Wasim S El Nekidy, Maher M El-Masri, Greg S Umstead, Michelle Dehoorne-SmithABSTRACT
Background:
Methicillin-resistant Staphylococcus aureus is a leading cause of death in patients undergoing hemodialysis. However, controversy exists about the optimal dose of vancomycin that will yield the recommended pre-hemodialysis serum concentration of 15–20 mg/L.
Objective:
To develop a data-driven model to optimize the accuracy of maintenance dosing of vancomycin for patients undergoing hemodialysis.
Methods:
A prospective observational cohort study was performed with 164 observations obtained from a convenience sample of 63 patients undergoing hemodialysis. All vancomycin doses were given on the floor after completion of a hemodialysis session. Multivariate linear generalized estimating equation analysis was used to examine independent predictors of pre-hemodialysis serum vancomycin concentration.
Results:
Pre-hemodialysis serum vancomycin concentration was independently associated with maintenance dose (B = 0.658, p < 0.001), baseline pre-hemodialysis serum concentration of the drug (B = 0.492, p < 0.001), and interdialytic interval (B = −2.133, p < 0.001). According to the best of 4 models that were developed, the maintenance dose of vancomycin required to achieve a pre-hemodialysis serum concentration of 15–20 mg/L, if the baseline serum concentration of the drug was also 15–20 mg/L, was 5.9 mg/kg with interdialytic interval of 48 h and 7.1 mg/kg with interdialytic interval of 72 h. However, if the baseline pre-hemodialysis serum concentration was 10–14.99 mg/L, the required dose increased to 9.2 mg/kg with an interdialytic interval of 48 h and 10.0 mg/kg with an interdialytic interval of 72 h.
Conclusions:
The maintenance dose of vancomycin varied according to baseline pre-hemodialysis serum concentration of the drug and interdialytic interval. The current practice of targeting a pre-hemodialysis concentration of 15–20 mg/L may be difficult to achieve for the majority of patients undergoing hemodialysis.
KEYWORDS: vancomycin, dosing, hemodialysis
RÉSUMÉ
Contexte :
Les infections à Staphylococcus aureus résistant à la méthicilline comptent parmi les principales causes de mortalité chez les patients traités par hémodialyse. Or, les avis sont partagés quant à la dose optimale de vancomycine qui permet d’atteindre la concentration sérique recommandée de 15–20 mg/L préalablement à l’hémodialyse.
Objectif :
Mettre au point un modèle guidé par des données afin d’optimiser l’exactitude de la dose d’entretien de vancomycine chez les patients qui subissent une hémodialyse.
Méthodes :
Une étude de cohorte prospective observationnelle a été menée à partir de 164 observations obtenues d’un échantillon de commodité de 63 patients traités par hémodialyse. Toutes les doses de vancomycine ont été données à l’unité de soins courants après la fin d’une séance d’hémodialyse. Une analyse de régression linéaire multiple par équation d’estimation généralisée a été effectuée pour cerner les variables indépendantes qui permettent de prévoir la concentration sérique de vancomycine préalablement à l’hémodialyse.
Résultats :
La concentration sérique de vancomycine avant hémodialyse a été associée de façon indépendante à l’intervalle entre deux dialyses (B = −2,133, p < 0,001), à la dose d’entretien (B = 0,658, p < 0,001) et à la concentration sérique initiale du médicament préalablement à l’hémodialyse (B = 0,492, p < 0,001). Selon les quatre meilleurs modèles élaborés, la dose d’entretien de vancomycine nécessaire pour atteindre une concentration sérique de 15–20 mg/L avant hémodialyse, si la valeur initiale était aussi de 15–20 mg/L, était de 5,9 mg/kg pour un intervalle de 48 h entre deux dialyses et de 7,1 mg/kg pour un intervalle de 72 h entre deux dialyses. Or, si la concentration sérique initiale préalablement à l’hémodialyse se situait entre 10 et 14,99 mg/L, la dose nécessaire augmentait à 9,2 mg/kg pour un intervalle de 48 h entre deux dialyses et à 10,0 mg/kg pour un intervalle de 72 h écoulé entre deux dialyses.
Conclusions :
La dose d’entretien de vancomycine variait en fonction de la concentration sérique initiale du médicament préalablement à l’hémodialyse et en fonction de l’intervalle entre deux dialyses. La pratique actuelle voulant qu’on vise une concentration préalable à l’hémodialyse de 15–20 mg/L peut être difficile à respecter chez la majorité des patients subissant une hémodialyse.
MOTS CLÉS : vancomycine, posologie, hémodialyse
Infections are among the leading causes of death among patients with stage 5 chronic kidney disease (CKD) who are receiving hemodialysis.1–3 Such infections are often caused by gram-positive bacteria, especially methicillin-resistant Staphylococcus aureus (MRSA).3–5 It has been reported that S. aureus infections account for 39% of all cases of catheter-related bacteremia among patients undergoing hemodialysis and that MRSA accounts for about 60% of all S. aureus infections.6,7
For more than 5 decades, vancomycin has been the treatment of choice for MRSA in patients undergoing hemodialysis.4,8,9 However, dosing of this drug in this patient population is still not well established. One study correlated a pre-hemodialysis vancomycin concentration of 5–10 mg/L with clinical and microbiological failure in patients with permanent catheter-related soft-tissue infections.4 Current vancomycin guidelines recommend that the trough serum concentration should be maintained between 15 and 20 mg/L for serious infections,9–11 but they do not address dosing strategies or appropriate pre-hemodialysis concentration for administration of vancomycin to patients undergoing hemodialysis.3,12 Furthermore, the recommended serum concentration of 15–20 mg/L was validated with patients who had normal kidney function, but the accuracy of this range for patients with stage 5 CKD who were receiving hemodialysis was not clearly addressed.
Several factors have been shown to affect the removal of vancomycin during hemodialysis sessions: the use of high-flux dialysis membranes, blood flow rate, dialysate flow rate, timing of vancomycin administration, duration of the session, presence of residual renal function, and actual body weight.2,3,6,12 For instance, in various studies, different high-flux dialysis membranes removed different amounts of the drug (25% to 50%) in each dialysis session.13–16 This variability may have been related to the differing composition of the dialysis membranes and whether or not vancomycin was infused during the last 30–90 min of dialysis.17 Use of these high-flux filters was implemented at St John Hospital and Medical Center, in Detroit, Michigan, in 2009. The institution’s protocol targets a pre-hemodialysis serum vancomycin concentration of 15–20 mg/L for all patients requiring vancomycin therapy.
The primary purpose of this study was to develop a prediction equation to assist clinicians in deciding on the optimal maintenance dose of vancomycin needed for hemodialysis patients to achieve the target pre-hemodialysis serum concentration of the drug. The secondary purposes of the study were to develop a data-driven vancomycin dosing nomogram and to explore the clinical feasibility of achieving a pre-hemodialysis serum vancomycin concentration of 15–20 mg/L in these patients.
Upon approval of the study protocol by the St John Hospital and Medical Centre Institutional Review Board, a prospective observational cohort study was conducted at this 772-bed teaching hospital in east Detroit, Michigan, with a convenience sample of 63 established hemodialysis patients, between January and June 2010. This sample exceeded the minimum sample of 55 patients needed to detect a modest effect size of 0.15, assuming α of 0.05 and 80% power for a linear regression model of 5 independent predictors (the model developed during the course of the study had 3 independent predictors). Patients were included in the study if they were 18 years of age or older, had stage 5 CKD with established hemodialysis (3 sessions per week), had suspected or confirmed MRSA infection, received at least 2 doses of vancomycin during the hospital admission (one loading dose and at least the first maintenance dose), and had serum vancomycin concentration measured after the first maintenance dose and before subsequent hemodialysis sessions. For the hemodialysis, high-flux filters (Optiflux F-160, Fresenius), with a surface area of 1.6 m2, were used.
Eligible patients with suspected or confirmed MRSA infection who received maintenance doses of vancomycin were enrolled in the study. All of the doses were given on hospital floors within a maximum of 6 h after completion of hemodialysis (i.e., doses were not given during the last 30–90 min of hemodialysis). The institutional protocol recommends the administration of vancomycin 500 mg IV after each hemodialysis session; however, pharmacists were left to use their clinical judgment for selection of the maintenance dose, as per the institution’s medial directive.
Patient body weight was obtained from electronic medical records. Interdialytic interval was the approximate time between 2 consecutive hemodialysis sessions. Baseline pre-hemodialysis serum vancomycin concentration was the concentration of drug that resulted from the loading dose or maintenance dose measured within a maximum of 6 h before commencement of the first hemodialysis session during the particular admission (either during morning laboratory testing or just before the hemodialysis session). Established hemodialysis patients were those with stage 5 CKD who were receiving hemodialysis. The vancomycin dose was measured in milligrams per kilogram, and no individual dose exceeded 2000 mg. Patients with suspected MRSA infection were those with an infection such as cellulitis, abscess, or osteomyelitis that was treated with vancomycin in the absence of any confirmatory culture results.
Data were analyzed using SPSS statistical software, version 22.0 (IBM, Armonk, New York). Before analysis, the data were explored for accuracy and possible violations of statistical assumptions, including multicollinearity, multivariate outliers, and multivariate normality. Descriptive statistics, such as general frequencies, means, and standard deviations, were calculated to describe the demographic and prognostic characteristics of the study sample. Given the clustered nature of the data (i.e., individual patients having multiple observations), multivariate linear generalized estimating equation (GEE) analysis was conducted to examine independent predictors of the actual pre-hemodialysis serum vancomycin concentration. Although it might be intuitive to use vancomycin dose as the outcome variable in the GEE analysis, pre-hemodialysis serum concentration was used instead, because this measure is a function of the dose that the patient receives.
Specifically, the analysis was performed on 164 observations obtained from the 63 patients. Insignificant variables were removed from the final GEE model before the analysis was repeated to yield a parsimonious model that was explained only by the significant variables. To evaluate the optimal cut-off point for pre-hemodialysis serum vancomycin concentration, the predicted values (generated by the GEE analysis) and the actual measured baseline values were categorized according to various cut-off points. This allowed cross-tabulation and calculation of the classification indices (i.e., sensitivity, specificity, and negative and positive predictive values) of the resulting models, which in turn allowed assessment of the most clinically relevant cut-off point based on the study data. Statistical significance was established using a 2-tailed α of 0.05.
Sample and dialysis characteristics are presented in Table 1. The results of the GEE analysis (Table 2) suggest that pre-hemodialysis serum vancomycin concentration was independently associated with maintenance dose (B = 0.658, p < 0.001), baseline pre-hemodialysis serum concentration (B = 0.492, p < 0.001), and interdialytic interval (B = −2.133, p < 0.001). These results were associated with a regression constant of 6.329, which yielded the following prediction equation (where HD = hemodialysis):
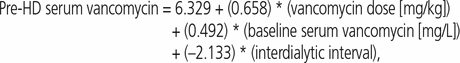
where interdialytic interval of 48 h had a value of 0 and inter-dialytic interval of 72 h had a value of 1.
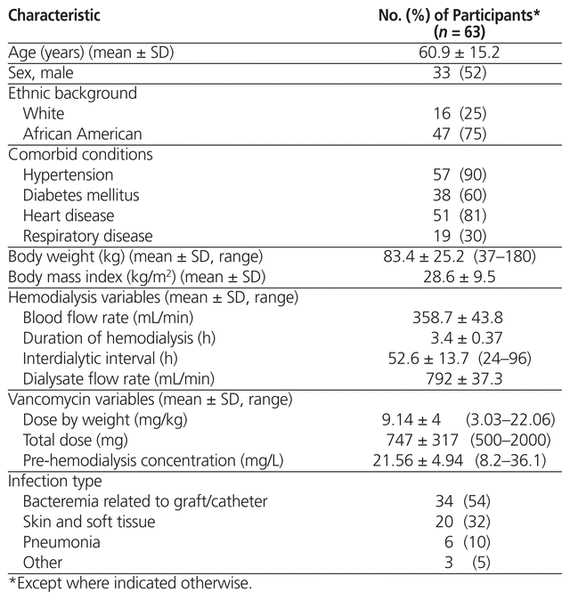
Table 1 Patient and Dialysis Characteristics
Table 2 Generalized Estimating Equation Model Identifying Predictors of Serum Vancomycin Concentration before Hemodialysis*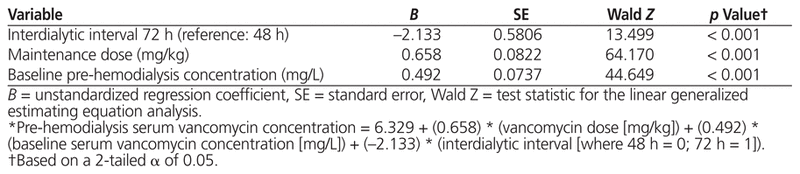
Table 3 displays the targeted vancomycin dose based on knowledge of the baseline pre-hemodialysis serum vancomycin concentration, the target pre-hemodialysis serum vancomycin concentration, and the interdialytic interval. According to this model, if the targeted serum concentration is 15–20 mg/L and the baseline pre-hemodialysis serum vancomycin concentration is also 15–20 mg/L, the mean required dose (and standard deviation) is 5.9 ± 0.82 mg/kg for an interdialytic interval of 48 h and 7.1 ± 1.99 mg/kg for an interdialytic interval of 72 h. However, if the baseline serum concentration is between 10 and 14.99 mg/kg, the required dose increases to 9.2 mg/kg for an interdialytic interval of 48 h and 10.0 mg/kg for an interdialytic interval of 72 h.
Table 3 Classification of Vancomycin Dose and Pre-HD Serum Concentration for Different Interdialytic Intervals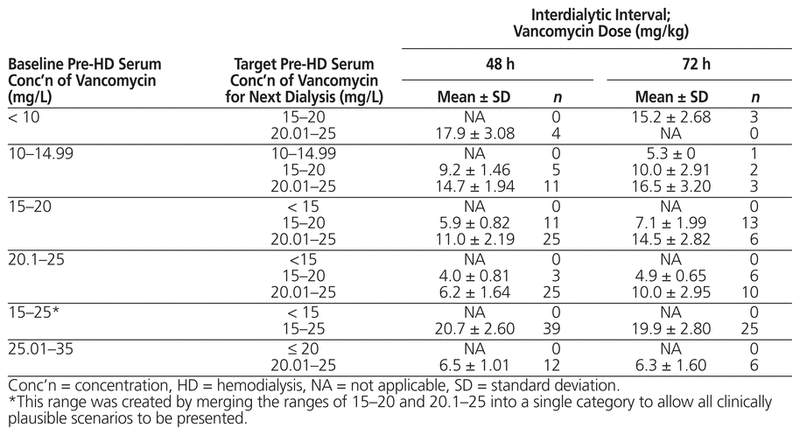
Table 4 shows 4 classification models based on different cut-off points for the actual and predicted pre-hemodialysis serum vancomycin concentrations, reflecting the sensitivity and specificity of each model with regard to the dose associated with those concentrations. Model 4 had the best characteristics overall: sensitivity of 96%, positive predictive value of 75%, and negative predictive value of 75%. It can also be inferred from Model 4 that the vancomycin dose for the 65.9% of patients (108/164) whose predicted serum concentrations matched their actual serum concentrations within the range of 15–25 mg/L was 8.88 mg/kg. However, according to Model 2, only 12.8% of patients (21/164) were correctly classified when the target pre-hemodialysis serum vancomycin concentration was narrowed to 15–20 mg/L.
Table 4 Classification Indices Highlighting Sensitivity, Specificity, PPV, and NPV According to Different Ranges of Predicted and Measured Serum Concentration of Vancomycin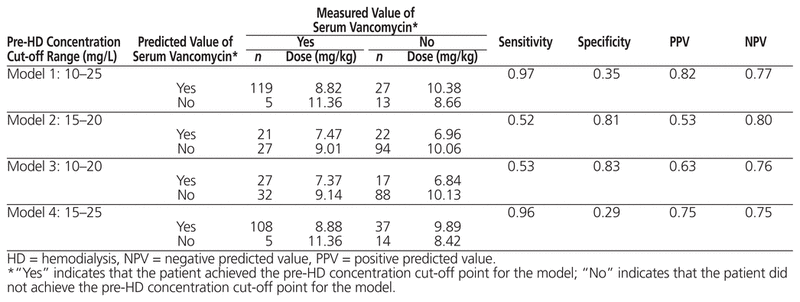
Optimal vancomycin dosing in patients who are undergoing hemodialysis has been controversial. Clinicians commonly select the dose of vancomycin on the basis of actual body weight (mg/kg) in accordance with data generated from patients with normal kidney function.2,9 Furthermore, the goal for trough serum vancomycin concentration of 15–20 mg/L that is recommended by current guidelines9–11 was validated with data from patients with normal kidney function. This situation creates uncertainty about the applicability of current practices pertaining to maintenance dosing of vancomycin and the associated pre-hemodialysis serum vancomycin concentrations in patients with stage 5 CKD. The current study used data from patients with stage 5 CKD who were receiving hemodialysis to provide additional evidence concerning the optimal vancomycin dose and the corresponding pre-hemodialysis serum vancomycin concentration in this patient population.
The analysis showed that pre-hemodialysis serum vancomycin concentration was a function of baseline pre-hemodialysis serum vancomycin concentration, the maintenance dose of vancomycin, and interdialytic interval. This information was used to generate a prediction equation (Table 2), which was then used to calculate the targeted vancomycin dose, given particular values for baseline pre-hemodialysis serum vancomycin concentration, targeted pre-hemodialysis serum vancomycin concentration, and interdialytic interval. In addition, a data-driven cross-tabulation (Table 3) was generated to outline the various vancomycin doses that would be associated with specific values for baseline and target pre-hemodialysis serum vancomycin concentrations and interdialytic interval of 48 h versus 72 h. According to this table, vancomycin dose should not be based exclusively on the targeted pre-hemodialysis serum concentration. Rather, clinicians must also factor in the baseline pre-hemodialysis serum vancomycin concentration and the inter-dialytic interval for a more precise estimate of the required dose.
These findings suggest that targeting a narrow pre-hemodialysis concentration of 15–20 mg/L is very restrictive, with an average required dose of 7 mg/kg or less when the pre-hemodialysis baseline value is around 15–20 mg/L and the interdialytic interval is 72 h, a range that is not feasible for the majority of patients. Specifically, only 29.3% (n = 48) of the observations in the current study had a measured pre-hemodialysis concentration of 15–20 mg/L. This finding is consistent with the arguments of Vandecasteele and De Vriese1 and Jeremiah and others,18 who explained that achieving such a narrow range in patients undergoing hemodialysis is not practical. In fact, a post hoc analysis in the current study showed that only 36.0% (n = 59) of the observations fit within the range of 10–20 mg/L, which further indicates that such a range of serum concentrations is not feasible in this patient population. Cut-off ranges of 10–25 mg/L and 15–25 mg/L were observed in 75.6% (n = 124) and 68.9% (n = 113) observations, respectively.
The goal was to report the range of cut-off points associated with the greatest sensitivity (i.e., the proportion of observations correctly classified as having a cut-off point within the specified range) and the highest positive predictive value (i.e., the likelihood that a given observation falls within the range of cut-off points into which the model has deemed the observation to fall). The study classification indices (Table 4) showed that the ranges of cut-off points of 15–20 mg/L and 10–20 mg/L were associated with low sensitivity (52% and 53%, respectively), which indicates that these 2 models have no better than a 50–50 chance of correctly classifying patients. Although Model 1 had the highest sensitivity (97%) and the best predictive values, the range of cut-off points associated with this model was very wide (10–25 mg/L), which limits its clinical application. Model 4 presented the most clinically relevant range of cut-off points, 15–25 mg/L, which was associated with an impressive sensitivity value of 96%. Furthermore, this model had reasonable positive and negative predictive values (both 75%), suggesting that it has 75% accuracy in correctly predicting the pre-hemodialysis serum vancomycin concentration in any given patient. Interestingly, the post hoc analysis indicated that the mean pre-hemodialysis concentration of the observations falling within this range of cutoff points was 20.5 mg/L (standard deviation 2.7; median 20.8).
The literature shows that variation in vancomycin dosing is associated with variability in pre-hemodialysis concentration and also that there is no consistency in reported pre-hemodialysis concentrations. For example, Pai and Pai19 and Ariano and others20 used a fixed maintenance dose of 500 mg during the last 30–60 minutes of each hemodialysis session and achieved a pre-hemodialysis concentration of 5–20 mg/L in 93% and 96% of patients, respectively. However, current guidelines target a much narrower range of 15–20 mg/L. Barth and DeVincenzo13 achieved a pre-hemodialysis serum vancomycin concentration of 10–25 mg/L in 82% of their observations, based on a maintenance dose of 500 mg following completion of the hemodialysis session. Foote and others21 administered 1.0 g of vancomycin as a loading dose in 5 healthy hemodialysis patients and recommended a loading dose of 25 mg/kg in the final 1–2 h of dialysis, administered at a rate of 1 g/h. However, these investigators tested only a single dose of vancomycin. Similarly, Pollard and others22 recommended a loading dose of 20 mg/kg after dialysis, followed by a maintenance dose of 15 mg/kg every 7 days. Furthermore, Crawford and others,23 who prospectively administered a single dose of vancomycin (35 mg/kg) at the end of dialysis, at a rate of 1 g/h, to 11 patients, reported that a once-weekly dose of vancomycin (35 mg/kg) did not achieve a vancomycin concentration of at least 10 mg/L before the third scheduled hemodialysis session. Zvonar and others24 retrospectively evaluated 163 courses of vancomycin therapy in 105 patients at their institution and found that the most common dose was 500 mg after or during the hemodialysis session. Panais and others25 administered a maximum loading dose of 1500 mg vancomycin in 38 courses, followed by 500 mg after each dialysis session and concluded that their protocol was successful in achieving a therapeutic trough concentration of the drug. The applicability of this dosing strategy is limited, because these authors used both high- and low-flux filters in their study. Taylor and Allon26 administered a maintenance dose of 1000 mg in the last hour of hemodialysis and reported that 56% of patients had pre-hemodialysis concentration between 10 and 20 mg/L and only 23% had a pre-hemodialysis concentration between 20 and 25 mg/L. Unlike the current study, all of the aforementioned studies either reported a wide range of pre-hemodialysis serum vancomycin concentrations or had a relatively small proportion of patients achieving the recommended range of 15–20 mg/L. In the current study, a clinically meaningful pre-hemodialysis concentration of 15–25 mg/L was achieved for 68.9% of observations, which was also associated with the impressive sensitivity value of 96%.
The current study and that of Vandecasteele and others6 identified similar variables (pre-hemodialysis vancomycin concentration, interdialytic interval, and body weight). Interestingly however, although both studies implemented a clustered approach to data analysis, the results of Vandecasteele and others6 were validated using a 3-phase approach that included testing the model on a new sample of patients. Their model was validated using sensitivity and predictive values based on predicted pre-hemodialysis cut-off values. In contrast, Zelenitsky and others27 developed a new vancomycin dosing protocol to achieve a pre-hemodialysis concentration of 10–20 mg/L, with an optimal goal of 15–20 mg/L. Similar to the current study, patients in the study by Zelenitsky and others27 received a maintenance dose that ranged between 500 and 1000 mg. However, the pre-hemodialysis concentration was 10–20 mg/L for 65.5% of patients, 10–22 mg/L for 89.7%, and 15–20 mg/L for only 37.9%. These findings further support the argument that a pre-hemodialysis concentration of 15–20 mg/L is restrictive and therefore may not be clinically feasible for a majority of patients undergoing hemodialysis. However, the models of both Vandecasteele and others6 and Zelenitsky and others27 were based on administration of vancomycin during the last 60 min of hemodialysis, whereas the model in the current study was based on administration of vancomycin after completion of the hemodialysis session. Moreover, Mason and others28 found that administering vancomycin 30 mg/kg in the last 1–2 h of hemodialysis achieved a similar pre-hemodialysis concentration as administering a vancomycin dose of 15 mg/kg after completion of the hemodialysis session. Similarly, Lucksiri and others15 detected that 24% of vancomycin was removed if the dose was administered during the last hour of dialysis, whereas none of the dose was lost if it was administered after completion of the session. Unlike most of the existing studies, maintenance doses of vancomycin in the current study and a few others13,28–30 were always administered after completion of hemodialysis. These differences are worthy of mention because they affect the net dose of vancomycin received. Thus, it is important that clinicians follow a uniform protocol for vancomycin dosing, including the timing of administration.
The limitations of this study include collection of data from patients with stage 5 CKD who were undergoing hemodialysis, without consideration of whether the patients might have had residual renal function. The interdialytic interval was not exactly 48 versus 72 h because samples for pre-hemodialysis measurements were drawn within 6 h before the procedure, whereas vancomycin doses were administered within 6 h after completion of hemodialysis. In addition, the cross-tabulation results (Table 3) for determining the required dose (based on knowledge of baseline pre-hemodialysis serum vancomycin concentration, target pre-hemodialysis serum vancomycin concentration, and interdialytic interval) did not include all possible dose scenarios. It is recommended that these findings be interpreted with caution and that they be replicated and validated before any implementation in practice settings. Finally, data for patient weight was calculated from the average of wet and dry weights as documented in the electronic medical records. In addition, mean patient weight had a relatively large standard deviation, which resulted in a wide range and large standard deviation for the weight-based dose (mg/kg); this might have compromised the predictive ability of the regression equation. Therefore, it is recommended that these findings be validated with samples that are more homogeneous with regard to body weight.
The findings of the current study and those of several other authors indicate that targeting a pre-hemodialysis concentration of 15–20 mg/L may be highly restrictive; this target is unlikely to be achieved in the majority of patients, and the associated outcomes have not yet been established in clinical studies. The current findings support a more liberal, yet clinically meaningful, range of 15–25 mg/L, which yielded considerable sensitivity (96%) and a positive predictive value of 75%. However, the current findings were based on an observational cohort study and are therefore subject to potential bias. Furthermore, they have not yet been validated or replicated with another sample. In addition, the effect that a range of 15–25 mg/L could have on the residual renal function of this particular patient population is unclear. It is therefore recommended that these findings be replicated and their effect on the residual function of patients with stage 5 CKD be investigated before any practice generalizations.
1 Vandecasteele SJ, De Vriese AS. Recent changes in vancomycin use in renal failure. Kidney Int. 2010;77(9):760–4.

2 Pallotta KE, Manley HJ. Vancomycin use in patients requiring hemodialysis: a literature review. Semin Dial. 2008;21(1):63–70.

3 El Nekidy WS, El-Masri MM, Umstead GS, Dehoorne-Smith M. Factors influencing vancomycin loading dose for hospitalized hemodialysis patients: prospective observational cohort study. Can J Hosp Pharm. 2012;65(6): 436–42.
4 Vandecasteele SJ, De Vriese AS. Vancomycin dosing in patients on intermittent hemodialysis. Semin Dial. 2011;24(1):50–5.

5 Jones RN. Key considerations in the treatment of complicated staphylococcal infections. Clin Microbiol Infect. 2008;14 Suppl 2:3–9.

6 Vandecasteele SJ, De Bacquer D, De Vriese AS. Implementation of a dose calculator for vancomycin to achieve target pre-HD concentrations of 15–20 microg/mL in persons undergoing hemodialysis. Clin Infect Dis. 2011;53(2):124–9.

7 Crawford BS, Largen RF, Walton T, Doran JJ. Once-weekly vancomycin for patients receiving high-flux hemodialysis. Am J Health Syst Pharm. 2008;65(13):1248–53.

8 Zoer J, Schrander-van der Meer AM, van Dorp WT. Dosage recommendation of vancomycin during haemodialysis with highly permeable membranes. Pharm World Sci. 1997;19(4):191–6.

9 Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. Erratum in: Am J Health Syst Pharm. 2009;66(10):887.
10 American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.

11 Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. Erratum in: Clin Infect Dis. 2011;53(3):319.

12 Brown M, Polisetty R, Gracely EJ, Cuhaci B, Schlecht HP. Weight-based loading of vancomycin in patients on hemodialysis. Clin Infect Dis. 2011; 53(2):164–6.

13 Barth RH, DeVincenzo N. Use of vancomycin in high-flux hemodialysis: experience with 130 courses of therapy. Kidney Int. 1996;50(3):929–36.

14 Welage LS, Mason NA, Hoffman EJ, Odeh RM, Dombrouski J, Patel JA, et al. Influence of cellulose triacetate hemodialyzers on vancomycin pharmacokinetics. J Am Soc Nephrol. 1995;6(4):1284–90.
15 Lucksiri A, Scott MK, Mueller BA, Hamburger RJ, Sowinski KM. CAHP-210 dialyzer influence on intra-dialytic vancomycin removal. Nephrol Dial Transplant. 2002;17(9):1649–54.

16 Scott MK, Mueller BA, Clark WR. Vancomycin mass transfer characteristics of high-flux cellulosic dialysers. Nephrol Dial Transplant. 1997; 12(12):2647–53.
17 Scott MK, Macias WL, Kraus MA, Clark WR, Carfagna MA, Mueller BA. Effects of dialysis membrane on intradialytic vancomycin administration. Pharmacotherapy. 1997;17(2):256–62.
18 Jeremiah CJ, Wills C, Bayly A, Perry GJ, Davis JS, Tong SY, et al. Vancomycin dosing nomogram for haemodialysis patients. Nephrology (Carlton). 2014;19(8):513–4.
19 Pai AB, Pai MP. Vancomycin dosing in high flux hemodialysis: a limited sampling algorithm. Am J Health Syst Pharm. 2004;61(17):1812–6.
20 Ariano RE, Fine A, Sitar DS, Rexrode S, Zelenitsky SA. Adequacy of a vancomycin dosing regimen in patients receiving high-flux hemodialysis. Am J Kidney Dis. 2005;46(4):681–7.

21 Foote EF, Dreitlein WB, Steward CA, Kapoian T, Walker JA, Sherman RA. Pharmacokinetics of vancomycin when administered during high flux hemodialysis. Clin Nephrol. 1998;50(1):51–5.
22 Pollard TA, Lampasona V, Akkerman S, Tom K, Hooks MA, Mullins RE, et al. Vancomycin redistribution: dosing recommendations following high-flux hemodialysis. Kidney Int. 1994;45(1):232–7.

23 Crawford BS, Largen RF, Walton T, Doran JJ. Once-weekly vancomycin for patients receiving high-flux hemodialysis. Am J Health Syst Pharm. 2008; 65(13):1248–53.

24 Zvonar R, Natarajan S, Edwards C, Roth V. Assessment of vancomycin use in chronic haemodialysis patients: room for improvement. Nephrol Dial Transplant. 2008;23(11):3690–5.

25 Panais R, Hirsch DJ, Dipchand C, Storsley L, Finkle SN. A protocolized approach to vancomycin dosing in conventional hemodialysis. J Nephrol. 2010;23(5):569–74.
26 Taylor ME, Allon M. Practical vancomycin dosing in hemodialysis patients in the era of emerging vancomycin resistance: a single-center experience. Am J Kidney Dis. 2010; 55:1163–5.


27 Zelenitsky SA, Ariano RE, McCrae ML, Vercaigne LM. Initial vancomycin dosing protocol to achieve therapeutic serum concentrations in patients undergoing hemodialysis. Clin Infect Dis. 2012;55(4):527–33.

28 Mason NA, Neudeck BL, Welage LS, Patel JA, Swartz RD. Comparison of 3 vancomycin dosage regimens during hemodialysis with cellulose triacetate dialyzers: post-dialysis versus intradialytic administration. Clin Nephrol. 2003;60(2):96–104.

29 Lin SY, Shen MC, Hwang SJ, Chen YH, Chen TC, Chiu YW, et al. Evaluation of vancomycin dosing protocols to achieve therapeutic serum concentrations in patients receiving high-flux haemodialysis. Int J Antimicrob Agents. 2014;43(4):384–5.

30 Brown M, Polisetty R, Gracely EJ, Cuhaci B, Schlecht HP. Weight-based loading of vancomycin in patients on hemodialysis. Clin Infect Dis. 2011;53(2):164–6.

Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors sincerely acknowledge the invaluable contributions and insights of Bruce A Mueller, PharmD, FCCP, FASN, FNKF, Professor and Associate Dean of Academic Affairs, University of Michigan College of Pharmacy.
Canadian Journal of Hospital Pharmacy, VOLUME 69, NUMBER 5, September-October 2016