Reza Rafizadeh, Ricky D Turgeon, Josh Batterink, Victoria Su, Anthony LauABSTRACT
Background
Symptomatic venous thromboembolism (VTE) occurs in about 1% of patients within 3 months after admission to a medical unit. Recent evidence for thromboprophylaxis in an unselected medical inpatient population has suggested only a modest net benefit. Consequently, guidelines recommend careful risk stratification to guide thromboprophylaxis.
Objectives
To compare candidacy for thromboprophylaxis according to 4 risk stratification models: a regional preprinted order (PPO) set used in the study institution, the Padua Prediction Score, and the IMPROVE predictive and associative risk assessment models.
Methods
A retrospective review of health records was undertaken for patients with no contraindication to pharmacologic thromboprophylaxis who were admitted to the internal medicine service of a teaching hospital between April and July 2013.
Results
Of the 298 patients in the study cohort, 238 (80.0%) received pharmacologic thromboprophylaxis on admission, ordered according to the regional PPO. However, according to the Padua and the IMPROVE predictive risk assessment models, only 64 (21.5%) and 21 (7.0%) of the patients, respectively, were eligible for thromboprophylaxis at the time of admission. On the basis of risk factors identified during the subsequent hospital stay, 54 (18.1%) of the patients were eligible for thromboprophylaxis according to the IMPROVE associative model. Chance-corrected agreement between the PPO and the published risk assessment models was generally poor, with kappa coefficients of 0.109 for the PPO compared with the Padua Prediction Score and 0.013 for the PPO compared with the IMPROVE predictive model.
Conclusions
These data suggest that quantitative models such as the Padua Prediction Score and the IMPROVE models identify more patients at low risk of venous thromboembolism than do in-hospital qualitative risk assessment models. Adoption of these guideline-based risk assessment models for predicting thromboembolic risk in medical inpatients could reduce the use of pharmacologic thromboprophylaxis from 80% to as low as 7%. Further external prognostic validation of risk assessment models and impact analysis studies may show improvements in safety and resource utilization.
KEYWORDS: thromboprophylaxis, venous thromboembolism, Padua Prediction Score, IMPROVE assessment models
RÉSUMÉ
Contexte
La thromboembolie veineuse symptomatique se produit chez environ 1 % des patients dans les trois mois suivant leur admission à un service médical. Des données récentes portant sur la thromboprophylaxie chez une population non sélectionnée de patients hospitalisés ne suggéraient qu’un modeste avantage. Par conséquent, les lignes directrices recommandent une stratification du risque rigoureuse pour guider l’emploi d’une thromboprophylaxie.
Objectifs
Comparer l’admissibilité à la thromboprophylaxie en fonction de quatre modèles de stratification du risque : un ensemble d’ordonnances préimprimées adopté dans une région et utilisé dans l’établissement à l’étude, le score prédictif de Padua et les modèles prédictifs et associatifs d’évaluation du risque issus de l’étude IMPROVE.
Méthodes
Une analyse rétrospective des dossiers médicaux a été menée auprès des patients ne présentant pas de contre-indication à la thromboprophylaxie médicamenteuse qui ont été admis au service de médecine interne d’un hôpital universitaire entre avril et juillet 2013.
Résultats
Parmi les 298 patients de l’étude de cohorte, 238 (80,0 %) ont reçu une thromboprophylaxie médicamenteuse au moment de l’admission, prescrite conformément à l’ensemble d’ordonnances préimprimées en usage dans la région. Or, respectivement selon les modèles prédictifs d’évaluation du risque Padua et IMPROVE, seuls 64 (21,5 %) et 21 (7,0 %) des patients étaient admissibles à la thromboprophylaxie au moment de l’admission. En fonction de facteurs de risques identifiés pendant le séjour subséquent à l’hôpital, 54 (18,1 %) des patients étaient admissibles à la thromboprophylaxie selon le modèle associatif IMPROVE. L’accord corrigé pour le hasard entre l’ensemble d’ordonnances préimprimées et les modèles d’évaluation du risque publiés était généralement faible, les coefficients de kappa étant de 0,109 pour l’ensemble d’ordonnances préimprimées comparé au score prédictif de Padua et de 0,013 pour l’ensemble d’ordonnances préimprimées comparé au modèle prédictif IMPROVE.
Conclusions
Ces données suggèrent que les modèles quantitatifs comme le score prédictif de Padua et les modèles IMPROVE permettent de dépister plus de patients qui sont à faible risque de thromboembolie veineuse que ne le permettent les modèles qualitatifs d’évaluation du risque propres aux hôpitaux. L’adoption de ces modèles d’évaluation du risque mis de l’avant dans des lignes directrices pour prédire les risques d’événements thromboemboliques chez les patients médicaux hospitalisés pourrait réduire l’utilisation de la thromboprophylaxie médicamenteuse, qui pourrait passer de 80 % à aussi peu que 7 %. De plus amples validations externes quant à la valeur prédictive des modèles d’évaluation du risque et des études d’analyse d’impact pourraient montrer des améliorations à la sécurité et une réduction de l’utilisation des ressources.
MOTS CLÉS: thromboprophylaxie, thromboembolie veineuse, score prédictif de Padua, modèles d’évaluation IMPROVE
Venous thromboembolism (VTE) in hospital inpatients causes significant morbidity and mortality.1 VTE leading to clinical symptoms occurs in about 1% of patients after admission to a medical unit, although as many as 11% of patients with multiple risk factors for VTE may experience symptoms.2,3 Various modifiable and nonmodifiable characteristics have been identified as risk factors for VTE, with few being consistently observed across studies.4
Heparin-based regimens are most often used for thromboprophylaxis in medical inpatients. A meta-analysis of randomized controlled trials4 and one later randomized trial5 suggested that pharmacologic thromboprophylaxis had no net effect on mortality and produced a 0.28% absolute reduction in clinically detected pulmonary embolism at the cost of a similar 0.19% absolute increase in major bleeding events. Furthermore, a Cochrane review of randomized controlled trials showed that pharmacologic VTE prophylaxis reduced deep vein thrombosis and increased major bleeding events, but had no significant effect on mortality or pulmonary embolism in medical inpatients at risk of VTE.6
At St Paul’s Hospital, a large teaching hospital in Vancouver, British Columbia, initiation of thromboprophylaxis is guided by a regional, hospital-wide preprinted order (PPO; see Appendix 1, available at www.cjhp-online.ca/index.php/cjhp/issue/view/118/showToc). This PPO stratifies patients as having high, moderate, or low risk for VTE events and recommends thromboprophylaxis for all but low-risk patients. The tool, which was implemented in 2010, was designed to meet the required organizational practices of Accreditation Canada, which mandate that “the team must identify clients at risk for venous thromboembolism and provide appropriate evidence-based VTE prophylaxis”.7 VTE risk factors included in this PPO parallel the list of risk factors in the previous edition of the American College of Chest Physicians (ACCP) guidelines for prevention of VTE, published in 2008.8
Given the narrow benefit-to-risk balance of thromboprophylaxis for medical inpatients, professional organizations such as Accreditation Canada7 and the ACCP2 have recommended careful stratification and treatment of patients on the basis of risk of VTE and bleeding events. Thus, several investigators have developed clinical prediction rules to provide objective, quantifiable estimates of VTE risk based on independent risk factors.3,9
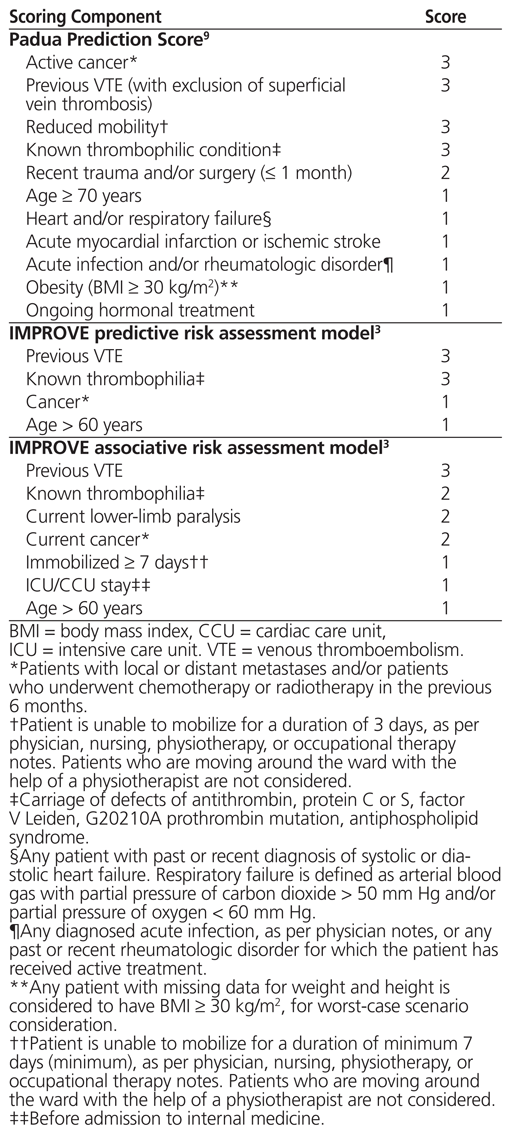
One such risk assessment model is the Padua Prediction Score,9 which was endorsed in the ninth edition of the ACCP guidelines.2 This model was generated empirically by integrating the Kucher model10 with additional items and by slightly modifying the assigned scores. With this tool, users assign points to each patient on the basis of 11 VTE risk factors and determine whether the patient is at high or low risk for VTE in relation to a cutoff of 4 points (Table 1). This risk assessment model was validated by Barbar and others9 in a prospective cohort of 1180 patients who were admitted to the internal medicine service of a hospital in Padua, Italy, between January 2007 and December 2008. Among the patients who did not receive pharmacologic thromboprophylaxis, rates of symptomatic VTE in the high-risk and low-risk groups were 11.8% and 0.3%, respectively.9
Table 1 Padua, IMPROVE Predictive, and IMPROVE Associative Risk Assessment Models, Customized for Comparison with Preprinted Order Used in the Study Hospital
Another set of risk assessment models included in the ACCP guidelines comes from the international IMPROVE cohort.3 Based on data for 15 156 prospectively and retrospectively enrolled medical inpatients, 2 variants of the IMPROVE risk assessment model were derived. The first model, termed the IMPROVE predictive model, is based on 4 variables that are typically known at hospital admission, which were independently associated with incidence of VTE up to 90 days after admission (Table 1). A second model, the IMPROVE associative model, is based on 7 factors that may be identified at any point during the hospital stay (after admission) (Table 1). Together, the IMPROVE risk assessment models allow continuous assessment of VTE risk over the entire hospital stay.3 In the IMPROVE cohort, with each of the predictive and associative models, those with a score of 0 to 1 had an observed 90-day incidence of symptomatic VTE less than or equal to 1%, whereas those with a score of 2 or above had a 3-month VTE risk of 2% or higher.3 Notably, the investigators externally validated the IMPROVE associative risk assessment model in 2 additional databases, both of which showed accurate identification of patients at sufficiently low risk of VTE to omit thromboprophylaxis.11,12
The primary objective of the current study was to compare the proportion of patients considered at high risk for VTE (and thus eligible for VTE prophylaxis) according to 3 published risk assessment models (the Padua Prediction Score and the IMPROVE predictive and associative models) and to compare these results with the proportion identified by the study institution’s PPO for VTE. In addition, the study aimed to evaluate concordance of the institutional PPO with the guidelinerecommended risk assessment models. The secondary objective was to characterize the prevalence of individual risk factors for VTE among patients admitted to the medical unit of this large teaching hospital.
This study was a retrospective chart review of patients admitted to the internal medicine service of St Paul’s Hospital between April and July 2013. The inclusion criteria were age 18 years or older and admission to the internal medicine service. Patients were excluded if VTE or bleeding was present at the time of admission, if they were receiving therapeutic anticoagulation before or at the time of admission, or if they had any contraindication to pharmacologic thromboprophylaxis. Ethics approval for this study was obtained from the Providence Health Care and Fraser Health authorities.
Data on the presence of risk factors for VTE and the rate of VTE and major bleeding events were retrospectively collected from the patients’ scanned and electronic health records. The main data collected from scanned admission, consultation, progress, and discharge notes were diagnosis on admission; history of VTE, thrombophilia, heart and/or respiratory failure, acute myocardial infarction or ischemic stroke, acute infection and/or rheumatologic disorder, immobility or reduced mobility; body weight; stay in the intensive care unit or cardiac care unit; presence of peripherally inserted central catheter line; concomitant antiplatelet therapy; and transfusion of packed erythrocytes or whole blood. Hemoglobin levels were ascertained from electronic laboratory records.
Two of the investigators (R.R., A.L.) were responsible for collecting the data as listed above. The same 2 investigators then applied the risk assessment models to determine each patient’s risk for VTE. This work was subdivided, such that for each patient, the risk was scored by one investigator.
The investigators found that some of the risk factor definitions in the original Padua tool3 were ambiguous. To improve the consistency of data collection, these definitions were refined by consensus after pilot data collection for the first 6 patients (Table 1).
A convenience sample size of 300 patients was chosen by consensus, without formal sample size calculation. Continuous and nominal patient data are reported as means and percentages, respectively.
Chance-corrected agreement between each of the IMPROVE predictive score, the Padua Prediction Score, and the institutional PPO was determined by paired comparisons and calculation of the Cohen kappa coefficient. Possible values for the kappa coefficient range from 0 to 1, with higher values representing greater levels of chance-corrected agreement.
Overall, charts for 398 patients were reviewed. Of these, 100 patients were excluded for the following reasons: receipt of therapeutic anticoagulation on admission (n = 55), admission with active bleeding (n = 38), and diagnosis of VTE on admission (n = 7).
Patient characteristics on admission and relevant VTE risk factors are presented in Table 2. Overall, 238 (80.0%) of the patients received thromboprophylaxis during their hospital stay. The mean age of all patients was 59 years, and 136 (45.6%) were female. The median risk assessment values were 2 for the Padua Predictive Score, 1 for the IMPROVE predictive score, and 1 for the IMPROVE associative score.
Table 2 Characteristics of Patients in a Review of VTE Risk Assessment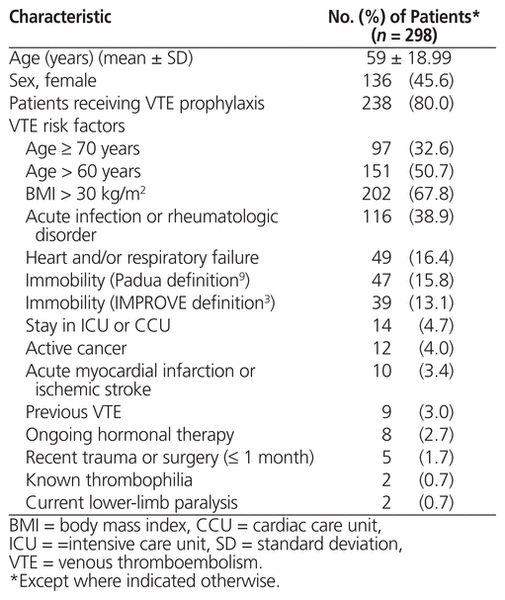
The proportion of patients for whom thromboprophylaxis was indicated was 80.0% (238/298) according to the PPO, 21.5% (64/298) according to the Padua Prediction Score, 7.0% (21/298) according to the IMPROVE predictive model, and 18.1% (54/298) according to the IMPROVE associative model.
Table 3 shows the kappa coefficient for comparisons between the PPO and the Padua Prediction Score and the IMPROVE predictive model, all of which are used at the time of admission. The IMPROVE associative model was not compared with the PPO because it was not used at the time of admission in the original derivation study.3 The kappa coefficients were 0.109 for comparison of the PPO and the Padua Prediction Score and 0.013 for comparison of the PPO and the IMPROVE predictive model; these values suggest a low level of agreement. In a separate analysis comparing the 3 risk assessment models (without reference to the PPO), there was only moderate agreement between the Padua Prediction Score and the IMPROVE predictive model (kappa coefficient 0.373) (Table 4). However, agreement between the Padua Prediction Score and the IMPROVE associative model was high (kappa coefficient 0.726) (Table 4).
Table 3 Agreement between Preprinted Order and Other Risk Assessment Models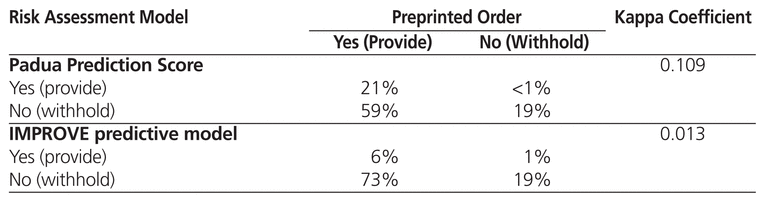
Table 4 Agreement between Padua Prediction Score and IMPROVE Predictive and Associative Models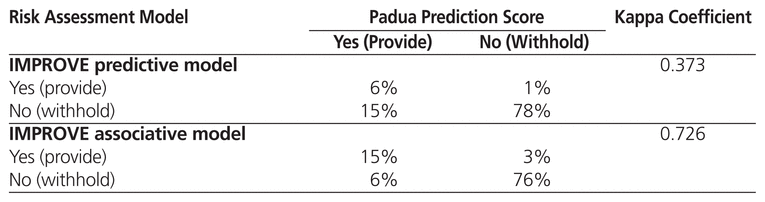
In the context of available evidence suggesting low overall rates of VTE among medical inpatients,2,3,6 pharmacologic thromboprophylaxis seems to be overutilized in the authors’ institution. The institutional PPO, which is not based on a validated set of VTE risk factors in medical inpatients and which does not assign risk scores to individual patients, led to prescription of pharmacologic thromboprophylaxis on admission for 80% of patients in the study cohort. To our knowledge, this PPO reflects the approach taken by many Canadian and international institutions. By retrospectively calculating risk using published, objective, patient-specific risk assessment models, we have shown that the use of thromboprophylaxis could be reduced from 80% to as low as 7%. Consequently, we hypothesize that modifying the institutional PPO to stratify patients according to one of the published risk assessment models would result in fewer patients receiving unnecessary pharmacologic thromboprophylaxis. Published risk assessment models have false-negative rates of less than 1%, which suggests that they can be used to safely select patients who will not benefit from thromboprophylaxis.
The IMPROVE predictive model was the most exclusive risk assessment model at the time of admission, classifying 7.0% of patients as having a high risk of VTE. Because the IMPROVE models were derived with the intention of ongoing VTE risk assessment, both of these models were assessed in the current study. Calculating scores for the IMPROVE associative model using data known at any point during hospitalization, we identified a further 11% of patients who would become candidates for thromboprophylaxis during their hospital stay; as a result, the overall percentage identified by both IMPROVE models was comparable to the 21.5% classified as having high risk with the Padua Prediction Score.
A major contributor to overuse of prophylaxis with the institutional PPO is imprecise definitions for the component risk factors. In practical terms, this lack of clarity in definitions can be expected to result in high inter-rater variability, leading in turn to inconsistencies in care, as well as low specificity in risk stratification. Some of the risk factors in the Padua Prediction Score also suffer from this imprecision. As a result, we found it necessary to redefine some of the risk factors for the purpose of our own data collection. Because of these changes, the sensitivity and specificity of the modified Padua Prediction Score used in the current study may differ from those in the original validation study.9 Indeed, a prospective external validation study of the Padua Prediction Score in 1080 medical inpatients with sepsis13 failed to replicate the excellent findings of the original validation study.9 One explanation for this failure is the fact that the authors of these 2 validation studies used different definitions of the risk factors included within the scoring system. Conversely, the definitions used in the IMPROVE models were clear and reproducible.
The kappa coefficient used for this analysis is a statistic that evaluates dichotomous agreement adjusted for chance between 2 raters or decision tools.14 Here, the kappa coefficient was used to evaluate concordance between the institutional PPO and the guideline-recommended risk assessment models. The PPO had generally poor agreement with the published models, whereas the Padua Prediction Score calculated at the time of admission corresponded well with the combined IMPROVE predictive and associative models. In practical terms, this finding would translate to similar rates of thromboprophylaxis initiation over the course of the hospital stay with these 2 approaches to risk assessment.
Several study limitations should be noted. First, this study was a retrospective chart review, and data collection was limited to what was available in the chart. Second, the convenience sample size reflects only a single time period. Third, this study was not a validation study or an impact analysis study; consequently, it is not possible to evaluate the effect on outcomes or cost of changing or using these tools.
The data presented here suggest that quantitative models such as the Padua Prediction Score and the IMPROVE risk assessment models identify more patients at low risk of VTE than do in-hospital qualitative risk assessment models. Adoption of these guideline-based risk assessment models for prediction of VTE risk in medical inpatients may reduce the use of pharmacologic thromboprophylaxis from 80% to as low as 7%. Further external prognostic validation of these risk assessment models and impact analysis studies may demonstrate improvements in safety and resource utilization.
Pharmacologic thromboprophylaxis has some disadvantages, including increased risk of bleeding, patient discomfort from subcutaneous injections, cost, and heparin-induced thrombocytopenia. Thus, future studies should evaluate model-guided initiation of thromboprophylaxis in medical inpatients with regard to efficacy, safety, cost-effectiveness, and overall impact on the efficiency of the health care system.
1 Prandoni P, Samama MM. Risk stratification and venous thromboprophylaxis in hospitalized medical and cancer patients. Br J Haematol. 2008; 141(5): 587–97.
2 Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, et al. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e89S–119S.
3 Spyropoulos AC, Anderson FA Jr, FitzGerald G, Decousus H, Pini M, Chong BH, et al.; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011; 140(3):706–14.
4 Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146(4):278–88.
5 Kakkar AK, Cimminiello C, Goldhaber SZ, Parakh R, Wang C, Bergmann JF; LIFENOX Investigators. Low-molecular-weight heparin and mortality in acutely ill medical patients. LIFENOX study. N Engl J Med. 2011; 365(26):2463–72.
6 Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database Syst Rev. 2014;(5):CD003747.
7 Required organizational practices handbook 2014. Ottawa (ON): Accreditation Canada; 2013.
8 Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al.; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):381S–453S.
9 Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–7.
10 Kucher N, Koo S, Quiroz R, Cooper JM, Paterno MD, Soukonnikov B, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–77.
11 Mahan E, Liu Y, Turpie G, Vu J, Heddle N, Cook R, et al. External validation of a risk assessment model for venous thromboembolism in the hospitalized acutely-ill medical patient (VTE-VALOURR). Thromb Haemost. 2014; 112(4):692–9.
12 Rosenberg D, Eichorn A, Alarcon M, McCullagh L, McGinn T, Spyropoulos AC. External validation of the risk assessment model of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) for medical patients in a tertiary health system. J Am Heart Assoc. 2014; 3(6):e001152.

13 Vardi M, Ghanem-Zoubi NO, Zidan R, Yurin V, Bitterman H. Venous thromboembolism and the utility of the Padua Prediction Score in patients with sepsis admitted to internal medicine departments. J Thromb Haemost 2013;11(3):467–73.
14 Bakeman R, Gottman JM. Observing interaction: an introduction to sequential analysis. 2nd ed. Cambridge (UK): Cambridge University Press; 1997.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 69, NUMBER 6, November-December 2016