Nina Kassett, Rosalind Sham, Rosanne Aleong, Daisy Yang, Michael Kirzner, Aidlee CraftABSTRACT
Background
There is a paucity of literature describing the implementation of antimicrobial stewardship programs (ASPs) in long-term care (LTC) facilities. The current study evaluated the impact of an ASP that was implemented across a geriatric facility, which included an inpatient specialty hospital and an LTC facility. The program included prospective audits with feedback, multidisciplinary education, information technology interventions, and guideline development.
Objective
To investigate the impact of the ASP on physicians’ prescribing practices in this geriatric facility.
Methods
Utilization data for antibiotics commonly used to treat urinary tract infections were retrieved for the period September 1, 2011, to August 31, 2013. The study examined whether there were significant changes in overall antibiotic use, ciprofloxacin use, and physician prescribing behaviour after program implementation in September 2012.
Results
There was no significant change in the total number of antibiotic prescriptions for urinary tract infections in the hospital or the LTC facility after ASP implementation. Significant reductions were seen in the average days of therapy initially prescribed and the actual days of therapy after ASP implementation in the LTC facility but not the hospital. Across both facilities, significant reductions were seen in the number of ciprofloxacin prescriptions.
Conclusions
The current study showed that an ASP can affect physicians’ antibiotic prescribing behaviour and antibiotic usage in an LTC environment.
KEYWORDS: antibiotics, long-term care, urinary tract infection, practice modification, antimicrobial stewardship
RÉSUMÉ
Contexte
Il n’existe que très peu de documentation qui porte sur la mise en œuvre de programmes de gérance des antimicrobiens dans les établissements de soins de longue durée. La présente étude a évalué l’effet d’un programme de gérance des antimicrobiens mis en œuvre dans l’ensemble d’un centre gériatrique, qui comprenait un hôpital spécialisé et un établissement de soins de longue durée. Le programme comprenait des audits prospectifs accompagnés de rétroaction, des séances de formation multidisciplinaire, des interventions s’appuyant sur les technologies de l’information et l’élaboration de lignes directrices.
Objectif
Évaluer les effets du programme de gérance des antimicrobiens sur les habitudes de prescription des médecins travaillant dans ce centre gériatrique.
Méthodes
L’étude s’appuie sur les données d’utilisation des antibiotiques les plus employés pour traiter les infections urinaires recueillies entre le 1er septembre 2011 et le 31 août 2013. L’étude a examiné si des changements significatifs à l’utilisation des antimicrobiens et de la ciprofloxacine ainsi qu’aux habitudes de prescription des médecins ont résulté de la mise en œuvre du programme en septembre 2012.
Résultats
Aucun changement significatif n’a été noté dans le nombre total de prescriptions d’antibiotiques destinés à traiter les infections urinaires à l’hôpital et dans l’établissement de soins de longue durée. D’importantes réductions ont été observées dans la moyenne des jours de traitement initialement prescrits et le nombre réel de jours de traitement après la mise en œuvre du programme à l’établissement de soins de longue durée, mais pas à l’hôpital. Aux deux établissements, des réductions significatives du nombre de prescriptions de ciprofloxacine ont été observées.
Conclusions
La présente étude a montré qu’un programme de gérance des antimicrobiens peut avoir un effet sur les habitudes de prescription d’antibiotiques des médecins et sur l’utilisation des antibiotiques dans un établissement de soins de longue durée.
MOTS CLÉS: antibiotiques, soins de longue durée, infection urinaire, changement dans la pratique, gérance des antimicrobiens
Antibiotics are often overprescribed in long-term care (LTC) facilities.1 Studies have shown that 47% to 79% of LTC residents are exposed to at least one antibiotic course over a 12-month period.2 Urinary tract infections (UTIs) account for 20% to 60% of systemic antibiotic courses in the LTC setting,3,4 with about one-third of antibiotics prescribed for the treatment of asymptomatic bacteriuria.5 For elderly patients in LTC facilities, diagnostic difficulty arises when the UTI presentation is atypical.6
Antimicrobial stewardship programs (ASPs) are being implemented in response to rising antibiotic resistance rates. Through various interventions, ASPs serve to optimize antibiotic use by ensuring appropriate diagnosis and drug selection, as well as appropriate dosing, route, and duration of antibiotic therapy. ASPs aim to achieve the best patient outcomes and to minimize unnecessary exposure to antibiotics, thereby reducing or stabilizing levels of antibiotic resistance. Since 2014, Accreditation Canada has included ASPs as a Required Organizational Practice for organizations that provide complex continuing care services.7
Despite a plethora of guidelines for developing an ASP in an acute care setting,8 there remains a paucity of literature describing ASP implementation in LTC facilities.9,10 Successful Canadian ASPs include programs implemented at large acute care teaching hospitals, such as Mount Sinai Hospital,11 the University Health Network,12 and Sunnybrook Health Sciences Centre.13 In 2012, an ASP was implemented at Baycrest Health Sciences, a geriatric facility in Toronto, Ontario, that includes both an inpatient specialty hospital and an LTC facility. This ASP was modelled on successful strategies that have been reported in the literature, with consideration of the local culture and support resources available. The ASP included development of guidelines, multidisciplinary educational interventions, information technology interventions, and prospective audits with feedback, and focused on 4 major areas of learning: correct diagnosis of UTIs, appropriate choice of antibiotic, appropriate duration of therapy, and modification of therapy on the basis of culture and sensitivity results. The purpose of the current study was to evaluate the effectiveness of the ASP.
The ASP was implemented at an academic geriatric health care facility that included both an LTC facility and a specialty hospital. These were the 2 units of observation involved in both the intervention and the evaluation study.
The LTC facility has 472 beds, and the average age of residents is 88 years. Patient services do not include IV therapy, long-term ventilation therapy, or dialysis. The hospital is a 255-bed specialty hospital, and the average age of patients is 83 years. The hospital houses a range of care services, including complex continuing care units, which serve medically complex patients (e.g., patients with chronic tracheostomy who are not receiving ventilation). The hospital also has rehabilitation, palliative care, psychiatry, and behavioural neurology units.
At the time of ASP implementation, there were 21 attending family physicians on staff (mostly part-time). The ASP team consisted of 1 part-time pharmacist and 2 part-time family medicine physicians.
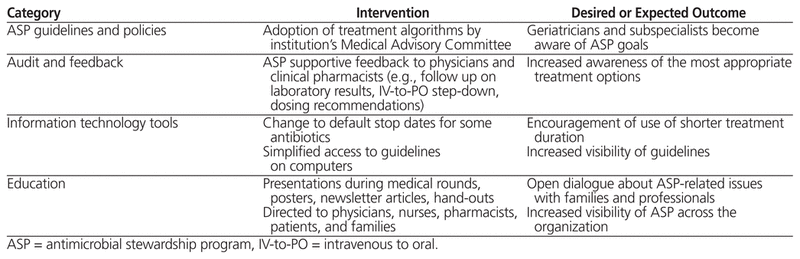
The institutional ASP was initiated in September 2012. Four complementary intervention strategies (Table 1) were employed to promote awareness of best-practice ASP policies and to effect behaviour change among health care providers. The interventions targeted all medical and nursing staff, pharmacists, patients, and their families.
Table 1 Antimicrobial Stewardship Strategies Implemented
Three research questions were identified to determine whether the ASP was effective: (1) Were there differences in over-all antibiotic use before and after ASP implementation? (2) Were there differences in physician prescribing behaviour, in terms of either initial duration of therapy or later modification of duration? (3) Were there differences in ciprofloxacin use? Ciprofloxacin was chosen for investigation because it is commonly used in the treatment of UTIs and because fluoroquinolones are associated with high resistance rates.
As an index of overall antibiotic use, the UTI rate was calculated. Selection of any UTI antibiotic (Box 1) was used as a proxy for identification of UTI cases. The UTI rate was calculated as the number of UTI cases in a given month divided by the unit occupancy for that month:
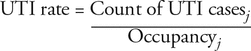
where j = month.
Box 1 Antibacterial Agents Used for Treatment of Urinary Tract Infection at Study InstitutionAmikacin
Amoxicillin
Amoxicillin–clavulanic acid
Ampicillin
Ceftriaxone
Ciprofloxacin
Gentamicin
Levofloxacin
Meropenem
Nitrofurantoin
Norfloxacin
Piperacillin and tazobactam
Tobramycin
Trimethoprim (TMP)
Trimethoprim–sulfamethoxazole (TMP-SMX)
Two metrics were retrieved from patient records as indices of physician prescribing behaviour: prescribed days of therapy (PDOT) and actual days of therapy (ADOT). PDOT refers to the duration of treatment indicated on the physician’s medical (written) orders. ADOT refers to the actual number of days that an antibiotic drug (regardless of dose) was dispensed. These two days-of-therapy (DOT) metrics were calculated independent of the dosage or specific antibiotic that a given patient received.
The PDOT and ADOT values were adjusted to control for differences in patient days and the number of UTI cases in a given month. The adjusted PDOT and ADOT variables for a given month were calculated by first determining, for each patient, the DOT for the individual patient divided by total patient days for that patient, then summing these values across all patients and dividing by the number of UTI cases:

where i = individual patient and j = month.
Ciprofloxacin use was determined from the count of ciprofloxacin prescriptions. Two measures of ciprofloxacin use were considered. The ciprofloxacin rate represented the number of ciprofloxacin prescriptions in a given month per unit occupancy for that month, and the ciprofloxacin proportion represented the number of ciprofloxacin prescriptions in a given month per UTI case:

where j = month.
Data on antibiotic drug use were retrieved from hospital and LTC facility records for individual patients for the period September 1, 2011, to August 31, 2013. Individual patient records were extracted if the patient had received a prescription for a medication most commonly associated with UTI. Antibacterial agents that the institution used for UTI treatment were identified (Box 1).
Additional data collected included monthly patient days (defined as the number of days a given patient spent at the institution) as well as monthly occupancy data for the hospital and LTC facility (defined as the total number of patient days across all patients at the institution).
It would have been prohibitively difficult to match patient identification across the pre- and post-implementation periods. Therefore, data extracted for the 2 periods were treated as independent observations. Independent-sample t-tests were performed for each of the outcome measures (i.e., the variables UTI rate, PDOT, ADOT, ciprofloxacin rate, and ciprofloxacin proportion), comparing the pre-intervention period (September 2011 to August 2012) and the post-intervention period (September 2012 to August 2013) separately for the hospital and the LTC facility. All hypothesis tests were performed at an α level of 0.05.
Data were extracted from 2638 patient records that met the study criteria. Independent-sample t-test results and summary statistics are reported in Table 2.
Table 2 Results of Independent-Sample t-Tests of Outcome Measures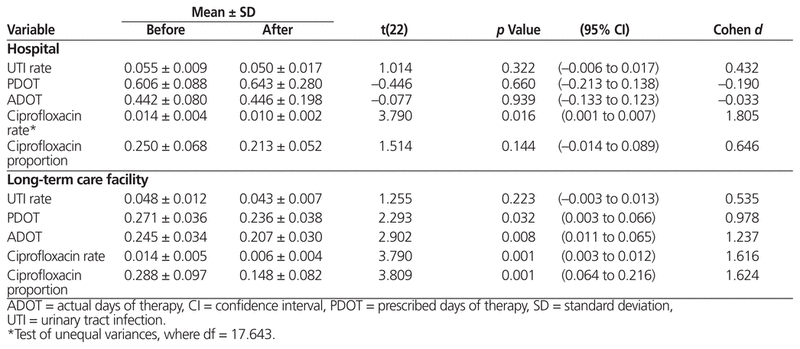
There was no significant difference in the rate of UTI cases (the indicator of antibiotic use) before and after implementation of the ASP.
There was no significant change in PDOT or ADOT after implementation of the ASP.
The independent-sample t-test indicated that there was a significant decrease in the rate of ciprofloxacin prescriptions after implementation of the ASP. However, there was no significant change in terms of the ciprofloxacin proportion (i.e., prescriptions per UTI case).
There was no significant difference in the rate of UTI cases before and after implementation of the ASP.
There were significant decreases in both PDOT and ADOT following implementation of the ASP.
Both ciprofloxacin rate and ciprofloxacin proportion were significantly lower following implementation of the ASP.
The current study has shown that physician prescribing behaviour in an LTC environment can be influenced by implementation of an ASP. Although no reduction in the number of antibiotic prescriptions for UTIs was observed in either the hospital or the LTC facility, there were significant reductions in both PDOT and ADOT after ASP implementation in the LTC facility (but not the hospital). In addition, significant reductions were seen in the number of ciprofloxacin prescriptions in both facilities.
The few previous studies that examined ASPs in LTC environments showed that ASP interventions can reduce total antibiotic consumption.14–16 The current study had similar overall results but suggests the existence of significant barriers to change. For example, the ASP at the study institution involved a small team, functioned at the program level rather than as part of the clinical team, used an external pharmacy for prescriptions generated in the LTC facility (which resulted in a lack of control over the formulary), and did not have on-site infectious disease services.
The current study showed no change in the number of UTI cases after implementation of the ASP. These negative results could be attributable to several factors. The existing literature supports the notion that diagnosis of UTI in elderly patients is difficult.6,17,18 Physicians often initiate empiric treatment of suspected UTI, attributing certain symptoms such as increased agitation and change in urine quality to a UTI, as well as initiating treatment for positive urine culture results in the absence of symptoms.
The measure used as an index of the number of UTI cases (i.e., number of antibiotic prescriptions for UTI) could be a poor indicator of UTI diagnosis. Chart reviews were not conducted to confirm diagnoses, nor was there any analysis of information about orders for urine culture.
The ASP attempted to combat these clinical barriers through the development of diagnostic guidelines. However, it is well recognized that there is considerable resistance to guidelines among front-line general practitioners.19–21 Reinforcement of education was also difficult because no ASP committee member was embedded in the clinical teams.
Despite the absence of detectable change in the number of UTI cases, further analyses were conducted to examine specific measures of physician prescribing behaviour, including PDOT, an index of the decision to treat and the intended duration of treatment. This study used 2 DOT variables, as opposed to defined daily dose (the assumed average maintenance dose per day for a drug used for its main indication in adults22), as outcome measures because they more accurately reflect antibiotic usage in a geriatric population, where renal dosing is often required.
There was a significant decrease in both PDOT and ADOT in the LTC facility but not the hospital. The decreases in the LTC facility can most likely be attributed to multiple aspects of the ASP, including implementation of short default stop dates for select antibiotics used for uncomplicated UTIs and audit with feedback for all UTI antibiotic prescriptions. The lack of any effect on DOT variables in the hospital may reflect the greater patient complexity and physicians’ ability to treat severe infections on site, where longer durations of antibiotic treatment are recommended.23–25 However, no subanalyses were performed on the use of parenteral antibiotics, which made any direct measure of disease severity difficult.
Another key focus of the ASP initiative was to reduce the use of ciprofloxacin, and significant reductions in ciprofloxacin use were observed at both sites. Change in the use of ciprofloxacin in particular may have resulted from widespread dissemination of institution-specific antibiograms. The anti-biograms showed high ciprofloxacin resistance, which may have motivated staff to change empiric antibiotic selection.
This observational study had a number of limitations. Chart reviews were not conducted because of resource constraints. The antibiotics assessed were assumed to be used exclusively for UTI treatment, but some patients may have had other types of infection. Urine culture results, as an indicator of suspected UTIs, were not analyzed. The study population was not stratified by disease severity. Finally, the study duration may have been insufficient to allow for behaviour change.
Based upon the process and findings of this evaluation study, it is recommended that future evaluations be conducted 2–3 years after program implementation, to allow sufficient education regarding the diagnosis of UTI and asymptomatic bacteriuria.
The current study showed that an ASP initiative in an LTC environment can affect physicians’ antibiotic prescribing behaviour and antibiotic usage. The findings indicate initial success in a nursing home environment, but more work must be done to recognize and overcome barriers to identification and appropriate management of UTIs.
1 Daneman N, Bronskill SE, Gruneir A, Newman AM, Fischer HD, Rochon PA, et al. Variability in antibiotic use across nursing homes and the risk of antibiotic-related adverse outcomes for individual residents. JAMA Intern Med. 2015;175(8):1331–9.
2 Benoit SR, Nsa W, Richards CL, Bratzler DW, Shefer AM, Steele LM, et al. Factors associated with antimicrobial use in nursing homes: a multilevel model. J Am Geriatr Soc. 2008;56(11):2039–44.
3 Rhee SM, Stone ND. Antimicrobial stewardship in long-term care facilities. Infect Dis Clin North Am. 2014;28(2):237–46.
4 Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control. 2008;36(7):476–80.
5 Loeb M, Brazil K, Lohfeld L, McGeer A, Simor A, Stevenson K, et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669.

6 Woodford HJ, George J. Diagnosis and management of urinary tract infection in hospitalized older people. J Am Geriatr Soc. 2009;57(1):107–14.
7 Required organizational practices handbook 2014. Ottawa (ON): Accreditation Canada; 2013.
8 Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77.
9 Fleming A, Bradley C, Cullinan S, Byrne S. Antibiotic prescribing in long-term care facilities: a meta-synthesis of qualitative research. Drugs Aging. 2015;32(4):295–303.

10 Fleming A, Bradley C, Cullinan S, Byrne S. Antibiotic prescribing in long-term care facilities: a qualitative, multidisciplinary investigation. BMJ Open. 2014;4(11):e006442.

11 Hurford A, Morris AM, Fisman DN, Wu J. Linking antimicrobial prescribing to antimicrobial resistance in the ICU: before and after an antimicrobial stewardship program. Epidemics. 2012;4(4):203–10.
12 Dresser L, Nelson S. Practice spotlight: pharmacists in an antimicrobial stewardship program. Can J Hosp Pharm. 2010;63(4):328–9.

13 Elligsen M, Walker SAN, Simor, A, Daneman N. Prospective audit and feedback of antimicrobial stewardship in critical care: program implementation, experience, and challenges. Can J Hosp Pharm. 2012;65(1):31–6.

14 Fleet E, Gopal Rao G, Patel B, Cookson B, Charlett A, Bowman C, et al. Impact of implementation of a novel antimicrobial stewardship tool on antibiotic use in nursing homes: a prospective cluster randomized control pilot study. J Antimicrob Chemother. 2014;69(8):2265–73.
15 Jump RL, Olds DM, Seifi N, Kypriotakis G, Jury LA, Peron EP, et al. Effective antimicrobial stewardship in a long-term care facility through an infectious disease consultation services: keeping a LID on antibiotic use. Infect Control Hosp Epidemiol. 2012;33(12):1185–92.

16 Pate PG, Storey DF, Baum DL. Implementation of an antimicrobial stewardship program at a 60-bed long-term acute care hospital. Infect Control Hosp Epidemiol. 2012;33(4):405–8.
17 Nicolle LE; SHEA Long-Term-Care Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22(3):167–75.
18 Genao L, Buhr GT. Urinary tract infections in older adults residing in long-term care facilities. Ann Longterm Care. 2012;20(4):33–8.
19 Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–65.
20 Lugtenberg M, Burger JS, Han D, Westert GP. General practitioners’ preferences for interventions to improve guideline adherence. J Eval Clin Pract. 2014;20(6):820–6.
21 Cranney M, Warren E, Barton S, Gardner K, Walley T. Why do GPs not implement evidence-based guidelines? A descriptive study. Fam Pract. 2001; 18(4):359–63.
22 DDD: definition and general considerations. Geneva (Switzerland): World Health Organization; [cited 2015 Jun 25]. Available from: www.whocc.no/ddd/definition_and_general_considera/
23 Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2013; 39(2):165–228.
24 Green RS, Djogovic D, Gray S, Howes D, Brindley PG, Stenstrom R, et al.; CAEP Critical Care Interest Group. Canadian Association of Emergency Physicians sepsis guidelines: the optimal management of severe sepsis in Canadian emergency departments. CJEM. 2008;10(5):443–59.
25 Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530–8.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors would like to acknowledge the following groups and individuals for their support: the institution’s Antimicrobial Stewardship Steering Committee and Dr Andrew Morris for study conception; Dr Malcolm Binns for data analysis; and Dexter Garcia and Jonathan Light for data acquisition.
Canadian Journal of Hospital Pharmacy, VOLUME 69, NUMBER 6, November-December 2016