Mycobacterium abscessus Lung Infection: A Case Report
Pan Pan Wang, Charles-André Bray, Simon Grandjean Lapierre, Hafid Soualhine, Fanny Arbour
INTRODUCTION
Nontuberculous mycobacteria (NTM) are ubiquitous, and more than 150 species have been identified.1,2 These low-virulence mycobacteria are known to cause mostly opportunistic infections in immunocompromised patients, although they are increasingly seen in normal hosts.1,2Mycobacterium abscessus complex is a rapidly growing NTM, which was the second most commonly isolated NTM pulmonary pathogen in British Columbia and Alberta in 2013.2 In Quebec, M. abscessus complex represented 11.4% of the 474 mycobacteria samples received for susceptibility testing by the Laboratoire de santé publique du Québec in the period April 2012 to March 2013.3 Multiple-drug resistance and frequent relapses make the treatment of M. abscessus lung infections clinically challenging.1 This case report discusses the management of pulmonary M. abscessus infection in a patient who underwent prolonged antimicrobial treatment and surgery and in whom several antimicrobial adverse effects were suspected.
CASE REPORT
In April 2014, a 36-year-old man presented with a 1-year history of fever, dyspnea, pleuritic pain, chronic productive cough, and 15-kg weight loss (body weight at time of presentation 55 kg).* He had immigrated 6 months earlier from West Africa and had received medical coverage 2 days before the initial consultation. His medical history included treated pulmonary tuberculosis in 2002 and pulmonary aspergilloma leading to right superior lobectomy in March 2013. He had no history of HIV infection or other forms of immunosuppression. He was not taking any medication and had no known allergies. Initial examination showed fever (38.4°C), tachycardia (135 beats/min) without hemodynamic instability, a normal respiratory rate, and the presence of multiple cutaneous fistulas with mucopurulent discharge at the site of the prior lobectomy incision.
Initial chest computed tomography (CT) showed the following features: multiple cavities and nodules in the right inferior and middle lobes; several fistulas between the skin, the pleural space, and the lung parenchyma; and lytic lesions of the right sixth rib. Both lungs showed important atelectasis and cylindrical bronchiectasis, especially on the right side. The left lung also contained multiple small nodules and a 20-mm air cavity. Bone scintigraphy later corroborated the bone infection.
Bronchoscopy showed no sign of hemoptysis. Spirometry showed a decreased forced expiratory volume in 1 s of 1.88 L (44% of the predicted value), with total pulmonary capacity of 3.11 L (56% of the predicted value). The initial leukocyte count was 11.3 × 109/L (normal range 4.0–11.0 × 109/L), hemoglobin was 75 g/L (normal range 140–175 g/L), and platelet count was 768 × 109/L (normal range 150–400 × 109/L). C-reactive protein was elevated, at 85.8 mg/L. Repeat culture of blood, induced sputum, fistula discharge, and bronchoa l veolar lavage (BAL) fluid yielded negative results for aerobic, anaerobic, and fungal organisms. Acid-fast colouration, Mycobacterium tuberculosis polymerase chain reaction assay, and HIV serology testing all gave negative results.
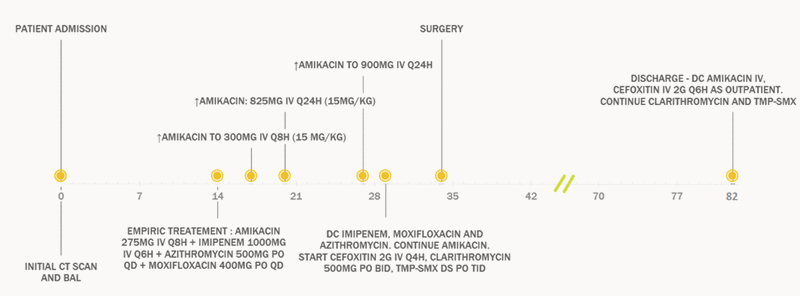
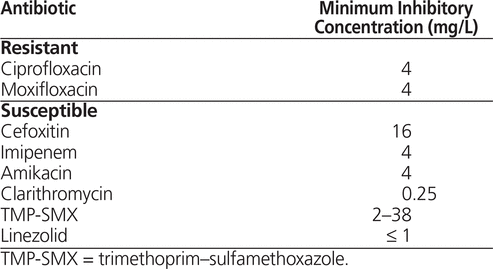
The patient was admitted to the pneumology unit. On day 9 of the hospital stay, after blood transfusions and supportive care, mycobacterial culture of the BAL fluid confirmed the presence of a rapidly growing mycobacterium. On day 14, the Laboratoire de santé publique du Québec identified the pathogen as M. abscessus complex. Empiric treatment with amikacin, imipenem, azithromycin, and moxifloxacin was started immediately (Figure 1), following the guidelines of the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) and according to the clinical expertise of the medical microbiologist and infectious disease clinician. On day 20, the National Microbiology Laboratory (Public Health Agency of Canada) identified the subspecies as M. abscessus ssp. abscessus. On day 28, treatment was adjusted, according to the susceptibility profile (Table 1), to amikacin, cefoxitin, clarithromycin, and trimethoprim–sulfamethoxazole (TMP-SMX). At that time, the patient still had fever, right pleuritic pain, and mild dyspnea, although the fistula discharge had decreased, and he had gained 5 kg body weight.
| |
|
|
|
Figure 1. Treatment timeline for a 36-year-old man with Mycobacterium abscessus lung infection. BAL = bronchoalveolar lavage, CT = computed tomography, DC = discontinue, DS = double strength, IV = intravenous, PO = by mouth, QD = daily, TMP-SMX = trimethoprim–sulfamethoxazole.
|
Table 1. Antibiotic Susceptibility of Identified Mycobacterium abscessus ssp. abscessus
On day 34, the patient underwent right pneumonectomy and partial removal of the right third, fifth, and sixth ribs, the latissimus dorsi muscle, and the right scapula. An open thoracic window was left in place, in case further intervention was needed. Specific polymerase chain reaction and gene sequencing assays (for rrs and hsp 65 genes) on pathology specimens later confirmed the diagnosis of pulmonary M. abscessus.
One month after the surgery, repeat chest CT showed decreased infiltrates and shrinkage of the nodules in the left lung. Culture results for control BAL fluid and induced sputum were still negative at 7 and 10 months, respectively, after the surgery. At about day 70, the patient had mild neutropenia (nadir of 1.1 × 109/L [normal range 1.8–8.2 × 109/L]). However, he remained clinically stable, and the treatment was continued.
The patient was discharged on day 82, and the open thoracic window was closed surgically with a muscle flap on day 95. There was close postdischarge follow-up by the medical microbiologists and infectious disease team. Because of elevation of serum creatinine (from baseline of 65 µmol/L to 117 µmol/L at discharge), the amikacin was stopped at discharge, without rechallenge. Soon after discharge, the patient reported peripheral neuropathy, with a painful burning sensation in the limbs. The condition was relieved by low-dose pregabalin. The patient completed 6 months of outpatient treatment with parenteral cefoxitin. Oral clarithromycin and TMP–SMX were stopped after 18 months of treatment. He was followed monthly for the first 4 months and then every 2 months until recently (for a total of 16 months of outpatient follow-up). At the most recent appointment, the patient had resumed normal activity and was looking for a job. He weighed 70 kg, and his inflammatory parameters had normalized.
DISCUSSION
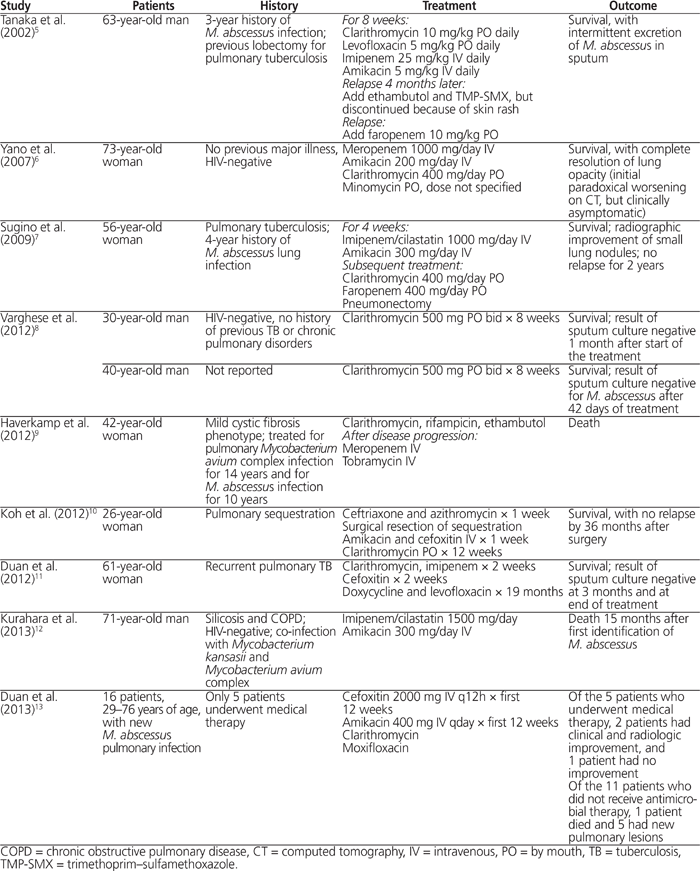
Mycobacterium abscessus complex is an ubiquitous, rapidly growing mycobacterium.1,2 The lungs are the most frequent site of infection, and M. abscessus infections progress slowly if left untreated.1,4 A history of chronic cough is often present initially, whereas fever and constitutional symptoms are seen with disease progression.1 Typical radiologic findings include nodular opacities and multifocal bronchiectasis.1,4 Previous pulmonary tuberculosis and bronchiectasis are predisposing conditions for M. abscessus pulmonary infection.1Table 2 summarizes the published case reports of M. abscessus pulmonary infections in immunocompetent patients.5–13 These cases were identified in PubMed with the keywords “Mycobacterium abscessus” and “pulmonary”, and selection was limited to cases in immunocompetent hosts without cystic fibrosis. Two of the patients were treated with macrolide alone for 8 weeks8; however, the efficacy of these regimens to prevent relapse cannot be determined because of the short duration of follow-up. In most of the published case reports at least 2 agents, often including a macrolide and an aminoglycoside, were combined for a variable duration of treatment.5–7,9,11,13 However, the variations in follow-up duration and clinical outcome make it difficult to compare the efficacy of these regimens. Publication of additional case series is needed to document the optimal treatment of M. abscessus lung infections.
Table 2. Published Case Reports of Mycobacterium abscessus Lung Infection in Immunocompetent Hosts without Cystic Fibrosis
In 2007, ATS and IDSA published a joint guideline on the management of NTM diseases, including M. abscessus infections.1 Given the lack of clinical studies, treatment in the case presented here was based on these guidelines and expert opinion. Because M. abscessus is resistant to many drugs, including antituberculous agents,1 identification of the subspecies and determination of drug susceptibility were crucial to choosing the appropriate treatment. Consistent with the guideline’s recommendation and pathogen susceptibility, the patient received quadruple therapy with amikacin, cefoxitin, clarithromycin, and TMP-SMX. Surgery is essential in cases of extensive disease, focal abscess formation, and when drug therapy alone cannot control disease progression.1 The patient in this case was initially deemed too frail to undergo surgery. Therefore, this medical treatment was given for 34 days to lessen the microbial burden, and surgery was performed later.
Once surgery has been performed, further antibiotic treatment is necessary to maintain negative results on sputum culture for at least 12 months. However, there is currently no antibiotic regimen that has been shown to consistently achieve this goal.1 Amikacin (10–15 mg/kg per day) and high-dose cefoxitin (12 g/day) are the cornerstones of therapy for M. abscessus infections.1 Macrolides are the only oral agents demonstrating consistent in vitro activity against M. abscessus, although they are insufficient, on their own, to cure the disease.1Mycobacterium abscessus ssp. abscessus carries an erm gene that provides in vivo inducible resistance to macrolides, but this may not be revealed by in vitro susceptibility tests.14 In contrast, M. abscessus ssp. massiliense is most often sensitive to macrolides.15 The TMP-SMX was added as a fourth agent, for extra coverage in case of resistance to any of the other medications.
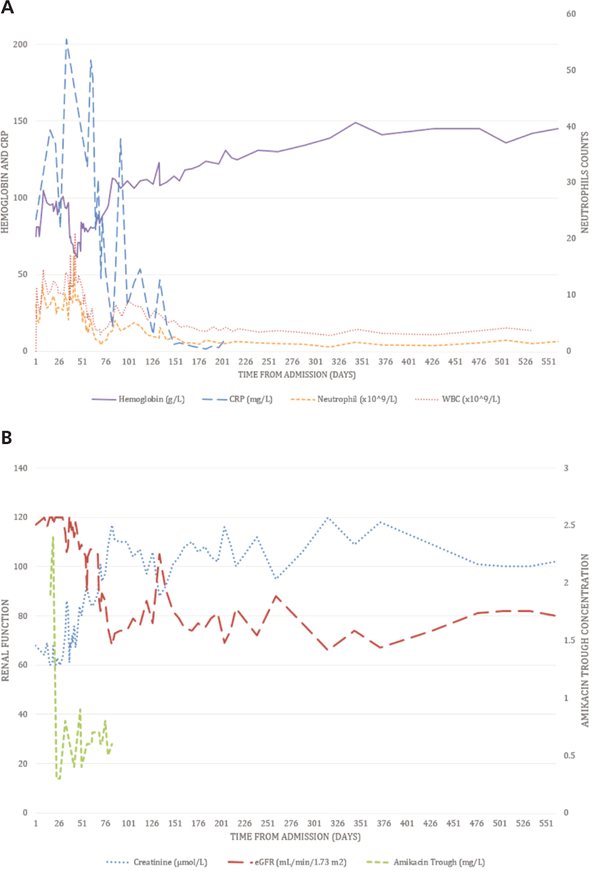
Despite the occurrence of adverse drug reactions in this case, the antibiotic therapy was maintained because of the seriousness of the patient’s condition and the lack of alternatives. His neutropenia might have been associated with the use of high-dose cefoxitin1,16 but might also have been attributable to the chronic infection. The patient remained mildly neutropenic (neutrophil count between 1 × 109/L and 1.5 × 109/L), even after the cefoxitin was stopped on day 202 (see Figure 2A). However, no infection developed, nor did the patient require other specific treatment.
| |
|
|
|
Figure 2. Clinical results over time. A: Hemoglobin, neutrophils, white blood cells (WBC), and C-reactive protein (CRP). B: Creatinine, estimated glomerular filtration rate (eGFR, according to the Modification of Diet in Renal Disease formula), and amikacin trough concentration. The patient was discharged on day 82. The 3 times daily regimen of amikacin was replaced by once-daily dosing on day 27, and amikacin was stopped on day 82.
|
The patient’s serum creatinine rose gradually, despite adequate fluid repletion and hemodynamic stability. Other than the antibiotics, the patient did not receive any nephrotoxic medication. The results of urinalysis and other laboratory tests were unremarkable. Possible causes of the renal injury were nephrotoxicity associated with amikacin,17 concomitant use of cephalosporin and aminoglycoside,17 and the competition of creatinine tubular secretion with TMP-SMX (Naranjo score 3, indicating possible adverse reaction).18 The therapeutic concentration of amikacin (see Figure 2B) was monitored by a clinical pharmacist. Although without strong evidence, once-daily dosing of amikacin was used as a reasonable alternative to 3 times daily dosing in the treatment of M. abscessus infection.1 A target trough concentration of ≤ 1 mg/L was used for once-daily dosing, which is a good predictor of drug clearance and avoidance of nephrotoxicity.19,20 At follow-up, the patient’s serum creatinine remained stable (≤ 120 µmol/L [normal range 53–120 µmol/L], with estimated glomerular filtration rate, according to the Modification of Diet in Renal Disease formula, of about 80 mL/min per 1.73 m2), even though he had gained considerable muscle mass by the time amikacin was stopped.
The neurology service was consulted for the peripheral neuropathy, but the results of electromyography were inconclusive. Prolonged treatment with both TMP-SMX (Naranjo score 4)18,21 and cefoxitin (Naranjo score 4)16,21 indicated a possible adverse reaction. In addition, local inflammation and nutritional deficiencies could not be excluded as possible causes of the peripheral neuropathy.
CONCLUSION
Treatment of M. abscessus pulmonary infection is clinically challenging because of the organism’s resistance to many antibiotics and the unfavourable adverse reaction profile of the currently recommended therapy.1 This article has discussed the management of severe M. abscessus lung infection in a 36-year-old man who received prolonged drug therapy and surgery and who had no clinical relapse after 18 months of follow-up.
References
1. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al.; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4): 367–416. Erratum in: Am J Respir Crit Care Med. 2007;175(7):744–5 [dosage error].

2. Behr M, Jarand J, Marras TK. Nontuberculous mycobacteria. In: Menzies D, editor. Canadian tuberculosis standards. 7th ed. Ottawa (ON): Public Health Agency of Canada; 2013. p. 279.
3. Tremblay C, Fauvel M. Rapport d’activités 2012–2013 du Laboratoire de santé publique du Québec. Québec (QC): Institut national de santé publique du Québec; 2013.
4. Philley JV, Wallace RJ Jr. Chapter 132: Diseases due to nontuberculous mycobacteria. In: Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI, Senior RM, editors. Fishman’s pulmonary diseases and disorders. New York (NY): McGraw-Hill Education; 2015 [cited 2016 May 27]. Available from: http://accessmedicine.mhmedical.com/content.aspx?bookid=1344&Sectionid=81199911. Subscription required to access content.
5. Tanaka E, Kimoto T, Tsuyuguchi K, Suzuki K, Amitani R. Successful treatment with faropenem and clarithromycin of pulmonary Mycobacterium abscessus infection. J Infect Chemother. 2002;8(3):252–5.

6. Yano S, Kobayashi K, Kato K, Tokuda Y, Ikeda T, Takeyama H. Paradoxical worsening of pulmonary Mycobacterium abscessus. Respir Med. 2007; 101(4):868–70.

7. Sugino K, Kobayashi M, Iwata M, Gocho K, Kaburaki K, Muramatsu Y, et al. Successful treatment with pneumonectomy for pulmonary Mycobacterium abscessus infection. Intern Med. 2009;48(6):459–63.

8. Varghese B, Shajan SE, Al MO, Al-Hajoj SA. First case report of chronic pulmonary lung disease caused by Mycobacterium abscessus in two immunocompetent patients in Saudi Arabia. Ann Saudi Med. 2012; 32(3):312–4.
9. Haverkamp MH, van Wengen A, de Visser AW, van Kralingen KW, van Dissel JT, van de Vosse E. Pulmonary Mycobacterium abscessus: a canary in the cystic fibrosis coalmine. J Infect. 2012;64(6):609–12.

10. Koh WJ, Hong G, Kim K, Ahn S, Han J. Pulmonary sequestration infected with nontuberculous mycobacteria: a report of two cases and literature review. Asian Pac J Trop Med. 2012;5(11):917–9.

11. Duan HF, Huang XR, Wang J, Chu NH. Mycobacterium abscessus lung disease in a patient with previous pulmonary tuberculosis. Southeast Asian J Trop Med Public Health. 2012;43(4):959–63.
12. Kurahara Y, Tachibana K, Tsuyuguchi K, Suzuki K. Mixed pulmonary infection with three types of nontuberculous mycobacteria. Intern Med. 2013;52(4):507–10.

13. Duan HF, Chu NH, Wang QF, Wang J, Huang HR, Liang Q. [Mycobacterium abscessus group lung disease: case reports and review of the literature]. Zhonghua Jie He He Hu Xi Za Zhi. [Chinese J Tuberc Respir Dis.] 2013;36(9):671–4. In Chinese.
14. Choi GE, Shin SJ, Won CJ, Min KN, Oh T, Hahn MY, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med. 2012; 186(9):917–25.

15. Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183(3):405–10.

16. Cephalosporins. In: Aronson JK, editor. Meyler’s side effects of drugs: the international encyclopedia of adverse drug reactions and interactions. 15th ed. Amsterdam (Netherlands): Elsevier; 2006. pp. 688–701.
17. Aminoglycoside antibiotics. In: Aronson JK, editor. Meyler’s side effects of drugs: the international encyclopedia of adverse drug reactions and interactions. 15th ed. Amsterdam (Netherlands): Elsevier; 2006. pp. 118–36.
18. Trimethoprim and co-trimoxazole. In: Aronson JK, editor. Meyler’s side effects of drugs: the international encyclopedia of adverse drug reactions and interactions. 15th ed. Amsterdam (Netherlands): Elsevier; 2006. pp. 3510–24.
19. Hatala R, Dinh T, Cook DJ. Once-daily aminoglycoside dosing in immunocompetent adults: a meta-analysis. Ann Intern Med. 1996; 124(8):717–25.

20. Murphy J, Matthias K. Aminoglycosides. In: Murphy JE, editor. Clinical pharmacokinetics. Bethesda (MD): American Society of Health-System Pharmacists; 2011. pp. 91–119.
21. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.

Pan Pan Wang, PharmD, was, at the time of this study, an MSc candidate and Pharmacy Resident at Hôpital du Sacré-Coeur de Montréal (affiliated with Université de Montréal), Montréal, Quebec. She has now completed the MSc program and residency and is a pharmacist at CIUSSS de l’Ouest-de-l’Île-de-Montréal.
Charles-André Bray, PharmD, was, at the time of this study, an MSc candidate and Pharmacy Resident at Hôpital du Sacré-Coeur de Montréal (affiliated with Université de Montréal), Montréal, Quebec. He has now completed the MSc program and residency and is a pharmacist at CIUSSS Centre-Sud-de-l’île-de-Montréal.
Simon Grandjean Lapierre, MD, is a Fellow in Infectious Disease and Medical Microbiology at Hôpital du Sacré-Coeur de Montréal (affiliated with Université de Montréal), Montréal, Quebec.
Hafid Soualhine, PhD, is Supervisor of the Mycobacteriology and Aerobic Actinomycetes Laboratory, Institut national de santé publique du Québec, Sainte-Anne-de-Bellevue, Quebec.
Fanny Arbour, BPharm, MSc, is a Clinical Pharmacist (Pneumology) with the Department of Pharmacy, Hôpital du Sacré-Coeur de Montréal, Montréal, Quebec.
Competing interests: None declared.
Address correspondence to: Fanny Arbour, Department of Pharmacy, Hôpital du Sacré-Coeur de Montréal, 5400, boulevard Gouin ouest, Montréal QC H4J 1C5, e-mail: fanny.arbour@umontreal.ca
(Return to Top)
*The patient gave written informed consent for publication of this case report. (
Return to Text
)
Funding: None received.
Acknowledgements
This article was written as part of the “Scientific Communication” course for the Faculty of Pharmacy, Université de Montréal. The authors would like to thank the course instructors, especially Mrs Louise Mallet.
Canadian Journal of Hospital Pharmacy, VOLUME 69, NUMBER 3, May-June 2016