication systems, such as Vocera® Collaboration Suite, improve the speed of, and satisfaction with hospital communication when compared to pagers. Reduced phone battery life should be considered when implementing VCS. This project has been recognized by the provincial Minister of Health and has already informed the future communication strategy for 10 acute care hospitals in Canada.
Headline News: A Simulation Game for Hospital Pharmacy Leaders
JF Bussières1, K Hall2
1Pharmacy Practice Research Unit and Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Consulting pharmacist
ABSTRACT
Background:
Pharmacists work in a complex environment. To make sound decisions in a changing environment, pharmacists must access and pursue quality information.
Objectives:
To evaluate the ability of pharmacy leaders to discriminate true and false information in a simulated headline news bulletin.
Methods:
This is a prospective descriptive study. Participants at a Canadian pharmacy management seminar were exposed to a 10-minute news bulletin with 20 headlines. Respondents were asked to score their level of awareness and their evaluation of truthfulness of the material on a 1–10 scale (1-not familiar or untrue and 10-very familiar or totally true). Average score of awareness and truthfulness were calculated for each headline. Thereafter, respondents scored 18 statements regarding their exposure to false information with a 5-level frequency scale and 12 statements strategies to identify the true from the false with a 2-level scale
Results:
Forty participants took part in the simulation game (male 52%, >40y.o. 92%). Of the 20 headlines, three were false (score:0), four were partly true (score:5) and the rest were true. In most cases (13/20), participants were unable to identify the true from the false with precision (i.e. an absolute difference of >3). However, the more aware respondents were about a topic, the lesser the gap was between the true score and the respondents’ score. There was low correlation between awareness score and truthfulness score (r2:0,628). At least 50% of respondents have used 8/12 strategies proposed to identify the true from the false in their life. A majority of respondents (95%) believed many decisions taken by hospital administrators do not rely on good science.
Conclusions:
A simulation using a headline news bulletin format can help support the reflection of pharmacy managers about finding the true and the false in their professional life.
Medication Incident Analysis Knowledge Mobilization Tool: Medication Safety Expertise at Your Fingertips
M Chan, R Cheng, K Duwyn, J Greenall, M Hamilton, C Ho, S Hyland, J Kong, C Lee, G Lee, H Leung, S Liang, G Mutti, M Ng, V Pan, A Reidel, C Salsman, K Streitenberger, A Watt, J Yu
Institute for Safe Medication Practices Canada, Toronto, ON
ABSTRACT
Background:
Learning from medication incidents is a fundamental step in improving medication use systems. However, many Canadian hospitals lack the resources and expertise to effectively analyze and learn from their medication incidents. The Knowledge Mobilization Tool (KMT) fills this gap by providing hospital practitioners with access to timely, context-specific medication safety information to facilitate medication incident analyses.
Description:
The KMT will be available as a website-based tool hosted by the organization. When a medication incident is entered into the KMT, it will generate a report containing incident-specific information units as extracted from the organization’s online safety bulletins. The information output will include similar medication incidents, potential contributing factors and recommendations for consideration. Each information unit will include a link to the full bulletin.
Action:
Development of the KMT involved extracting information from safety bulletins (information units) and incorporating them into a sophisticated information matching algorithm to create the KMT prototype. The prototype is then tested and refined for its ability of providing relevant results and user-friendliness. Enhancements to the user interface and refinement of the information matching algorithm will continue post-launch.
Evaluation:
To evaluate the information matching algorithm, medication incidents from previously published bulletins were entered into the KMT and the data output indicated an 84.4% sensitivity for retrieving an exact match within the organization’s safety bulletins. With respect to the overall concept and user-friendliness, a preliminary demonstration of the KMT prototype at a workshop garnered positive feedback from workshop participants.
Implications:
This innovative tool is the first of its kind, and will contribute to the safety of medication use by providing healthcare practitioners in hospitals with the needed tools and information to effectively analyze and learn from medication incidents.
Comparison of Information Available in the Medication Profile of an Electronic Health Record and the Inpatient Best Possible Medication History
J Daupin1, G Rousseaux1, D Lebel1, S Atkinson1, P Bédard1, JF Bussières1,2
1Pharmacy Practice Research Unit, Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Faculty of Pharmacy, Université de Montréal, Montréal, QC
ABSTRACT
Background:
Medication reconciliation (MedRec) can improve patient safety. In Canada, most provinces are implementing electronic health records (EHR). The Quebec Health Record (QHR) can theoretically be used for medication reconciliation. However, the quantity and the quality of information available in this HER have not been studied.
Objectives:
The aim of the study was to compare the quantity and quality of the information collected between the inpatient best possible medication history (BPMH) and the QHR.
Methods:
This is a descriptive prospective study conducted in a 500-bed tertiary mother-and-child university hospital center. All inpatients from May 19th to 26th 2015 were considered for inclusion. Every prescription line in the BPMH and QHR was compared.
Results:
The study included 344 patients. A total of 1,039 prescription lines were analyzed. The medications’ name and dosing were more often available in the QHR (95%) than in the BPMH (61%). However, fewer medications were reported in the QHR than in the BPMH, with averages of 1.30 vs. 1.84 medications per patient, respectively. Concordance between the medication names between QHR and BPMH was found in 48% of the prescription lines; this rate fell to 29% when also factoring daily dosage. When analyzing discrepancies, 29% of the QHR lines that did not match (85/290) referred to as needed medications and 20% of the BPMH unmatched lines (89/443) referred to natural health products.
Conclusions:
This study suggests that the QHR can provide high-quality information to support the MedRec hospital process. However, it should be used as a second source to optimize the BPMH obtained from a thorough interview with the patient or his family. More studies are required to confirm the most optimal way to integrate the QHR to the MedRec process in hospitals.
A Comparative Study of the Conformity of the Documentation of Drug Doses Administered Pre and Post Implementation of an Electronic Medication Record
A Rousseau1, D Lebel1, G Mercier2, JF Bussières1,3
1Pharmacy Practice Research Unit, Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Electronic Health Record Team, CHU Sainte-Justine, Montréal, QC
3Faculty of Pharmacy, Université de Montréal, Montréal, QC
ABSTRACT
Background:
There is a growing need for better documentation of drug doses in patient records, including higher traceability of steps performed by healthcare professionals. Electronic medication administration records (eMAR) are expected to increase the documentation of drug administration.
Objectives:
To compare the conformity of the documentation of drug doses administered pre-post implantation of an eMAR.
Methods:
This retrospective pre-post study was conducted in a 45-bed pediatric surgery unit. The pre-implementation phase occurred between October 19th–26th, 2014. The post-implementaion phase occurred between May 3rd–14th, 2015. Medication administration records were extracted from the digitalized patient healthcare record (Chartmax®). We compared the documented steps for each planned drug administration. A chi square test was used to compare conformity. We calculated the ratio of the number of steps documented on the number of steps that should be documented.
Results:
A total of 135 patient charts were consulted. A total 4157 doses (2551 pre-implementation, 1606 post-implementation) were included. They represented 543 regular drug orders (280 pre-implementation, 263 post-implementation), 388 as needed drug orders (260 pre-implementation, 128 post-implementation). Post-implementation, the conformity of the documentation of drug dose administered increased from 79.5% (689/867) to 88.1% (796/904) (p < 0.0001) for regular drug doses and from 16.3% (272/1665) to 43.5% (298/685) for as needed drug doses (p < 0.0001). The ratio of steps documented increased from 0.40 to 0.56 for regular drug doses and from 0.13 to 0.28 for as needed drug doses. However, for drug infusions (n=19 doses pre-implementation, n=17 doses post-implementation), it decreased from 0.63 to 0.51.
Conclusions:
This study showed an increase in conformity of the documentation of drug doses administered pre and post implementation of an eMAR. While eMAR can contribute to better documentation and traceability, further gain relies on human factor, enforcement of policy and procedures and periodical practice audits.
Drug Shortages in Health Care Institutions: Perspectives in 2014–2015
A Rousseau1, D Lebel1, JF Bussières1,2
1Pharmacy Practice Research Unit, Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Faculty of Pharmacy, Université de Montréal, Montréal, QC
ABSTRACT
Background:
Health Canada and many stakeholders recognize the importance and the negative impacts of drug shortage on patients, healthcare professionals and the healthcare system.
Objectives:
The aim of this study was to describe Canadian drug shortages.
Methods:
This is a retrospective descriptive study. All drug shortages identified on the list provided by a Canadian wholesaler/distributor between Aug 1st, 2014 and Aug 17th, 2015 were included. These data were compared to previous annual data starting in 2006–2007. The number of drug shortages and their therapeutic classes, average duration of drug shortages and number and type of manufacturers involved were analyzed.
Results:
From 2006–2007 to 2014–2015, the annual number of drug shortages were, respectively, 493, 400, 441, 679, 429, 1081, 497, 611 and 662. Most therapeutic classes were represented throughout the years. In 2014–2015, they were, in descending order: central nervous system agents (22%), cardiovascular drugs (16%), anti-infective agents (13%), autonomic drugs (6%), gastrointestinal drugs (6%), antineoplastic drugs (5%), blood formation, coagulation and thrombolysis agents (5%), hormones and synthetic substitutes (5%) and others (22%). The average duration of drug shortages increased from 108±130 days in 2006–2010 to 197±166 in 2014–2015. The number of drug manufacturers involved was reduced from 70 in 2006–2007 to 51 in 2014–2015, which was associated to numerous mergers. The proportion of drug shortages associated to generic manufacturers increased from 67% in 2011–2012 (first year captured) to 86% in 2014–2015. The proportion of drug shortages associated to the injectable was 33% in 2011–2012 and 36% in 2014–2015. The top-five manufacturers were Apotex (21%), Teva (14%), Sandoz (8%), Mylan (8%) and Pharmascience (7%).
Conclusion:
Drug shortages remain a daily issue for pharmacists and other stakeholders. The annual monitoring of the current portrait of drug shortages should contribute to the debate and the identification of viable solutions.
Comparaison de la perception des pharmaciens québécois et français vis-à-vis des données sur les rôles et retombées des activités pharmaceutiques
M Breton1, A Guérin1,2, JF Bussières1,3
1Unité de recherche en pratique pharmaceutique, Département de pharmacie, CHU Sainte-Justine, Montréal, QC
2Service de pharmacie, Hôpital Béclère, Paris, France
3Faculté de pharmacie, Université de Montréal, Montréal, QC
ABSTRACT
Contexte :
Compte tenu des dépenses élevées en santé, tous les professionnels sont confrontés à une révision de leurs modèles de pratique. Cette révision devrait notamment tenir compte des meilleures données publiées sur les rôles et les retombées d’une activité professionnelle.
Objectif :
L’objectif principal a été d’évaluer et de comparer la perception et la sensibilité des pharmaciens au Québec et en France vis-à-vis des données sur les rôles et retombées des activités pharmaceutiques (RRAP). L’objectif secondaire a été de mesurer leur appréciation du site impactpharmacie.org.
Méthodologie :
Pour cette étude transversale descriptive, revue de littérature et séances de remue-méninges entre les membres de l’équipe de recherche ont permis d’identifier les variables d’intérêt permettant d’évaluer la perception et la sensibilité aux RRAP. Un questionnaire en ligne de 33 questions a été créé puis envoyé aux pharmaciens québécois et français.
Résultats :
Les taux de participation ont été de 13,6% (217/1600) au Québec et de 5,0% (145/2914) en France. Une différence statistiquement significative a été mise en évidence pour 10/15 variables évaluant la lecture des données scientifiques relatives aux RRAP par les pharmaciens, et pour 4/12 items évaluant leur perception de l’utilité et de l’utilisation en pratique de ces données. Globalement, les répondants les ont estimées importantes concernant leur utilité et leur intégration dans la pratique.
Conclusion :
Cette étude met en évidence la perception et la sensibilité des pharmaciens de deux milieux de pratique différents quant aux données relatives aux RRAP. Les études évaluatives et comparatives sont essentielles pour développer de nouvelles activités, valoriser et améliorer la qualité des services pharmaceutiques. Les pharmaciens ont besoin d’outils pour les aider. Ainsi, le site impactpharmacie.org, pourrait aider à favoriser la lecture de la littérature scientifique sur les RRAP et axer davantage l’enseignement de la pharmacie sur le rôle du pharmacien en pratique.
Réactions des étudiants en pharmacie à la mise en place d’un code de bonnes pratiques pharmaceutiques en ligne et dans les réseaux sociaux
JF Bussières
Unité de recherche en pratique pharmaceutique, Département de pharmacie, CHU Sainte-Justine, Montréal, QC
Faculté de pharmacie, Université de Montréal, Montréal, QC
ABSTRACT
Contexte :
Tous les citoyens possèdent désormais une empreinte numérique. Les étudiants en pharmacie et les pharmaciens doivent s’en préoccuper.
Objectifs :
L’objectif de cette étude est de décrire les réactions des étudiants en pharmacie à la mise en place d’un code de bonnes pratiques pharmaceutiques en ligne (CBPPL).
Méthodologie :
Il s’agit d’une étude descriptive transversale. Dans le cadre du cours « Le pharmacien et la loi » (donné en 1ère année du Pharm.D. dans une Faculté de pharmacie), les étudiants étaient exposés à un article synthèse sur le sujet, puis ils étaient invités à signer un CBPPL. Quatre semaines après la signature du code, les étudiants pouvaient répondre à sept questions relatives à leurs perceptions sur le sujet. Une échelle Likert et une échelle dichotomique (oui/non) ont été utilisées selon les questions.
Résultats :
Au total, 198 étudiants (taux de réponse : 100%) ont signé le CBPPL en août 2015 et 197 étudiants ont répondu aux questions quatre semaine plus tard. Dans l’ensemble, 99% étaient en accord avec le fait que la lecture de l’article synthèse les a sensibilisés aux risques des comportements en ligne en exerçant la pharmacie. La majorité (94%) était d’avis que cet article les a exposés aux opportunités des médias sociaux et autres outils en ligne en pharmacie. Un total de 96% a confirmé que la lecture du CBPPL les a fait réfléchir et a remis en question certaines pratiques. Un total de 73% ont modifié certains paramètres d’accès de leurs comptes en ligne et 28% ont abandonné certaines plates-formes. De plus, 89% se sont dits intéressés à un atelier structuré sur l’utilisation responsable des médias sociaux.
Conclusion :
La signature d’un code de CBPPL est faisable et contribue à la sensibilisation et à des changements de comportements d’étudiants en pharmacie de 1er cycle.
Chambre des erreurs : une simulation afin de sensibiliser le personnel soignant aux risques du circuit du médicament
J Daupin1, V Pelchat2, S Atkinson1, JF Bussières1,3
1Unité de recherche en pratique pharmaceutique, Département de pharmacie, CHU Sainte-Justine, Montréal, QC
2Direction des soins infirmiers, CHU Sainte-Justine, Montréal, QC
3Faculté de pharmacie, Université de Montréal, Montréal, QC
ABSTRACT
Contexte :
Agrément Canada détermine ses exigences en ce qui concerne le circuit du médicament à partir de la norme sur la gestion des médicaments.
Objectifs :
Évaluer la capacité du personnel soignant à identifier des erreurs reliées au circuit du médicament dans le cadre d’une simulation et évaluer la satisfaction du personnel exposé à cette activité.
Méthodologie :
Il s’agit d’une étude descriptive transversale. En préparation à la visite d’agrément, nous avons scénarisé 30 vignettes relatives au circuit du médicament de la prescription à l’administration de doses de médicaments. Ving-quatre erreurs (pratiques non conformes) étaient dissimulées. Un aménagement représentant une pharmacie d’étage et une chambre de patient a permis de scénariser de façon réaliste le circuit (p.ex. lit, comptoirs, pompe, ordonnances, étiquettes, seringues, chariots). Des plages horaires de jour-soir-nuit ont été offertes avec publicité afin d’inciter la participation. Chaque participant avait en main une grille afin d’indiquer la présence ou l’absence d’erreur par vignette ainsi qu’un questionnaire de satisfaction (13 questions).
Résultats :
Au total, 175 personnes (moyenne 12,1±10,5 années d’expérience) se sont présentées à l’activité (70,4%-infirmières, 8,3%-pharmaciens, 6,5%-médecins/résidents, 4,7%-assistant-technique, 10,7%-autres) durant 9 plages horaires pour 75 heures. Le taux moyen d’exactitude des participants était de 65,9%±13,1%. Sept vignettes comportaient un taux d’exactitude <50% pour les thèmes suivants : seringue, lavage des mains, identification du patient, port de gants, relevé de température, abréviation et incompatibilité. Parmi les énoncés, les participants ont considété la simulation comme étant très pertinente (96%), très efficace (97%) et lui accordait en moyenne une note de 8,9±1,2. Une majorité de répondants (84%) envisageait d’apporter des changements à sa pratique.
Conclusion :
Cette étude démontre la pertinence et l’efficacité d’une simulation de type « chambre des erreurs » afin de sensibiliser le personnel d’un établissement de santé aux risques du circuit du médicament.
Evaluation of the Sterility of Single-Use Vials Undergoing Multiple Access Following Application of a Closed System Transfer Device
W Perks, H Carating, J Iazzetta, LF Charbonneau, SE Walker
Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON
Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
Closed system transfer devices (CSTD) are designed for safe handling of hazardous drugs from preparation to administration. According to NIOSH, these devices are airtight and leak-proof. While these devices protect staff, as a closed system they could also minimize microbial contamination.
Objective:
To test whether attaching a CSTD (Equashield®) to single-use vials can minimize microbial contamination and extend the “use-by” date following multiple withdrawals under extreme-use-conditions.
Methods:
An Equashield® vial adapter was attached to three 20 mL vials (A, B, C) containing sterile TSB growth medium and placed in each of 6 biological safety cabinets weekly for 16 weeks. Vial A (control) had no medium removed during the week. One mL of medium was removed once daily x5 days from vial B, and twice daily x5 days from vial C. At day 5, vials were collected, incubated at 37°C for 14 days and inspected visually every 2 days for microbial growth. As a positive control, TSB vials were inoculated with less than 102 of S. epidermidis ATC 12228. As a negative control, an unopened vial of TSB was incubated for the duration of the study.
Results:
All positive control vials demonstrated growth within 48 hours. All negative control vials showed no growth throughout the study. During the 16-week study all accessed vials remained sterile following storage at room temperature for 5 days and subsequent incubation for 14 days. None of the 192 vials accessed 1440 times or the 96 vials that had the CSTD attached but had no broth removed demonstrated contamination. The 95% confidence interval of the contamination rate is 0.000 to 0.035%.
Conclusions:
Attachment of a CSTD adapter to single-use vials within an ISO-5 environment has the ability to maintain sterility following multiple withdrawals during 5 days and stored in worse than ISO-5 conditions.
Evaluation of a Critical Incident: Simulating Hydromorphone Concentrations Using Population-Based Pharmacokinetic Parameters
H Zheng2, L Wong2, C Bailey3, J Sawyer4, T Wu4, L Zhou4, M Van Der Vyver4, S Belo4, SE Walker1,2
1Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
3Quality & Patient Safety, Sunnybrook Health Sciences Centre, Toronto, ON
4Acute Pain Service, Sunnybrook Health Sciences Centre, Toronto, ON
ABSTRACT
Background:
Opioids are commonly used for pain management in a postoperative setting but use can be associated with life-threatening respiratory depression. Naloxone administration is used to rescue patients suffering from respiratory depression. An unexpected death of a patient after the hydromorphone dose was increased triggered an investigation into the safety of opioids in pain management.
Objective:
To determine if simulated hydromorphone concentrations, using population based mean pharmacokinetic parameters, are predictive of respiratory depression and naloxone use.
Methods:
A literature search was conducted to determine the weighted mean of pharmacokinetic variables (volume, half-life, Tmax and bioavailability) for three hydromorphone formulations (IV, oral immediate release, oral controlled release) described by one compartment model. The pharmacokinetic variables were used to simulate the concentration-time profile administration of each dose of hydromorphone to the patient to create a total hydromorphone concentration-time profile for all doses for patients administered naloxone (cases) and those not administered naloxone (controls). Cases and controls were identified prospectively between October 2014 and June 2015 and were compared for demographics (age, gender), highest hydromorphone concentration and relationship to highest concentration.
Results:
Three naloxone cases were identified over a 9-month period, indicating a rate of less than 0.5%. Nine controls were identified. The average highest simulated concentration of hydromorphone in controls was 46±17ng/mL, which is not significantly (p=0.22) higher than the 31±13ng/mL simulated for naloxone cases. In one of the naloxone cases, a simulated concentration twice that of the concentration simulated when naloxone was administered occurred 48 hours prior.
Conclusion:
Administration of naloxone for opioid induced respiratory depression is a rare event that cannot be predicted by hydromorphone concentration. The use of naloxone in patients receiving opioids is a medication safety event that should be tracked ideally through an electronic Medication Administration Record.
Rhabdomyolysis during Treatment with Thrice-Weekly Daptomycin
Y Cheng1, N Wilcox1, GW Thompson1,2, P Dool1
1London Health Sciences Centre, London, ON
2St. Joseph’s Healthcare, London, ON
ABSTRACT
Background:
Daptomycin is an antimicrobial effective against multi-drug resistant gram-positive organisms. We report a case of rhabdomyolysis in a patient treated with daptomycin for vancomycin-resistant enterococci (VRE).
Case Description:
An adult male presented with tachycardia, worsening fever and neutropenia. Computerized tomography scans revealed right hip osteomyelitis and a soft tissue abscess. Due to growth of VRE from a prior right hip intraoperative sample, daptomycin 6mg/kg IV was initiated on August 26, 2015 for administration post-dialysis thrice weekly. Baseline creatine kinase (CK) was 14U/L. Daptomycin was well-tolerated until September 5 when he complained of left upper limb myalgia. Over the next 3 days, pain worsened and was accompanied by considerable inflammation, erythema, and decreased hand sensation. Deep vein thrombosis and compartment syndrome were ruled out. On September 9, his CK level was 33,540U/L, and pain had spread to his right side. At that point, daptomycin was discontinued and an additional round of hemodialysis was administered. CK levels subsequently improved within the next week.
Assessment of Causality:
The proposed mechanism for this reaction may involve the leakage of CK from myocytes. There was a temporal relationship between daptomycin initiation and the observed reaction. A Naranjo score of 7 suggested a probable adverse drug event.
Literature Review:
While up to 7% of patients in phase III clinical trials experienced CK elevation on daptomycin, few cases of rhabdomyolysis have been described in the literature. To our knowledge, this is the first case of rhabdomyolysis reported in a dialysis patient receiving thrice weekly daptomycin.
Importance to Practitioners:
Pharmacists should be aware of this infrequent yet serious adverse event associated with daptomycin. At a minimum, CK should be monitored at baseline and then once weekly. More frequent monitoring is warranted in those patients who experience myalgia or weakness, CK elevation >1000U/L, or deteriorating renal function.
Clinical and Economic Outcomes of Outpatient Parenteral Antimicrobial Therapy: Experience at a Community Hospital
A Lee, S John, R Lovinsky
The Scarborough Hospital, Toronto, ON
ABSTRACT
Background:
Outpatient parenteral antimicrobial therapy (OPAT) facilitates the administration of intravenous antimicrobials to clinically stable patients in the home. OPAT is defined as the intravenous, intramuscular, or subcutaneous administration of at least 2 doses of an antimicrobial agent on different days without intervening hospitalization. An OPAT program was developed by the Scarborough Hospital’s (TSH) Antimicrobial Stewardship team, as an extension of their inpatient stewardship activities.
Objectives:
To describe the clinical outcomes of patients enrolled into an OPAT program at a community hospital and to determine the impact of OPAT on the duration of hospitalization and health care costs from the perspective of both the hospital and the Ministry of Health (MoH).
Methods:
The medical records of 151 patients who received 183 courses of antimicrobial therapy through the Scarborough Hospital’s OPAT program (Toronto, Canada) were reviewed to collect patient demographic information, and to determine the indication for parenteral antimicrobial therapy, therapy outcomes, and the occurrence of complications. Parenteral antimicrobial regimens and duration of therapy were obtained from Bayshore Specialty Rx Health prescription records.
Results:
Osteomyelitis/septic arthritis (32%), soft skin and tissue infections (22%), and urinary tract infections (14%) were the most common types of infections treated. The most commonly used antimicrobial was cefazolin (38%). 86% of patients completed therapy as planned. 78% of patients experienced clinical cure, 10% experienced control or improvement in their infection while 12% had clinical failure. OPAT saved a total of 3,586 hospital bed days, with an average of 20 days saved per patient. Antimicrobial cost reduction from the hospital’s perspective was $47,455 while the net cost avoidance of the OPAT program from the MOH’s perspective was $3,073,050.
Conclusion:
Results from this study support the safety and effectiveness of OPAT for patients. OPAT also substantially reduces the duration of hospitalization and health care costs.
Varicella Zoster Virus Meningitis Possibly Associated with Zoster Vaccination
S Li, AM Bombassaro, E Spicer, K Gupta
London Health Sciences Centre, London, ON
ABSTRACT
Background:
The zoster vaccine is recommended in adults 50 years and older for prevention of shingles. It has been shown to reduce disease burden and postherpetic neuralgia.
Case description:
An immunocompetent adult developed a pounding headache and burning sensation over the scalp on day 1 after receiving the zoster vaccine. The patient presented to hospital on day 6 with aphthous tongue ulcers, an occipital scalp rash and nuchal rigidity. Forty-eight hours later, audiovisual hallucinations and a vesicular eruption on the anterior neck were noted. Cerebrospinal fluid (CSF) analysis revealed elevated protein and nucleated cell count (lymphocytic predominance) and normal glucose. Polymerase chain reaction of CSF was positive for varicella zoster virus (VZV), and negative for herpes simplex, enterovirus and bacteria. The patient responded to acyclovir 10mg/kg IV q8h for 48 hours and was discharged on oral acyclovir.
Assessment of Causality:
The presence of a vesicular rash and central nervous signs and symptoms, including VZV in the CSF, temporally following vaccination with no other identifiable medical causes suggests an association. The lack of strain typing (wild-type or vaccine), in addition to the absence of published case reports of meningitis post-vaccination due to the vaccine-strain, suggests a “possible” relationship according to the Naranjo scale.
Literature Review:
A case report described a 79-year-old presenting with varicella meningitis 6 hours post-zoster vaccination, confirmed to be due to the wild-type strain. Post-marketing surveillance of a large adult cohort identified 4 cases of meningitis, encephalitis and encephalopathy occurring within 1–14 days post-zoster vaccination, however, strain relatedness was not specified.
Importance to Practitioners:
The benefit of the varicella zoster vaccine has been reported in large well-designed randomized controlled trials without evidence of excess harm. Ongoing post-marketing surveillance will assist in determining if the vaccine, in rare cases, may cause primary infection or reactivation of latent VZV.
Compliance to a Febrile Neutropenia Protocol for Leukemia Patients Based on Antimicrobial Stewardship Principles and Human Factors Engineering
M So1,2, B Yeats1, S Lu2, C Bell1,3,4, A Morris1,3,4, S Husain1,3,4
1University Health Network, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, ON
3Mount Sinai Hospital, Toronto, ON
4Department of Medicine, University of Toronto, ON
ABSTRACT
Background:
We implemented a locally developed febrile neutropenia protocol (FNP) for leukemia patients applying principles of antimicrobial stewardship and human factors engineering (HFE). The World Health Organization and Agency for HealthCare Research and Quality have recommended HFE as a tool to improve patient safety through workflow optimization. Clinicians can self-navigate to relevant sections of the FNP at point of clinical decision through embedded hyperlinks. The protocol was introduced in Sep 2013, updated and re-launched in Sep 2014.
Objective:
To determine the uptake of a human factor-engineered, stewardship-based febrile neutropenia protocol amongst prescribers.
Methods:
We conducted prospective point-prevalent surveys (audits) on 4 randomly selected dates between October 2014 and June 2015. All leukemia in-patients were included. Compliance checkpoints were antifungal and antiviral prophylaxis; surveillance serum galactomannan (GM); empiric antibiotics with piperacillin-tazobactam and tobramycin (P+T) at onset of febrile neutropenia; and de-escalation of empiric tobramycin after 72 hours in patients without focus of infection. Fisher’s exact test or Chi square test were used to compare compliance, depending on sample size; significance level p<0.05.
Results:
Audit dates were October 24, 2014, March 12, May 8 and June 22, 2015. A total of 147 charts were reviewed. Between October and March, antiviral prophylaxis increased from 8.7% (2/23) to 68.2% (15/22) (p<0.05), and antifungal prophylaxis increased from 55% (12/22) to 95% (18/19) (p<0.05). Surveillance serum GM increased from 26% (7/27) to 70% (18/26) in eligible patients (p<0.05). Compliance to those recommendations was maintained subsequently. Empiric P+T and de-escalation did not change significantly over the 4 audits, with compliance ranging between 40–53%, and 55–75%, respectively (p=0.83 for both).
Conclusions:
A locally developed guideline designed with HFE and antimicrobial stewardship principles increased the compliance of prophylaxis and surveillance GM. Audit findings will inform the ASP team to target knowledge translation strategies on appropriate empiric regimen and de-escalation.
Once Daily Aminoglycoside Pharmacokinetics and Optimal Dosing in the Burn Population: A Prospective Study
C Lee1,2, SAN Walker1,2,3,4, SE Walker1,2, W Seto2,5, S Simor3,4, R Cartotto6, M Jeschke6
1Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
3Division of Infectious Diseases, Sunnybrook Health Sciences Centre, Toronto, ON
4Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, ON
5Sick Kids Hospital, Toronto, ON
6Ross Tilley Burn Centre, Sunnybrook Health Sciences Centre, Toronto, ON
ABSTRACT
Background:
Once-daily aminoglycoside dosing (ODA) is used in most patient populations to optimize antibacterial activity and reduce toxicity. Unfortunately, burn patients are excluded from ODA due to concerns over altered pharmacokinetics resulting in a shortened half-life and low peak aminoglycoside concentrations. Retrospective studies suggest that ODA may be appropriate if higher milligram/kilogram doses are used. However, no prospective clinical trials in burn patients exist to confirm these findings.
Objective:
To determine the adequacy of once daily tobramycin dosed at 10 mg/kg in adult burn patients.
Methods:
This prospective single dose pharmacokinetic clinical trial was conducted at the Ross Tilley Burn Centre. Patients with a total burn surface area (TBSA) of < 20% and creatinine clearance > 50 ml/min were eligible. A first-order one compartment model was used to determine the pharmacokinetic profile from 3 or 5 tobramycin levels over a 24 hour period per patient. Monte Carlo simulation (MCS) was performed to determine the probability of target level attainment.
Results:
The mean percent TBSA, partial, and full thickness burn were 10%, 7%, and 4%, respectively. Seven of the eight patients recruited achieved peak concentrations of > 20 mg/L (mean of 28.9 ± 6.3 mg/L) and all patients had a trough level < 0.5 mg/L. The mean half-life, volume of distribution, and clearance were 2.40 hours, 0.33 L/kg, and 7.31 L/hour, respectively. The MCS determined probability of attaining target peak concentrations with the 10 mg/kg dose was 93%, which almost doubled that predicted with the usual 7 mg/kg dose.
Conclusion:
Burn patients with adequate renal function and < 20% TBSA are candidates for ODA. Tobramycin half-life was similar to healthy, non-burn patients. The larger than normal volume of distribution supports the use of the higher empiric dose of 10 mg/kg with further dose adjustment based on therapeutic drug monitoring.
Multicenter Study of Environmental Contamination with Antineoplastic Drugs in 47 Canadian Hospitals
C Poupeau1, C Tanguay1, NJ Caron2, JF Bussières1,3
1Pharmacy Practice Research Unit, CHU Sainte-Justine, Montréal, QC
2Centre de toxicologie du Québec, Institut national de santé publique du Québec, Québec, QC
3Faculty of Pharmacy, Université de Montréal, Montréal, QC
ABSTRACT
Context:
Oncology workers are occupationally exposed to antineoplastic drugs. This exposition can lead to health risks. In order to reduce their exposure, contamination on surfaces should be kept as low as possible.
Objectives:
To describe a multicenter environmental monitoring study of cyclophosphamide, ifosfamide and methotrexate in oncology pharmacy and patient care areas in Canada.
Methods:
This is a descriptive and comparative study. Hospitals of more than 50 beds were invited to participate. Twelve standardized sites were sampled in each participating center (6 in the pharmacy and 6 in patient care areas). Samples were analyzed for the presence of cyclophosphamide, ifosfamide and methotrexate by ultra-performance liquid chromatography tandem mass spectrometry technology. The limit of detection (LOD) was 0.36 pg/cm2 for cyclophosphamide, 0.95 pg/cm2 for ifosfamide and 0.97 pg/cm2 for methotrexate. Descriptive statistical analyses (75th percentile) were carried out.
Results:
In 2015, 47 hospitals from Canada participated in this study (participation rate 47/202, 23%). A total of 513 samples were quantified. Overall, 35% (177/513) of the samples were positive for cyclophosphamide, 8% (39/513) of the samples were positive for ifosfamide and 6% (31/517) of the samples were positive for methotrexate. The 75th percentile value of cyclophosphamide surface concentration was 6.7 pg/cm2. The 75th percentiles for ifosfamide and methotrexate concentrations were lower than the LOD. The most frequently contaminated sites were the front grille of the hood (64%, 30/47), the floor in front of the hood (62%, 29/47) and the armrest (61%, 25/41).
Conclusion:
In comparison with other multicenter studies that were conducted in Canada, the concentration of antineoplastic drugs measured on surfaces is decreasing. However, some sites are still frequently contaminated. Regular environmental monitoring is a good practice in order to maintain contamination as low as reasonably achievable. It is also an opportunity to increase workers’ awareness on this issue.
Investigation of the Face and Content Validity, and Perceived Usefulness of the Pharmacy Residency Competency Based Assessment Tools
H Halapy
St. Michael’s Hospital, Toronto, ON
Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
In 2011 to 2012, a hospital residency program developed and implemented, in collaboration with other provincial residency programs, competency based assessment tools for all its general pharmacy residency pharmacy residency rotations in response to changes to the Canadian Pharmacy Residency Board (CPRB) 2010 residency standards. At the time of development of the residency assessment tools, no validated and studied competency based pharmacy residency assessment tools existed.
Description:
The residency program developed the assessment tools, carefully selecting competency domains based on the 2010 CPRB residency standards. Education principles based on the Structure of the Observed Learning Outcome taxonomy served as the foundation for the assessment scale in the form a 5 point Likert scale.
Action:
Recently, the residency program sought to explore opinions regarding the face and content validity, the ease of use and practicality regarding the pharmacy residency assessment tools. Residency rotation preceptor focus groups were conducted at one institution by a residency coordinator utilizing a semi-structured interview guide. Unabridged transcripts were analyzed by another residency coordinator by manually forming coded units. Coded units were summarized using inductive thematic analysis.
Evaluation:
Six residency preceptors volunteered for focus group participation (5 females, 13.5 years of average work experience, age range of 30–50 years). Thematic analysis revealed three major categories: the assessment tools were considered 1. easy to use overall; 2. to have face validity; and 3. to have content validity. Regarding practicality, preceptors indicated an average completion time of 60 to 90 minutes, while a 30 minute completion time was considered reasonable.
Implications:
Preceptors indicated that the pharmacy residency assessment tools were valid and were overall easy to use. But completion time for the tools could be shortened. Improving preceptor training and tool formatting could help with addressing the short-comings of the tools and ultimately improve resident assessment.
Development and Validation of a Screening Tool for Bacteremia in Acute Burn Injury Patients
A Cooper1, SAN Walker1,2,3,4, M Elligsen1, J Lo1, C Lee1,2, SE Walker1,2, L Palmay1, R Cartotto5, M Jeschke5
1Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
3Division of Infectious Diseases, Sunnybrook Health Sciences Centre, Toronto, ON
4Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, ON
5Ross Tilley Burn Centre, Sunnybrook Health Sciences Centre, Toronto, ON
ABSTRACT
Background:
Diagnostic criteria for infection and Systemic Inflammatory Response Syndrome are not discriminatory in identifying burn patients at risk for bacteremia.
Objective:
To develop and validate a screening tool using common laboratory, and clinical parameters to assist in the early identification of bacteremia following acute burn injury (within 10 days post burn).
Methods:
A retrospective chart review of matched patients admitted to the Ross Tilley Burn Centre from March 12th, 2011 - January 11th, 2015, and prospective assessment of patients admitted from January 12th, 2015 - April 30th, 2015 were completed. A Pearson’s Correlation matrix was completed to identify independent variables associated with infection (p<0.05, univariate analysis). Significant parameters were analyzed using iterative binary logistic regression to identify the simplest significant model (p<0.05). The optimal probability cut-off for bacteremia was determined using a Receiver Operating Characteristic curve. The predictive value of the model was assessed and then validated on a separate historic burn population.
Results:
Thirty-seven of 156 patients with a complete data set were bacteremic (24%). The best binary logistic regression model was (p<0.05): Ln [odds of bacteremia] = −96.749+3.230(Platelet Volatility)+2.235(MaxTemperature (°C))+0.339(%FullBurn)+0.242 (%PartialBurn)+ 0.045(MaxHeartRate (bpm)) with a threshold probability categorizing bacteremia of >48%. The sensitivity, specificity, accuracy, false positive rate (FPR) and false negative rate (FNR) were 89%, 98%, 96%, 2% and 11%, respectively. At the study bacteremia prevalence of 24%, the positive predictive value, negative predictive value, positive and negative likelihood ratio, and positive and negative post-test probability were 94%, 97%, 53, 0.11, 94% and 3%, respectively. The sensitivity, specificity, accuracy, FPR, and FNR of the tool in the validation cohort (n=26) was 100%, 100%, 100%, 0% and 0%, respectively.
Conclusion:
The developed and validated bacteremia screening tool in acute burn injury patients has excellent predictive ability to assist in identifying patients for whom blood cultures should be requested.
A Study to Determine the Pharmacological Management of Delirium in the Cardiovascular Intensive Care Unit
L Tsang, O Ng, N Adhikari
Sunnybrook Health Sciences Centre, Toronto, ON
ABSTRACT
Background:
Delirium is a recognized post-operative adverse event whose significance has intensified due to effects on morbidity and mortality. Currently, the rate of delirium post cardiac surgery in the cardiovascular intensive care unit (CVICU) at our institution is unknown and the pharmacological treatment modalities are not standardized and vary greatly.
Objective:
To determine the rate of delirium, the presence of risk factors that may predict the development of delirium and pharmacological treatment modalities used in post-operative cardiovascular surgery patients admitted to the CVICU.
Methods:
In this retrospective chart review, all patients undergoing cardiovascular surgery from April 1 to December 31, 2014 were reviewed. Differences between patients were analyzed using Chi-square analysis.
Results:
The review included 420 patients of which 17% (n=73) developed delirium post-cardiovascular surgery in the CVICU. No significant differences were found between groups with respect to pre-operative co-morbidities and the development of delirium. Of those who became delirious, 83.6% (n=61) received a pharmacological agent for the treatment of delirium, with 52.5% (n=32) receiving 1 medication, 31.1% (n=19) receiving 2 and 16.4% (n=10) receiving 3 or more medications. Haloperidol was most commonly used (82%) followed by quetiapine (60.7%) and dexmedetomidine (13.1%). Those who experienced delirium had significantly greater exposure to sedatives including midazolam (57.5 vs 17.3%, p<0.00001), lorazepam (26 vs 1.4%, p<0.00001) and zopiclone (26 vs 6%, p<0.00001). No differences in mortality were found however length of CVICU stay was significantly longer in delirious patients (5.7 vs 1.8 days, p<0.00001).
Conclusions:
Delirium can commonly develop post cardiovascular surgery in the CVICU and it is difficult to predict who will develop it based on preoperative comorbidities. Therefore, the development of strategies to prevent delirium and to optimize pharmacological management is warranted.
Design and Implementation of Pharmacy Services for the Toronto 2015 Pan Am and Parapan Am Games
L Tsang1, R Ojo2
1Sunnybrook Health Sciences Centre, Toronto, ON
2Corporate Pharmacy, Toronto, ON
ABSTRACT
Background:
The Toronto 2015 Pan American and Parapan American games were the largest multi-sport games ever held in Canada. The goal was to design and implement comprehensive pharmacy services for 10 000 athletes and officials and to support an inter-professional medical team of 2000 volunteers.
Description:
Pharmacy services were primarily based in the athletes’ village medical polyclinic. An accredited community pharmacy was opened for outpatient prescriptions and a unit-dose medication system was implemented to support the polyclinic emergency department. Medication kits were also created for use at 5 satellite accommodation sites for athletes staying outside of Toronto and 47 competition venues. Pharmacist services were provided onsite at the polyclinic and via telephone to satellites and venues.
Action:
A comprehensive medication formulary was developed to standardize prescribing and usage. Medication decisions were made in coordination with the chief medical officer and sport specific physicians. An accompanying pharmacy guide was created outlining drug name, dosing, adverse effects, therapeutic use and compliance to World Anti-Doping Agency (WADA) guidelines. This guide encouraged appropriate prescribing and helped educate all practitioners on sport pharmacy. Policies were also developed for the handling of WADA prohibited medications to avoid inadvertent prescribing and dispensing. Partnerships were formed with the Ontario College of Pharmacists and the Ministry of Health to ensure pharmacy accreditation and opening. Finally, 50 pharmacy volunteers were recruited to carry out daily operations.
Evaluation:
Following the Pan Am and Parapan Am games, statistics were collected. In total, 1953 prescriptions were dispensed for 1189 patients. Top usage nations included Brazil (290 prescriptions), Canada (276), Venezuela (170). Most commonly dispensed drug classes were analgesics & anti-inflammatories (658), antibiotics (233) and dermatological (182).
Implications:
This project created a model for comprehensive pharmacy services that can be used for future large, multi-sport games.
Ciprofloxacin-Induced Stevens-Johnson Syndrome Treated with Cyclosporine: A Case Report
J AuYeung, M Lee
North York General Hospital, Toronto, ON
ABSTRACT
Background:
Stevens-Johnson Syndrome (SJS) is an idiosyncratic reaction involving skin sloughing traditionally treated with systemic corticosteroids. We describe a probable case of ciprofloxacin-induced SJS treated with cyclosporine and corticosteroid.
Case Description:
A 66-year-old Caucasian female presented with a rash on her trunk, neck and extremities. She had a fever, progressive dysphagia, odynophagia, dysuria and painful ulcerations to her genitalia. Her past medical history included chronic obstructive pulmonary disease, osteoporosis, osteoarthritis, stress urinary incontinence and migraines. She reported a history of rash to sulfa drugs and azithromycin. Oral ciprofloxacin was initiated 8 days prior, following a urological procedure. Laboratory results were unremarkable. Her Nikolsky’s sign was positive. A skin biopsy confirmed the diagnosis. Her symptoms got worse despite initial treatment with methylprednisolone. Five days later, intravenous cyclosporine 3 mg/kg daily was added. Her skin improved dramatically in the first few weeks of cyclosporine, but her dysphagia was slower to improve. She was eventually switched to oral cyclosporine and prednisone to complete her therapy. She did not experience adverse effects from cyclosporine but developed hiccups from corticosteroid therapy.
Assessment of Causality:
The Naranjo score of 6 indicates a probable adverse drug reaction. The exact mechanism is unknown but is thought to be a cytotoxic reaction to drug antigens resulting in keratinocyte apoptosis. CD8+ T cells, and the cytolytic molecules FasL and granulysin are thought to be involved.
Literature Review:
Twelve case reports involving ciprofloxacin-induced SJS or toxic epidermal necrolysis were found. Two observational studies found statistically significant reduction in time to re-epithelialization with cyclosporine compared to a historical control group using usual care. Only enteral cyclosporine has been reported in the literature.
Importance to Practitioners:
This case adds to the literature of ciprofloxacin-induced SJS. It demonstrates that intravenous or oral cyclosporine can be considered if a patient is not responding to corticosteroids.
Enjeux relatifs à I’exercice de la pharmacie : perception des résidents en pharmacie
A Guérin1, JF Bussières1,2
1Unité de recherche en pratique pharmaceutique, Département de pharmacie, CHU Sainte-Justine, Montréal, QC
2Faculté de pharmacie, Université de Montréal, Montréal QC
ABSTRACT
Contexte :
L’exercice de la pharmacie est confronté à de nombreux changements. Les résidents en pharmacie doivent se préparer à ces changements dans le cadre de leur formation de 2ème cycle.
Objectifs :
L’objectif principal était d’évaluer la perception des résidents en pharmacie face à 48 enjeux relatifs à l’exercice de la pharmacie.
Methodologie :
II s’agit d’une étude descriptive transversale. L’étude ciblait tous les résidents en pharmacie de la cohorte 2015–2016 inscrit au programme de maîtrise en pharmacothérapie avancées de deux faculté de pharmacie. Nous avons recueilli des données démographiques et le niveau d’accord (échelle de Likert à 4 choix – très en accord, partiellement en accord, partiellement en désaccord, totalement en désaccord) de probabilité d’occurrence d’ici 2025 de 48 pratiques émergentes et vraisemblables reliées à l’exercice de la pharmacie.
Résultats :
Un total de 91% (57/63) des résidents ont répondu à I’enquête. Un total de 60% (34/57) étaient des femmes et 97% (55/57) se destinaient à la pratique hospitalière. Plus de 80% des répondants étaient d’avis que 24 des 48 pratiques émergentes évoquées seraient implantées pour dans la majorité des cas d’ici 2025 (p.ex. distribution des médicaments sans pharmaciens en hôpital, majorité du temps du pharmacien au chevet des patients). Pour autant de pratiques émergentes, une majorité des répondants pensaient que leur réalisation était peu probable ou trés peu probable (p.ex. poursuites judiciaires de pharmaciens hospitaliers, départements de pharmacie gérés par des non pharmaciens).
Conclusion:
Des résidents en pharmacie hospitalière canadiens ont émis leur opinion face à 48 enjeux relatifs à l’exercice de la pharmacie d’ici 2025. II est important de tenir compte de 1’opinion des futurs résidents en pharmacie et de les sensibiliser à 1’évolution de leur profession.
Stability of 1.0 and 2.5 mg/mL Bortezomib Solution in Vials and Syringes Following Reconstitution with 0.9% Sodium Chloride at 4°C and Room Temperature (23°C)
SE Walker, LF Charbonneau, I Tyono, S Law
Department of Pharmacy Sunnybrook Health Sciences Centre, Toronto, ON
Leslie Dan Faculty of Pharmacy University of Toronto, Toronto, ON
ABSTRACT
Background:
Previous publications have demonstrated the stability of l.0mg/mL and 2.5mg/mL of bortezomib for 42 days and 21 days respectively. The introduction of a generic version of bortezomib raised questions of the stability of the generic formulation and the validity of extending stability from one brand to another.
Objective:
To evaluate the stability of bortezomib 3.5 mg vials reconstituted with 3.5 or 1.4 mL of 0.9% sodium chloride (NS) during storage over 42 days at room temperature and at 40C in syringes and manufacturer vials.
Methods:
On study day 0, 2.5mg/mL and l.0mg/mL concentrations of the TEVA formulation were prepared. 3 units of each container were stored at room temperature and 3 were stored in the refrigerator. Concentration and physical inspection were completed on study days 0, 1, 3, 7, 10, 14, 22, 28, 34, and 42. Bortezomib concentrations were determined by a validated stability-indicating liquid chromatographic method with UV detection. The recommended beyond-use-date was determined based on the intersection of the lower limit of the 95% confidence interval of the observed degradation rate and the time to achieve 90% of the initial concentration.
Results:
The analytical method separated degradation products from bortezomib such that the concentration was measured specifically, accurately (deviations from known averaged 2.5%) and reproducibly (replicate error was less than 1% (CV(%)). During the study period all solutions retained more than 95% of the initial concentration in vials and syringes at both temperatures and concentrations. The calculated beyond-use-date exceeded 42 days for all temperatures, concentrations and container combinations.
Conclusions:
We conclude that 3.5-mg vials of TEVA bortezomib reconstituted with 3.5 mL or 1.4 mL of NS (concentrations of 1.0 and 2.5mg/mL) are physically and chemically stable for at least 42 days at 4°C or room temperature (23°C) in both syringes and the original manufacturer’s glass vials.
Bridging the Gap from the Classroom to the Institutional Practice Site: Evaluation of an Online Transition Module for Pharmacy Students
P Tchen, L Leung, M Williamson, F Simpson, A Kim-Sing, M Pearson
Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC
ABSTRACT
Background:
In recent years, feedback from students and preceptors indicates that pharmacy students are ill-prepared for institutional practicums. They frequendy require a prolonged orientation period at the practice site and close clinical supervision, which negatively impacts their ability to achieve learning outcomes.
Description:
Literature suggests that medical students exhibit less anxiety and better clinical performance in institutional settings when supported with transitional learning activities, and we predicted the same holds true for pharmacy students. Accordingly, we designed and evaluated an online transitional learning module for pharmacy students to complete prior to their institutional practicum.
Action:
Focus groups with students, residents, faculty, and preceptors were conducted to determine desired content and learning outcomes. This input guided development of three modules: General Orientation to Institutional Practice, Introduction to Patient Charts, and Patient Workup and Report.
Evaluation:
The first module was evaluated by surveying focus group participants and student users of the module. Data on reactions to the module were collected through items rated on a 5-point Likert scale. Evaluation of the other two modules is ongoing. The module’s design was consistent with the learning needs identified in the focus groups. Responses from the student user survey (n=36) demonstrated a mean completion time of 2.20 hours (SD=1.83). Most agreed the first module was of appropriate difficulty (M=3.84, SD=0.74) and was logically organized (M=3.64, SD=0.78). Opinion was divided on appropriateness of length and contribution to improved understanding of institutional practice.
Implications:
While the transition modules were designed to meet learning needs identified by key stakeholders, evaluation data from the first module indicate mixed views of its learning value. Future iterations must address feedback regarding content and efficiency of delivery.
Comparison of Pharmacy Students’ and Pharmacists’ Activities Using a Clinical Pharmacist Workload Measurement Tool
J Chiu, M Lee
North York General Hospital, Toronto, ON
ABSTRACT
Background:
Pharmacy students in the final year of their program are required to complete 7 Advanced Pharmacy Practice Experience (APPE) rotations. Each rotation is 5 weeks in duration. Student’s activities during their direct patient care (DPC) in-patient rotations were analyzed and compared to clinical pharmacists’ activities in a community teaching hospital, to help understand how students contribute to patient care activities.
Description:
Pharmacy students completing a DPC APPE rotation were asked to document their activities using the clinical pharmacist workload measurement (WLM) tool. This is a Microsoft® Access database consisting of fields to record the volume and time spent on each type of clinical and non-clinical activity (excluding dispensing).
Action:
Data were collected between May 2014 and May 2015. Activities were classified into 4 main categories and the proportion of time spent on each was analyzed and compared to clinical pharmacists’ data for the same period.
Evaluation:
Twenty students completed 33 DPC rotations during this period. On average, students spent 23.9 days per rotation on site. Students documented 69.8% of their hours worked in the WLM database compared 63.2% of pharmacists. Table 1 shows the total time and proportion of time spent by students compared to pharmacists on various activities.
Table 1: Total time and proportion of time spent in activities
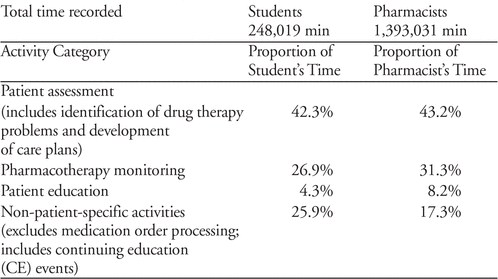
Implications:
Pharmacy students and pharmacists spent the majority of their time in patient assessment and pharmacotherapy monitoring. Students spent more time in non-patient specific activities such as CE events. Compliance with WLM entry into database was similar in both groups. This tool helped gain insight into where students spent their time during the rotation. Future research should aim at finding opportunities in pharmacist’s activities that students can contribute to.
Evaluation of Standardization of Pharmacist Attendance at Rounds
J Proceviat1, NF Dewhurst1,2, P Gillespie2, E Tom1, C Chant1,2
1St. Michael’s Hospital, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
Evidence supports active participation of pharmacists in medical rounds as an activity that improves patient outcomes. When numerous types of medical rounds are available on patient care units, prioritizing attendance at relevant rounds is necessary to ensure other clinical duties are fulfilled. A standardized policy was developed and implemented to prioritize round attendance and ensure balance with other pharmacist roles and responsibilities. Pharmacists were expected to attend “high priority” rounds regularly, dictated by round frequency, overall workload and staffing.
Description:
Following implementation of a standardized rounding policy for pharmacists, evaluation of policy adherence and rounds attendance was required.
Action:
Pharmacists were surveyed to determine adherence to policy, barriers to round attendance, and requests to attend other non-high priority rounds. Data was collected on type of rounds attended, average time spent in rounds and perceived value added to practice.
Evaluation:
Most, 27 of 29 (93%), of pharmacist respondents were able to adhere to the policy, attending the three “high priority” types of rounds outlined in the policy. Multidisciplinary, medical/bedside and bullet/ discharge rounds were attended by 61%, 26% and 23% of pharmacists, respectively. A small proportion of pharmacists attended more than one type of high priority rounds. Average time spent attending priority rounds is 30 to 70 minutes per weekday. A minority (24%) of pharmacists have been requested to attend other types of rounds including teaching, antimicrobial stewardship program (ASP) and interdisciplinary. Priority rounds are perceived to be of higher value than other round types, with the exception of ASP rounds. The main perceived barriers to pharmacist round attendance are scheduled evening shifts and heavy workload.
Implications:
Data suggests that pharmacists are adhering to the policy, and that it assists in standardizing pharmacist practice and attendance at high priority rounds.
Fatigue, Anxiety and Irritability with Apixaban
M Ramandt, P Dool
London Health Sciences Centre, London, ON
ABSTRACT
Background:
Apixaban is indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. The most commonly reported adverse drug reaction (ADR) is hemorrhage, with no noted reports of low energy or mood changes. We describe a case report of dose-related fatigue, anxiety and irritability associated with apixaban use.
Case Description:
A pleasant 76-year old male requiring anticoagulation due to atrial fibrillation was started on apixaban 5mg orally twice daily. Over the following 2 months, the patient and his wife began to notice a gradual decrease in his energy level, followed by significant anxiety and increased irritability. No bruising or bleeding was apparent. Self-adjustment of his dose to 2.5mg twice daily lead to a dramatic improvement in both the patient’s mood and energy level. These same symptoms quickly returned upon re-initiation of the higher dose. As a result, apixaban was discontinued and rivaroxaban was initiated.
Assessment of Causality:
Given the timing and improvement with dose reduction, it is likely that the reported symptoms are contributable to apixaban. The Naranjo causality scale indicates a definite adverse drug reaction with a score of 9. A theoretical drug interaction between diltiazem and apixaban, mediated through CYP3A4 and P-glycoprotein, may have occurred. However, an increased serum concentration of apixaban would be more likely to present as a hemorrhage rather than as a previously unreported ADR. Additionally, the patient reports no significant lifestyle changes that could have contributed to his alterations in mood.
Literature Review:
There have been no known case reports reporting studies of fatigue, anxiety or irritability with apixaban.
Importance to Practitioners:
Practitioners should be aware of the possibility that apixaban may produce fatigue, anxiety and irritability in some patients. This case report also highlights the importance of reporting adverse drug reactions for new drugs.
Evaluation of a Communities of Practice Program for Clinical Pharmacists
P Gillespie1, E Tom2, NF Dewhurst1,2, C Chant1,2
1Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
2St. Michael’s Hospital, Toronto, ON
ABSTRACT
Background:
A need for stronger intra-departmental relationships was identified given recent organizational changes and expanded scope of practice for pharmacists.
Description:
The Pharmacy CoP program was introduced in 2013. The goals of CoP are to share knowledge, facilitate collaboration and professional relationships, standardize professional practice approaches and create new knowledge through scholarly work.
Action:
Quantitative and qualitative data were used to evaluate the achievements and perceived value of the CoP program. Data was collected retrospectively from available CoP agendas and minutes since program inception until September 2015. Two surveys which included Likert scales were performed 17 months apart to evaluate pharmacists’ perception of the program in 4 domains: patient outcomes, educational value, professional relationships, and organizational format.
Evaluation:
Each CoP discussed 8 topics monthly, inviting 5 guest speakers per CoP group over the 17 month period. Distribution of topics varied between groups. Clinical practice discussion, practice advisory updates and medication management/goals and objectives accounted for 37%, 20% and 13% of topics, respectively. Most pharmacists attended at least 80% of meetings, with the majority (54%) of pharmacists agreeing that CoP has contributed towards improving patient outcomes. The majority (48%) of pharmacists agreed that they have gained valuable clinical pharmacy knowledge and skills through CoP. Most (45%) pharmacists agreed that CoP has helped them to 1) standardize practice with their peers and 2) share responsibility/workload amongst peers for activities related to pharmacist education and medication management. 54% of pharmacists agreed that the current meeting format was satisfactory and that CoPs are an effective forum for learning. Suggestions to format change include alternate timing and frequency of meetings.
Implications:
The CoP program is effective in expansion of knowledge and will continue in its current form. Each CoP chair will review and implement pharmacist suggested feedback to maximize attendance and perceived value.
A Canadian Survey of High-Dose Extended-Interval Gentamicin and Tobramycin in Pediatric Inpatients
C Strugari, C Gray, L Ruda, A Bell, J Bolt
Regina Qu’Appelle Health Region, Regina, SK
ABSTRACT
Background:
The use of high-dose extended-interval (HDEI) aminoglycosides (AMG), a common practice in adult populations, is less established for pediatric patients, where AMGs are often dosed utilizing a multiple daily dosing method.
Objectives:
The purpose of this project was to characterize AMG prescribing practices in pediatric inpatients across Canada. The objective was to determine current practice of Canadian health care delivery organizations regarding HDEI gentamicin and tobramycin for pediatric inpatients, including their criteria and indications for use, dosing and monitoring practices, and extent of pharmacist authority to independently dose or monitor AMG.
Methods:
This study was comprised of an electronic survey of pharmacists in Canadian health care delivery organizations providing pediatric inpatient services. Questions focused on demographics, criteria for HDEI tobramycin or gentamicin use in pediatric inpatients, empiric dosing, and monitoring parameters as well as extent of pharmacist authority to independently dose and monitor AMGs at their institution.
Results:
Of the 45 survey participants (48% response rate), 35 (78%) indicated their health region uses HDEI tobramycin or gentamicin in pediatric inpatients. The population characteristics for use of HDEI AMG were varied. Dosing recommendations included 10 to 15 mg/kg for pulmonary exacerbations in cystic fibrosis, 5 to 8 mg/kg in urinary tract infections, and 6 to 9 mg/kg in febrile neutropenia. 89% of participants monitor serum levels and 77% monitor for nephrotoxicity. For prescriptive authority, 15.6% (7/45) of participants are authorized to independently adjust dosing at their institution and 31.1% (14/45) are authorized to order monitoring parameters.
Conclusions:
HDEI AMG is frequently utilized for pediatric patients across Canada, although the dosages and monitoring practices varied greatly. The information will be useful for creating a HDEI AMG pediatric protocol for use in the local health region, as well as for cross-comparison of practice by other centres across Canada.
Strategies to Support Pharmacy Students’ Progress in Experiential Learning: A Literature Review
L Ferruccio1, D Cheng1,2, C Natsheh1,3
1Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto ON
2Department of Pharmacy, Mount Sinai Hospital, Toronto ON
3Department of Pharmacy, University Health Network, Toronto ON
ABSTRACT
Background:
The transition from formal classrooms to clinical placements can be challenging for healthcare students. When students encounter difficulty, supportive strategies tend to be drawn from the traditional didactic setting, but this may not address the additional competencies that are necessary for success in the clinical setting.
Description:
A literature review was conducted to determine which strategies are being used to support learners’ progress in experiential education at schools of pharmacy, nursing and medicine when students experience difficulty. The goal was to determine best practices and themes that can be applied to the pharmacy experiential education curriculum at our institution.
Action:
Searches of four databases (Embase, Medline, International Pharmaceutical Abstracts and SCOPUS) were conducted. References of identified articles were also examined. Articles included in the analysis addressed (1) the experiential education of nursing, pharmacy or medical learners, (2) methods of remediation used specifically for learners experiencing difficulty while on clinical placements, and (3) were written in the English language.
Evaluation:
Forty-seven articles were identified. Four were from pharmacy, 8 were from nursing and 35 were from medicine. Types of studies identified include a description of experiences at the authors’ institution(s) (31), surveys of program directors (6), case studies (4), review articles (3) and formal/informal recommendations (3). Common helpful strategies include student ownership, self-reflection, determining the root/underlying cause(s), deliberate practice for mastery of skills, involvement and support of preceptors/faculty, and using a well-constructed learning contract that contains a timeline, concrete end points and consequences of failure.
Implications:
A framework was identified to support students’ progress in experiential placements. The 4 main steps include assessment, planning, implementation and evaluation. For effective remediation, learning activities should be tailored to the specific competencies the student is struggling with, and the student must be engaged in each step.
Evaluation of Meropenem Usage Patterns in the Paediatric Intensive Care Unit and Cardiac Critical Care Unit
T Karnieg1,6, S Pong1,6, K Timberlake1,6, M Clarke5, M Science4,7, S Schwartz3,7, P Cox3,7, W Seto1,2,6
1Department of Pharmacy, The Hospital for Sick Children, Toronto, ON
2Child Health Evaluative Sciences, Research Institute, The Hospital for Sick Children, Toronto, ON
3Department of Critical Care Medicine, The Hospital for Sick Children, Toronto, ON
4Division of Infectious Diseases, Department of Paediatrics, The Hospital for Sick Children, Toronto, ON
5Infection Prevention and Control, The Hospital for Sick Children, Toronto, ON
6Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
7Faculty of Medicine, University of Toronto, Toronto, ON
ABSTRACT
Background:
Broad-spectrum antimicrobials use, such as meropenem, has been linked to increased rates of resistant organisms. The susceptibility of meropenem at our institution remained close to 100% for most isolates; however, there are concerns for increased resistance rates.
Objectives:
To describe meropenem use in patients admitted to the Paediatric Intensive Care Unit (PICU) and Cardiac Critical Care Unit (CCCU).
Methods:
A retrospective chart review was conducted to describe meropenum use over 12 months (January - December 2014) in patients who received meropenem for at least 72-hours during their stay. Research Electronic Data Capture (REDCap), Microsoft® Excel and R Project for Statistical Computing were used to collect and analyze data.
Results:
A total of 110 patient records were screened. Of these, 48 (43.6%) were included, 12.5% were bone marrow transplant patients, 12.5% were haematology/oncology patients, 18.8% were liver transplant patients, and 2.1% were lung transplant patients. The study revealed that 90.9% of courses received ID consultation; ID agreed with meropenem initiation in 93.8% of cases. The most common initial indication was empiric (58.2%), followed by microbiologically proven infection (41.8%). For indications after 72-hours of initiation, 50.9% remained empiric while microbiologically proven infections increased to 49.1%. Meropenem was continued past PICU/CCCU discharge in 34.6% courses. For 23.6% courses, meropenem was discontinued for resolution of indication or completion of duration. Meropenem was de-escalated to narrower-spectrum antibiotics for 20% courses. Patient death resulted in meropenem discontinuation in 10.9% courses. In the remaining 10.9% courses, meropenem was deemed no longer required due to repeated negative cultures or ruled-out infections. Mean duration of Meropenem was 9.7±6 days.
Conclusion:
Prolonged meropenem use was mainly in high-risk patients, which could contribute to the long duration of therapy and continued empiric use despite negative cultures. Future studies should explore reasons for continuation of meropenem empirically after 72-hours of initiation.
Adjunctive Ethanol-Lock Therapy in Paediatric Patients with Catheter-Related Bloodstream Infection
K Qi1, E Wong1, E Peebles2,3, P Atkison2,3
1London Health Sciences Centre, London, ON
2Children’s Hospital, London Health Sciences Centre, London, ON
3Department of Paediatrics, Western University, London, ON
ABSTRACT
Background:
Patients with long-term catheter access are at higher risk of catheter-related bloodstream infection (CRBSI). CRBSI is a major source of morbidity and may persist despite appropriate antibiotic therapy. It is reported that high concentration (70%) ethanol lock therapy (ELT) can prevent CRSBI, due to its bactericidal properties and penetration of biofilms. The role of ELT in combination with antibiotics for treatment of acute CRBSI is yet to be established.
Objective:
To describe ELT utilization in combination with antibiotics for treatment of CBSRI paediatric patients with long-term catheter access.
Methods:
A retrospective review of patient cases was conducted where at least one dose of ELT was given between September 2013 and September 2015. Clinical outcome of bacteriological cure after initiation of ELT, the identity of microorganisms in blood culture and number of catheter changes after ELT were defined a priori and assessed.
Results:
Six patients were treated with ELT and antimicrobials for a total of 12 events of acute CRBSI. Patients were 4 months to 15 years of age; all were TPN-dependent secondary to short bowel syndrome, with central catheter access. Persistently positive blood cultures in 11 events were observed prior to ELT. Duration of ELT ranged from 1 to 5 days. Bacteriological cure after ELT was achieved in 90.9% (10/11) of cases. Two patients required a catheter change after bacteriological cure
Conclusion:
ELT was demonstrated to have a promising role for adjunctive treatment of acute CRSBI in combination with antimicrobials for TPN-dependent paediatric patients with short bowel syndrome.
Utilisation des données relatives aux rôles et retombées de l’activité pharmaceutique : étude pilote de panels d’experts
M Breton1, E Ferreira1,2, N Letarte2,3, JF Bussières1,2
1Unité de recherche en pratique pharmaceutique, Département de pharmacie, CHU Sainte-Justine, Montréal, QC
2Faculté de pharmacie, Université de Montréal, Montréal, QC
3Département de pharmacie du Centre hospitalier de l’université de Montréal, Montréal, QC
ABSTRACT
Contexte :
Les pharmaciens sont peu exposés aux données publiées relatives aux rôles et retombées de l’activité pharmaceutique (RRAP). Suite à ce constat, le site Impact Pharmacie, outil visant à valoriser et diffuser ces données, a été lancé en 2013.
Objectifs :
Cette étude vise à connaître la position de pharmaciens experts quant à l’utilisation des données scientifiques sur les RRAP et leur avis sur la manière d’améliorer la place du site web dans ce contexte.
Méthodologie :
Étude descriptive prospective dans laquelle des pharmaciens experts ont été invités à visiter le site Impact Pharmacie, consulter les données relatives aux RRAP de leur domaine, puis à répondre à un questionnaire en ligne (pré testé) de 26 questions. Ils ont ensuite été conviés à une entrevue téléphonique de 60 minutes, semi-structurée, enregistrée et modérée par l’équipe de recherche.
Résultats :
Deux panels de cinq pharmaciens experts, hématologieoncologie (HO) et soins intensifs (SI), ont été consultés en septembre 2015. Les 10 panélistes exerçaient dans leur programme respectif depuis 6–10 ans (30%), 11–15 ans (60%) ou plus de 15 ans (10%). L’enquête en ligne a montré que 50% de l’ensemble des pharmaciens colligeaient des données descriptives sur leurs activités, mais aucune relative aux retombées. Seulement 20% des pharmaciens ont publié sur leurs activités. En SI, 80% du panel avaient réévalué leur pratique à partir des données de la documentation scientifique sur les RRAP mais 0% en HO. Pendant la téléconférence, les panélistes ont proposé des moyens d’accroitre la diffusion des études relatives aux RRAP, d’ajouter un résumé des interventions pharmaceutiques ayant davantage de retombées à la section « Que retenir » du site.
Conclusion :
La consultation de panels d’experts est une approche pertinente afin de sensibiliser et favoriser l’utilisation des données relatives aux RRAP. D’autres panels sont prévus dans d’autres disciplines.
Pharmacokinetics of Oral Ciprofloxacin at Steady State in Continuous Cycling Peritoneal Dialysis
C Lee1,2, SAN Walker1,2,3,4, SE Walker1,2, L Palmay1, J Ng1, S Tobe5, A Simor3,4
1Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
3Division of Infectious Diseases, Sunnybrook Health Sciences Centre, Toronto, ON
4Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, ON
5Division of Nephrology, Sunnybrook Health Sciences Centre, Toronto, ON
ABSTRACT
Background:
Peritoneal dialysis (PD) is typically delivered as continuous ambulatory dialysis (CAPD) or continuous cycling dialysis (CCPD). Peritonitis is a major complication of PD, which is associated with increased mortality and PD failure. Pseudomonas aeruginosa (PA) has been known to cause infection and catheter-related PD complications. Oral options for treating PA are limited to ciprofloxacin, but its pharmacokinetic profile has mostly been studied in CAPD.
Objective:
To determine the pharmacokinetics of oral ciprofloxacin and its adequacy for the treatment of PA infections in CCPD patients.
Methods:
Patients were enrolled from the outpatient PD clinic at Sunnybrook Health Sciences Centre. Ciprofloxacin 750mg was given twice daily for a total of 5 doses. Blood was drawn around the fourth and fifth doses to determine steady state concentrations. Dialysate samples were taken to observe attainable concentrations in the peritoneal cavity. Monte Carlo Simulation (MCS) was performed to determine the probability of target level attainment using pharmacokinetic parameter and bacterial minimum inhibitory concentration (MIC) mean and standard deviation.
Results:
Three patients were recruited for this study. The average steady state concentration and half-life were 4.44 ± 1.45 mg/L and 10.28 ± 2.58 hours, respectively. MCS predicted the probability of attaining target peak to MIC ratio of 10 and AUC to MIC ratio of 125 to be 15% and 40%, respectively, for treating PA bacteremia. The maximum concentration observed in the daytime long-dwell dialysate and overnight continuous cycling dialysate was 7.43 ± 1.24 mg/L and 3.25 ± 1.15 mg/L, respectively.
Conclusion:
Oral ciprofloxacin 750mg twice daily may be reasonable in CCPD patients for treating bloodstream or peritoneal infections due to gram-negative bacteria, including highly susceptible PA species. Levels achieved were higher than those typically observed in healthy, non-dialysis patients, but were not excessively high to be concerned with concentration related ciprofloxacin toxicity.
Evaluating the Short-Term Sustainability of Benzodiazepine Receptor Agonist Discontinuation Following Discharge from a General Internal Medicine Program: A Prospective Observational Study
AE Wright1, S Treleaven1, A Holbrook2, C Wallace1
1Pharmacy Department, St Joseph’s Healthcare Hamilton, Hamilton, ON
2Division of Clinical Pharmacology and Toxicology, McMaster University, Hamilton, ON
ABSTRACT
Background:
Long-term use of benzodiazepine receptor agonists (BRAs), including benzodiazepines, zopiclone and zolpidem, is associated with adverse outcomes. Abrupt discontinuation of BRAs is challenging due to withdrawal effects. Many different strategies have resulted in BRA discontinuation in the short-term; however, evidence supporting the sustainability of these interventions is limited.
Objectives:
To determine if BRA discontinuation during hospital admission is sustained 2 weeks after discharge and to determine if any patient, drug or situational factors are associated with sustaining discontinuation.
Methods:
Prospective observational study of patients admitted to the General Internal Medicine (GIM) service at our institution from March 25th–May 29th, 2015 who were using BRAs prior to admission. Participants were excluded if admitted for less than 48 hours or if transferred to or from another acute care service. Demographic, drug use and situational factor data was collected using self-reported questionnaires and chart review. Participants who had their BRA discontinued during admission were followed-up 2 weeks after discharge. Descriptive statistics were used to summarize findings.
Results:
Fifty-three patients admitted to GIM were using BRAs. Twelve met the inclusion criteria; most were excluded for admission less than 48 hours or not providing consent. Four participants (33%) had their BRAs discontinued during admission and 2 (50%) sustained discontinuation two weeks after discharge. No differences in patient or drug use factors were observed between groups however, documentation of BRA discontinuation on the discharge prescription was present for those who sustained BRA discontinuation and absent for those who did not.
Conclusion:
Few prescribers discontinued BRAs during admission. Studies on prescriber knowledge and attitudes may identify barriers to inpatient BRA discontinuation. The sustainability of BRA discontinuation may be facilitated by documentation on the discharge prescription however, the small sample size limits firm conclusions. Standardized communication of medication discontinuation upon discharge may improve sustainability of BRA discontinuation.
Antimicrobials Defined Daily Doses and Days of Therapy in a Mother-Child Teaching Hospital from 2010–2011 to 2014–2015
C Cotteret1,2, D Lebel1,2, H Roy1,2, P Ovetchkine2, JF Bussières1,3
1Pharmacy Practice Research Unit, Pharmacy Department, CHU Sainte-Justine, Montréal, QC
2Pediatric Department, CHU Sainte-Justine, Montréal, QC
3Faculty of Pharmacy, Université de Montréal, Montréal, QC
ABSTRACT
Background:
Antimicrobial stewardship programs include the monitoring of defined daily dose (DDD) and days of therapy (DOT) as surrogate markers of the use of antimicrobials. Such monitoring may contribute to optimal drug use and to limit the emergence of drug resistances.
Objectives:
To describe antimicrobial drug use through the profile of DDD and DOT from 2010–2011 to 2014–2015.
Methods:
This is a cross-sectional descriptive study conducted at a 500-bed mother-child teaching hospital. The study included 53 antibiotics, 10 antivirals and 8 antifungals. All pediatric inpatients from 2010–2011 to 2014–2015 were included. Data was extracted from the pharmacy information system. Inpatient data was extracted from the admission software. We calculated DDD/1000 patient-days (PD) ratios as well as DOT/1000 PD.
Results:
For the five-year period, DDD/1000 PD were 642, 693, 660, 637 and 652, respectively. DOT/1000 PD were 1068, 1150, 1084, 1039 and 1033, respectively. DOT/1000 PD remained stable over the study period. We observed wide variations in DOT/1000 PD between patient care units, as follows: 2619 in hematology-oncology, 1771 in infectious disease, 1508 in intensive care unit, 1000 in surgery units and 867 in pediatric units. Increased use between 2010–2011 and 2014–2015 was observed especially for linezolid (+469%), piperacillin-tazobactam combination (+103%) and carbapenems (+50%).
Conclusions:
There are limited data published about antimicrobial use in pediatrics in Canada. Measurement of DOT/1000 PD is preferred in pediatrics. While antimicrobial drug use appears stable throughout the five-year study period in our hospital, detailed data revealed specific trends per antimicrobial agents and patient care areas and possibly reflects a specific activity in a tertiary hospital. DDD/1000 PD and DOT/1000 PD should be trended per hospital to support antimicrobial stewardship programs and corrective actions.
Development of a Standard Assessment Tool for Field-Based Pharmacy Training
H Halapy1,4, A Lee1, D Spizzirri2, S Yau3, M Suleiman2
1Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
2Ontario College of Pharmacists, Toronto, ON
3School of Pharmacy, University of Waterloo, Waterloo, ON
4St. Michael’s Hospital, Toronto, ON
ABSTRACT
Background:
Field-based pharmacy training is conducted by schools of pharmacy, residency training sites and provincial licensing bodies. A standard assessment tool utilized by these training programs could improve consistency of trainee performance assessments and simplify documentation for preceptors.
Description:
A standard field-based assessment form was developed through a year-long collaboration with representatives of 4 training programs in the province. The assessment tool is competency-based derived from national and provincial standards for pharmacy practice. Education principles based on the Structure of the Observed Learning Outcome (SOLO) taxonomy served as the foundation for the assessment scale.
Action:
Representatives from the training programs met regularly to develop an assessment tool based on 5 main competency domains of patient care, communication and education, professionalism, professional collaboration and practice management. Domains were drawn from the Association of Faculties of Pharmacy of Canada 2010 Educational Outcomes document. The SOLO taxonomy served as the anchor for the 5 point Likert scale used in each domain. Domain selection and descriptors for each point on the Likert scale were decided by representatives of the training programs based on consensus. Feedback regarding assessment tool practicality and design was sought from preceptors, university faculty, and residency coordinators.
Evaluation:
The assessment tool was tested for content validity by asking residency coordinators to rate how relevant the domains were to pharmacy learner competence (mostly 5/5 ratings). Assessment tool descriptors were evaluated similarly through rating how well the descriptors described their corresponding domain item (range of 2–5/5 ratings). This latter finding indicated the need to refine descriptors. An accompanying glossary document was written as a result of this feedback.
Implications:
A standard assessment tool for field-based pharmacy training will provide consistency in assessment and facilitate preceptor training across provincial training programs. With positive feedback from various training programs, the implementation process is underway.
Attitudes and Beliefs towards Smoking Cessation Medications amongst Canadian Armed Forces Personnel
K Lui, R Reid, K Windl, R Comeau, K Wong, H Grenier, B Wierstra, J Ma
Canadian Forces Health Services Group
ABSTRACT
Background:
Despite the presence of organized multidisciplinary smoking cessation programs available at the workplace and comprehensive drug coverage, rate of tobacco use continues to be high amongst Canadian Armed Forces (CAF) personnel. Previous studies demonstrated associations between positive beliefs about smoking cessation medications (SCMs) and increased likelihood of continued abstinence and latency of relapse. There is currently limited information pertaining to the attitudes and beliefs towards smoking cessation medications amongst CAF personnel. Knowledge in this regard may aid in streamlining interventions to reduce tobacco use rate.
Objective:
This descriptive study aims to examine attitudes and beliefs of CAF personnel towards smoking cessation medications.
Methods:
An anonymous and voluntary questionnaire was included as part of departure administration procedures to gather data for this study. CAF members relocating from Canadian Forces Base Shilo, Wainwright, Petawawa and Valcartier were offered the questionnaire. Questions were developed by a focus group of health care professionals. It was subsequently reviewed by multidisciplinary clinicians, translated, and pretested on lay CAF members. Responses were collated centrally by a research nurse and descriptive analysis was done using SPSS.
Results:
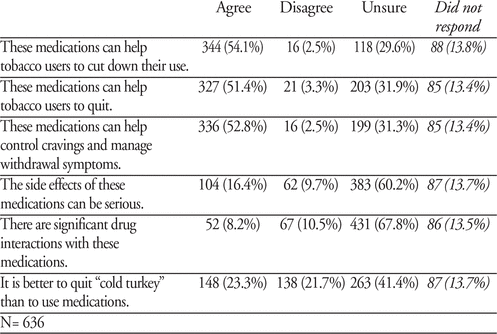
Results were similar when stratified by tobacco use history or by geographic location.
Conclusion:
Only half of the respondents agreed with the efficacy of SCMs. Very few respondents expressed significant concerns with side effects or disagreement with efficacy of SCMs. Unexpectedly, previous smokers who successfully remained abstinent at the time of the study did not display differences in beliefs compared to active smokers. Education pertaining to smoking cessation medications directed at the general CAF population may be helpful in the goal of decreasing tobacco use rates across the organization.
Characterizing the Role of Infectious Diseases Consultant Pharmacists and Antimicrobial Stewardship Pharmacists: A Survey of Canadian Tertiary Care Academic Hospitals
J Beach1, T Ramsey2,3, S Gorman1,4, T Lau1,5, P Calissi4
1The University of British Columbia, Vancouver, BC
2Nova Scotia Health Authority, Halifax, NS
3Dalhousie University, Halifax, NS
4Interior Health Authority, Kelowna, BC
5Vancouver General Hospital, Vancouver, BC
ABSTRACT
Background:
Infectious diseases consultant (IDC) pharmacists provide care to their IDC service inpatients. Increasing numbers of antimicrobial stewardship (AMS) pharmacists are being employed as a result of the Accreditation Canada Required Organizational Practice for hospital AMS programs. With pharmacists in IDC and AMS positions, there is potential for overlapping responsibilities. As there is no literature outlining the current tasks for each group in Canada, a survey was designed to illustrate their roles.
Objective(s):
To characterize the clinical, educational, administrative, and research roles of self-identified IDC and AMS pharmacists in tertiary care academic hospitals across Canada.
Methods:
A survey-based study of IDC and AMS pharmacists at Canadian tertiary care academic hospitals was conducted. The questionnaire was open to targeted participants for 8 weeks. An Internet-based questionnaire platform was used to collect, aggregate, and analyze responses. Descriptive statistics were used to summarize and report data.
Results:
The completed survey response rate was 77% (68/88) and respondents self-identified as IDC (21%), AMS (50%), and dual-role IDC and AMS (29%) pharmacists. IDC pharmacists reported more of the following unique clinical roles: communicating with patients, attending rounds, involving patients in decision-making, and providing patient education as compared to AMS pharmacists. IDC, AMS, and pharmacists who identify themselves as dual-role IDC and AMS pharmacists described similar educational responsibilities. AMS pharmacists performed more of the following administrative and research roles: antibiogram and pre-printed order development, antimicrobial metric collection, and antimicrobial drug use evaluations. Dual-role IDC and AMS pharmacists were involved in less of the unique activities described by those who practiced with a single specialty.
Conclusion(s):
Canadian IDC and AMS pharmacists in tertiary care academic hospitals reported performance of many similar roles. However, distinct differences were identified between IDC, AMS, and those who identified as dual-role IDC and AMS pharmacists within their clinical, administrative, and research domains.
Evaluation of Oseltamivir Dosing Recommendations for Elderly Patients
N Ma, SAN Walker, SE Walker
Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON
Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
The oseltamivir product monograph recommends dose adjustments based on changes in renal function. For treatment of influenza, the monograph recommends 75mg BID for patients with creatinine clearance (CrCl)>60mL/min, 30mg BID for patients with CrCl 30–60mL/min and 30mg daily for patients with CrCl 10–30mL/min. A 2013 population based pharmacokinetic analysis of oseltamivir and its active carboxylate metabolite (OC) reported that CrCl, age and weight were significant determinants of the OC concentration.
Objective:
To evaluate OC concentrations using product monograph recommended doses based on the 2013 population based pharmaco-kinetic analysis and an elderly population of 114 patients with known age, weight and CrCl.
Methods:
OC concentrations were simulated for 114 elderly patients (age range: 83–101) using product monograph recommended doses. Concentrations were compared to those simulated in a normal patient (55yr; 70kg, CrCl=100mL/min). The peak OC concentration at steady state (Cmaxss) and area under the curve at steady state (AUCss) was calculated for each elderly patient and grouped according to renal function. Differences were tested using analysis of variance.
Results:
In a normal patient the AUCss was 3.53mg*hr/L. The average AUCss for elderly patients with CrCl>60mL/min (n=18), 30–60mL/min (n=70) and 10–30mL/min (n=26) were 3.78mg*hr/L, 2.16mg*hr/L, and 3.14mg*hr/L, respectively, with all groups significantly different from each other (P<0.001). The concentration-time profiles also demonstrated elderly patients with CrCl 30–60mL/min and 10–30mL/min achieved lower concentrations compared to the normal patients. In order to achieve similar concentrations, elderly patients with CrCl 30–60mL/min should receive 45mg BID and patients with CrCl 10–30mL/min should receive 30mg BID.
Conclusion:
The current oseltamivir monograph dosing recommendations for the treatment of influenza in patients with renal impairment under doses patients.
Defining the Roles of an Experiential Education Facilitator: A Pilot Project
M Legal, M Leung, J Guenter, K Tse
The University of British Columbia, Vancouver, BC
ABSTRACT
Background:
With the recent and imminent changes to the pharmacy education program in British Columbia, placement capacity, teaching workload, and the need for preceptor and learner support are clearly identified as key areas of concerns. The implementation of Experiential Education Facilitators (EEFs) to provide on-the-ground support to preceptors and learners at the placement sites was identified as one of the strategies to help manage clinical workload and optimize student learning. In April 2015, a pilot EEF position was implemented as a trial to provide support to learners and preceptors at 4 different hospitals.
Objectives:
The objectives of this report are to explore learners’ and preceptors’ perceptions of the EEF roles, summarize the key findings and provide suggestions for implementation of the permanent EEFs.
Method:
A non-experimental descriptive exploratory design was used to explore learners’ and preceptors’ perceptions of the strengths and weaknesses of the EEF roles. Learners and preceptors were contacted at the end of the rotation for feedback. All interviews followed a semi-structured format and were audio recorded. Verbatim transcripts and/or detailed field notes were produced. Data were independently categorized, reassembled and managed in a systematic way to identify the issues and themes, such as the roles, benefits and challenges of the EEF experience.
Results:
The benefits of EEFs include direct support to learners to ease their transition from school to real world practice setting, which in turn, minimizes the workload for preceptors. Role delineation, clear expectations and frequent communications are critical to ensure all stakeholders share a common understanding of EEF roles.
Conclusions:
This report offers preliminary evidence for the roles of EEFs and provides some insight to potential benefits and challenges of such roles.
Non-Traditional Learner-Preceptor Models: Development of Preceptor Guidebooks
M Legal1, J Guenter1, K Tse1, M Leung1, A Thompson1, M MacDonald2,3, K Mulherin4
1The University of British Columbia, Vancouver, BC
2University of Alberta, Edmonton, AB
3Pharmacy Services, Alberta Health Services, Calgary, AB
4Association of Faculties of Pharmacy of Canada, Toronto, ON
ABSTRACT
Background:
There has been renewed interest in non-traditional learner-preceptor models in pharmacy experiential education in Canada due to increased placement capacity demands as well as support for these models in educational literature. Development of tools and resources will provide benefit to preceptors as they contemplate or begin adopting these models.
Description:
This project compiled the available knowledge, both theoretical and practical, into reference guidebooks for preceptors and experiential education coordinators. Peer assisted learning (PAL), near peer (NP) teaching and co-precepting models were chosen as the focus of each guidebook.
Action:
In May–July 2015, preceptors experienced in non-traditional learner-preceptor models were contacted to participate in either a survey or phone interview. Preceptors from British Columbia (n=18 and 14), Alberta (n=6 and 6), Ontario (n=8 and 2) and Newfoundland (n=2 and 0) participated in the national survey and interviews, respectively. Questions were asked in such a way to extract practical tips and solutions to any perceived challenges of these models. The insights and advice from these champion preceptors were used to create these guidebooks. Other information sources came from precepting guidebooks from other health disciplines at various Canadian Universities, experiential education faculty and a systematic literature review on the benefits and challenges of learner-preceptor models.
Evaluation:
Three guidebooks were created. Project team members reviewed each for content, format and ease of use. Secondly, suggestions from experienced preceptors were incorporated. The commonly cited advantages were peer support, clinical independence and enriched learning for PAL and NP models and reduced preceptor workload for co-precepting. Strategies for success were offered to help address possible increased workload with PAL and NP and to improve communication and expectations with co-precepting.
Implications:
Finalized guidebooks will be made available to all Canadian faculties/schools as a resource to provide practical tips and advice for preceptors adopting a non-traditional precepting model.
Standardization of Patient Education by Pharmacists
E Tom1, NF Dewhurst1,2, J Proceviat1, P Gillespie2, C Chant1,2
1St. Michael’s Hospital, Toronto, ON
2Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
ABSTRACT
Background:
Pharmacists play a vital role in educating patients on the safe and effective use of medications in a variety of situations. Pharmacists must select and prioritize which patients receive education within hospital and just prior to discharge. In order to balance performance of patient education and other clinical duties, standard criteria for prioritization was necessary.
Description:
In order to minimize variation amongst pharmacists in patient selection, the process of standardizing practice for patient education was required to allow all pharmacists to apply a consistent approach.
Action:
A formalized literature search was performed as a baseline assessment of existing patient education practices. Clinical pharmacists were surveyed to assess the current methods for patient selection, educational practice and documentation. This data was used to develop criteria for the selection of patients for pharmacist education.
Evaluation:
All inpatient pharmacists surveyed participated in the provision of patient education, averaging 2 hours of patient education per week over 6 patients. The majority of patients were self-referred, followed by physician, nurse and nurse practitioner referrals. Pharmacists targeted patients taking on an average 3 new or 9 medications in total. Drug-specific criteria were considered most important for prioritization of patient education, followed by those patients on a high number of medications on discharge. Medication counseling was the most common type of education provided, followed by medication schedule teaching. Therapeutic anticoagulants were the most common targeted drug for discharge education, followed by systemic corticosteroids and insulin.
Implications:
Prioritizing patients taking therapeutic anticoagulation was identified as the best criteria to standardize patient education. Standardization enables the discipline to set minimum criteria for when education will be provided by pharmacists to enable a balance of clinical duties.
Implementation of an Insulin Pen Trial in an Acute Medical and Surgical Patient Population
S Sanaz
Trillium Health Partners, Mississauga, ON
ABSTRACT
Background:
The use of insulin pens has been shown to improve patient safety, patient comfort, transition of care, staff satisfaction and cost savings.
Description:
An insulin pen protocol was developed with input of stakeholders including nurses, clinical educators, and pharmacy staff. In collaboration with our Industry Partners, nursing staff were trained in the safe use of insulin pens and a checklist was developed for certification. Insulin pens are high risk medications, and can pose serious patient safety risk in the hospital setting, if used incorrectly. To mitigate this risk, a safety campaign was adopted to emphasise ‘one pen, one patient’, and all staff completed the ‘Safe Use of Insulin Pens E-learning Module’
Action:
Nurses completed staff satisfaction surveys before and after the trial and quality audits were performed throughout, to ensure the policies and procedures were correctly and safely implemented.
Evaluation:
Significant improvement in needlestick injuries was observed on the medical ward, and none on the surgery ward. Additionally, no incidents of patients sharing pens were documented on either ward. Insulin wastage was noticeable with shorter stays and higher dispensing costs with the pen; however there was a reduction in nursing administration times. When evaluating administration, all nurses demonstrated correct technique, reflecting good knowledge. Nursing staff preferred the insulin pen, as did the patients due to convenience and ease of transition of care to the community. The trial did not demonstrate cost savings on the medicine ward with transition to insulin pens, however there was a cost savings observed on the surgical ward. The significance of cost savings requires a larger patient population to be established.
Implications:
Patient safety, staff and patient satisfaction will support the roll out of insulin pens organization wide. Education and proper training is imperative for the safe roll-out of insulin pens and support of staff.
Inappropriate Administration of Oral Valganciclovir to Infants with Congenital Cytomegalovirus Infection
S Harricharan1, VC Harris1,2
1London Health Sciences Centre, London, ON
2Department of Paediatrics, Western University, London, ON
ABSTRACT
Background:
Oral valganciclovir is a mono-valyl ester pro-drug of ganciclovir, available in a 50 mg/mL oral suspension. Its use for the treatment or prevention of CMV in paediatric patients has increased since oral formulations of ganciclovir were withdrawn from the market.
Description:
A neonate was discharged from hospital with a prescription for valganciclovir 40 mg/0.8 mL orally twice daily. The patient’s caregiver estimated she gave approximately 125 mg before realizing this was a larger volume than the baby had received in hospital. The caregiver brought the baby to the emergency department, where the baby was found to be clinically stable. Follow up blood work did not show any evidence of renal dysfunction or bone marrow suppression, although the latter may be a delayed effect requiring further monitoring.
Assessment of Causality:
The oral syringe provided by the manufacturer is marked in 100 mg gradations up to 500 mg, with small lines to denote 25 mg intervals. The patient’s caregiver believes she drew up 400 mg, as a 40 mg/0.8 mL dose is not measurable with this syringe.
Literature Review:
Administration errors are often reported when less than 1 mL of medication per dose is prescribed and can frequently involve infants under 1 year. Errors have been reported secondary to confusion between the desired mg and mL dose to be administered using an oral syringe with mL gradations.
Importance to Practitioners:
Pharmacists should be made aware that the syringe supplied by the manufacturer with valganciclovir is marked in 100 mg graduations rather than mL and is not appropriate to measure neonatal doses. Concern about this oral syringe was reported to ISMP. An oral syringe, with mL markings, should be supplied and appropriate counselling provided to caregivers to minimize the risk of accidental overdose in this vulnerable population.
Two Cases of New Onset Refractory Status Epilepticus: Prolonged Barbiturate and Ketamine Therapy
C Lougheed, D Debicki, E Althenayan, H Khosravani, T Gofton
London Health Sciences Centre, London, ON
ABSTRACT
Background:
New onset refractory status epilepticus (NORSE) is a difficult to control clinical condition with potentially poor outcomes. Coma induction, including the use of barbiturates and ketamine, is an acceptable therapeutic strategy. However, the use of general anesthetics has potential inherent systemic complications. Thus, the optimal therapeutic approach remains unclear.
Case Description:
Two cases of young healthy males presenting with new generalized tonic-clonic seizures following flu-like symptoms were managed in the intensive care unit. Continuous electroencephalography monitoring indicated ongoing non-convulsive status epilepticus refractory to numerous antiepileptic drugs. Seizure suppression was achieved through infusions of pentobarbital, thiopental, ketamine, propofol or midazolam. This continued for greater than 6 months with unsuccessful weaning attempts. Brain imaging demonstrated evidence of progressive white matter disease. Other systemic complications included recurrent infections, hepatomegaly and, in one case, ischemic gut requiring surgical intervention. Both patients received immunologic therapy for presumed autoimmune encephalitis. Ultimately, one patient remained in a persistent vegetative state while the other recovered with a significant reduction in seizure burden.
Assessment of Causality:
Treatment complications, specifically hepatomegaly and reversible cerebral changes (as seen on magnetic resonance imaging), were attributed to prolonged barbiturate and ketamine use respectively.
Literature Review:
Evidence in the form of randomized controlled trials in the management of refractory status epilepticus is limited to one study comparing barbiturates to propofol. As such, management of this severe disorder is directed largely by case reports and small case series. Prolonged barbiturate coma has been described in the order of weeks and not months.
Importance to Practitioners:
Current practices in the management of NORSE are guided by expert consensus, systematic reviews of the literature and cumulative practice. Further research into the risks and benefits of prolonged anesthesia is needed. In the meantime, case reports add to the small body of evidence currently used to direct therapy.
(Return to Top)
Canadian Journal of Hospital Pharmacy, VOLUME 69, NUMBER 1, January-February 2016