
Uzma Alim, Duane Bates, Ashten Langevin, Denise Werry, Deonne Dersch-Mills, Robert J Herman, Marcy Mintz, Sunita GhoshABSTRACT
Background
Thiamine (vitamin B1) is an essential cofactor responsible for the breakdown of glucose, and its deficiency is associated with Wernicke encephalopathy (WE). There is a lack of evidence from systematic studies on the optimal dosing of thiamine for WE. Objectives: The primary objective was to describe the prescribing patterns for IV thiamine in adult patients admitted to a large teaching hospital. The secondary objective was to evaluate the clinical resolution of WE symptoms (confusion, ataxia, and/or ocular motor abnormalities) in relation to the dose of IV thiamine prescribed.
Methods
A retrospective design was used to review data for adult patients admitted to an internal medicine service from June 1, 2014, to June 30, 2015. All patients included in the study received IV thiamine: low-dose therapy was defined as 100 mg IV daily and high-dose therapy was defined as dosage greater than 100 mg IV daily.
Results
A total of 141 patients were included; low-dose thiamine was prescribed for 115 (81.6%) and high-dose thiamine for 26 (18.4%). Patients for whom high-dose thiamine was prescribed were more likely to be those in whom a diagnosis of WE was being considered (12/26 [46.2%] versus 5/115 [4.3%], p < 0.001). Of the total 219 IV thiamine doses ordered, 180 (82.2%) were for 100 mg, and 143 (65.3%) were prescribed for once-daily administration. There was no statistically significant difference in the time to resolution of WE symptoms for patients receiving high-dose versus low-dose thiamine.
Conclusions
A wide variety of thiamine prescribing patterns were noted. This study did not show a difference in time to resolution of WE symptoms in relation to the dose of IV thiamine. Additional large-scale studies are required to determine the optimal dosing of thiamine for WE.
KEYWORDS: thiamine, vitamin B1, thiamine deficiency, Wernicke encephalopathy, alcohol-related disorders
RÉSUMÉ
Contexte
La thiamine (vitamine B1) est un cofacteur essentiel responsable du métabolisme du glucose. Une carence en thiamine est associée à l’encéphalopathie de Wernicke (EW). Or, on observe une absence de données probantes provenant d’analyses systématiques portant sur la posologie optimale de thiamine dans le traitement de l’EW.
Objectifs
L’objectif principal était de décrire les habitudes de prescription de thiamine à administrer par voie intraveineuse chez les patients adultes admis dans un important hôpital universitaire. L’objectif secondaire était d’évaluer la disparition clinique des symptômes de l’EW (confusion, ataxie ou troubles moteurs oculaires) en fonction de la dose prescrite de thiamine à administrer par voie intraveineuse.
Méthodes
Un plan d’étude rétrospectif a été utilisé pour étudier les données concernant les patients adultes admis à un service de médecine interne entre le 1er juin 2014 et le 30 juin 2015. Tous les patients à l’étude ont reçu de la thiamine par voie intraveineuse : le traitement à faible dose était de 100 mg par jour et le traitement à dose élevée excédait 100 mg par jour.
Résultats
Au total, 141 patients ont été admis à l’étude; l’on a prescrit une faible dose de thiamine à 115 (81,6 %) d’entre eux et une dose élevée aux 26 (18,4 %) autres. Les patients qui se sont vus prescrire une dose élevée de thiamine étaient vraisemblablement ceux pour qui l’on envisageait un diagnostic d’EW (12/26 [46,2 %] contre 5/115 [4,3 %], p < 0,001). Pour l’ensemble des 219 doses prescrites de thiamine à administrer par voie intraveineuse, 180 (82,2 %) étaient de 100 mg et 143 (65,3 %) devaient être injectées à une fréquence uniquotidienne. On n’a relevé aucune différence statistiquement significative quant au temps de disparition des symptômes de l’EW entre les patients ayant reçu une dose élevée de thiamine et ceux en ayant reçu une faible dose.
Conclusions
On a noté une grande variété d’habitudes de prescription de la thiamine. La présente étude n’a pas montré que la dose de thiamine administrée par voie intraveineuse changeait le temps nécessaire à la disparition des symptômes de l’EW. Il est nécessaire de mener de plus amples études à grande échelle afin de déterminer quelle est la posologie optimale de thiamine dans le traitement de l’EW.
MOTS CLÉS: thiamine, vitamine B1, carence en thiamine, encéphalopathie de Wernicke, affections liées à l’alcool
Thiamine pyrophosphate, the biologically active form of thiamine (vitamin B1), is an essential cofactor for the aerobic breakdown of glucose.1 One of the most common neurologic complications of thiamine deficiency is Wernicke–Korsakoff syndrome,2 which consists of 2 disorders: Wernicke encephalopathy (WE) and Korsakoff psychosis.3,4 Petechial hemorrhage and demyelination within periventricular structures account for the classic triad of WE—confusion, gait ataxia, and ocular abnormalities—which may occur together or in various combinations.1–3 WE can also progress to Korsakoff psychosis, which is characterized by severe memory impairment without any dysfunction in other intellectual parameters.3
Chronic alcohol use is one of the most common predisposing factors for WE.2,5 WE has been reported to occur in 12.5% of those with chronic alcohol use, although it is widely suspected that this is an underestimate of the true prevalence of the disorder.6,7 Importantly, WE can cause substantial morbidity and mortality if left untreated, leading to death in up to 20% of cases and progression to Korsakoff psychosis in 85% of survivors, of whom 25% require long-term institutionalization.3,8–10 Up to 90% of patients with suspected WE do not present with the classic triad of symptoms.3,8 Thus, a presumptive diagnosis of WE should be made in any patient with a history of alcohol abuse who may be at risk.6
Although administration of thiamine has been the treatment of choice for Wernicke–Korsakoff syndrome for more than 50 years, current recommendations for thiamine treatment are based on guided extrapolations from basic science, case reports, and expert opinion.3 A Cochrane review published in 2013 stated that there is currently insufficient evidence from randomized controlled trials to guide clinicians in determining the dose, frequency, route, and/or duration of thiamine treatment for the treatment of Wernicke–Korsakoff syndrome related to alcohol abuse.3 Historically, the treatment for WE has been thiamine 100 mg IV daily for 3–5 days, although this regimen has not been tested in any randomized controlled trials.2,5 The European Federation of Neurological Societies (EFNS) guidelines for diagnosis, therapy, and prevention of WE suggest thiamine 200 mg IV 3 times daily until there is no further improvement in signs and symptoms.11 The Royal College of Physicians (RCP) guideline on management of WE in the emergency department recommends thiamine 500 mg IV 3 times daily for 3 days.12 If there is no response, the medication should be discontinued, but if a response is noted, then the dose may be decreased to 250 mg IV once daily for 5 days or until clinical improvement ceases.12 Oral thiamine is not used for the acute management of WE, but is often prescribed after completion of an IV course of thiamine.
The primary objective of the study presented was to describe the prescribing patterns for IV thiamine administered to adult patients admitted under the Internal Medicine Service of the study hospital. The secondary objective was to evaluate the clinical resolution of WE symptoms (confusion, ataxia, and/or ocular abnormalities) in relation to the dose of IV thiamine prescribed.
A chart review was used to collect data related to the study objectives. Data were obtained for adult patients admitted to 1 of 3 Internal Medicine Services at the Foothills Medical Centre (Calgary, Alberta) from June 1, 2014, to June 30, 2015. This 1081-bed hospital, affiliated with the University of Calgary, provides advanced health care services to more than 2 million people from Calgary, southern Alberta, southeastern British Columbia, and southern Saskatchewan.
Patients were identified through the computerized physician order entry (CPOE) system, which contains detailed clinical information about all patients and their hospital stay, including demographic information, diagnostic imaging results, laboratory values, procedures and treatments received, progress notes, and discharge summaries. The CPOE system and paper charts were used to obtain all of the data for the study.
Patients who were 18 years of age or older, had been admitted to 1 of the 3 Internal Medicine Services at the study hospital, and had received at least one IV dose of thiamine were included in the study. Patients whose admission lasted less than 3 days and patients who were admitted under a different service and transferred to the Internal Medicine Service after 3 or more days in hospital were excluded. For patients with multiple admissions during the study period, only the first admission was included in the analysis, to avoid repeated measures.
The primary outcomes were the prescribing patterns of thiamine, including proportion of patients who received high-dose and low-dose IV thiamine, a description of the patient groups who received high-dose and low-dose thiamine, and a description of thiamine prescribing patterns among those for whom a diagnosis of WE was being considered, relative to the rest of the study population. The secondary outcome was the difference in time between presentation and clinical resolution of symptoms of WE (confusion, ataxia, and/or ocular abnormalities) in relation to whether high-dose or low-dose thiamine had been prescribed. For the purposes of this study, low-dose thiamine was defined as 100 mg IV daily for at least one day and high-dose thiamine was defined as more than 100 mg IV daily for at least one day.
Demographic data were collected for each patient, including age, sex, weight, presence of an alcohol-related admission diagnosis, length of hospital stay, and mortality. Data were also collected for the following baseline laboratory tests, performed at the time IV thiamine was ordered: serum creatinine, alanine aminotransferase, alkaline phosphatase, γ-glutamyl transferase, total bilirubin, international normalized ratio, and serum albumin. Documentation of estimated alcohol consumption (standard drinks defined according to criteria of the National Institute on Alcohol Abuse and Alcoholism13), history of liver dysfunction, use of the Emergency Department Alcohol Withdrawal protocol or the Clinical Institute Withdrawal Assessment—Alcohol, Revised (CIWA-AR) protocol, neurology consultation, and diagnostic imaging (specifically magnetic resonance imaging [MRI] of the head)11 was also collected.
The following data concerning IV thiamine therapy were collected: indication, dose, frequency, duration, and rationale for prescribing thiamine. In addition, the dosing and duration for thiamine administered by the oral route after discontinuation of IV thiamine and data on continuing thiamine at discharge were collected. Documentation of any symptoms of WE (confusion, ataxia, and/or ocular abnormalities [nystagmus, abnormal eye movement, visual field defect, gaze palsy, ptosis, and diplopia]) and the time to their resolution (if applicable) were collected.
Descriptive statistics were used to describe the study population. Continuous variables that were normally distributed were described using means and standard deviations, whereas continuous variables that were not normally distributed were described using medians and interquartile ranges (IQRs). Categorical variables were described using frequency counts and proportions. Independent t tests were used to compare the means of the low-dose and high-dose groups. χ2 tests were used to compare 2 categorical variables. Results of Fisher exact tests were reported when the cell frequency was less than 5. A p value less than 0.05 was considered statistically significant. SPSS version 15 (IBM, Armonk, New York) was used to perform all statistical tests.
Ethics approval was obtained from the Health Research Ethics Board of the Alberta Community Health Committee.
A total of 147 patients received IV thiamine during the study period. Six of these patients were excluded, 5 because they had been admitted to a different service and were transferred to the Internal Medicine Teaching Team outside of the specified time-frame and 1 because of incomplete documentation. Therefore, 141 patients were included in the final analysis (Table 1). There were no statistically significant differences between the high-dose (n = 26) and low-dose (n = 115) groups for most patient characteristics. Patients treated with high-dose thiamine had a lower mean weight and were more likely to have an alcohol-related admission diagnosis (alcohol withdrawal, alcoholic hepatitis, alcoholic pancreatitis, and/or WE) (Table 1). The indication for IV thiamine was alcohol history or withdrawal for 97 (84.3%) and 23 (88.5%) of patients in the low-dose and high-dose groups, respectively (p = 0.59). A diagnosis of WE was being considered for 17 patients, representing 5 (4.3%) of the low-dose group and 12 (46.2%) of the high-dose group.
Table 1 Baseline Characteristics of Patients (n = 141)
A neurology consultation was completed for 4 (3.5%) of the low-dose patients and 9 (34.6%) of the high-dose patients, and a diagnosis of WE was considered by the neurology service for 5 (19.2%) of the high-dose patients. MRI was completed for 10 (8.7%) of the low-dose patients and 8 (30.8%) of the high-dose patients. The MRI findings showed abnormal enhancement of mammillary bodies suggestive of WE for only 1 patient, who was receiving high-dose thiamine.
IV thiamine was prescribed at a high dose for 26 patients (18.4%) and a low dose for 115 patients (81.6%) (Table 2). The most common IV thiamine dose was 100 mg daily (n = 112) for a median duration of 3 days (IQR 3–4). Among the 17 patients in whom a diagnosis of WE was being considered, 12 (70.6%) had a prescription for high-dose thiamine and 5 (29.4%) a prescription for low-dose thiamine. In addition, 14 (11.3%) of the 124 patients for whom a diagnosis of WE was not being considered had a prescription for high-dose thiamine (Table 2). A variety of thiamine prescribing patterns were noted (Table 3). Documentation of any rationale for selection of a particular dosing strategy or for any dose changes was lacking. No adverse effects of IV or oral thiamine were reported.
Table 2 Prescription of High- and Low-Dose IV Thiamine
Table 3 Summary of Thiamine Prescribing Patterns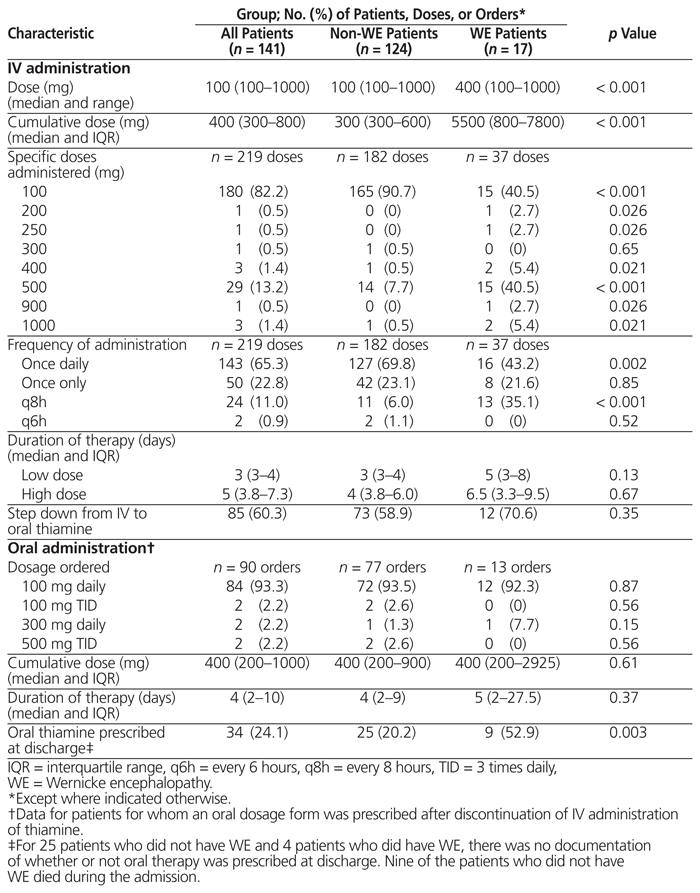
The patients for whom high-dose thiamine was prescribed had a significantly higher incidence of any of the 3 classic WE symptoms (confusion, ataxia, and/or ocular abnormalities) relative to those for whom low-dose thiamine was prescribed (Table 4). There was no significant difference in the time to resolution of any WE symptom for patients given high-dose versus low-dose IV thiamine (Table 5). Ataxia and ocular abnormalities appeared to resolve sooner than confusion (Table 5). Twenty patients died during the study period. Cause of death was documented as end-stage liver disease (n = 3), complications of metastatic lung cancer (n = 3), heart failure (n = 1), sepsis (n = 1), or renal/respiratory failure (n = 1). Cause of death was not documented for 11 patients.
Table 4 Incidence of Symptoms of Wernicke Encephalopathy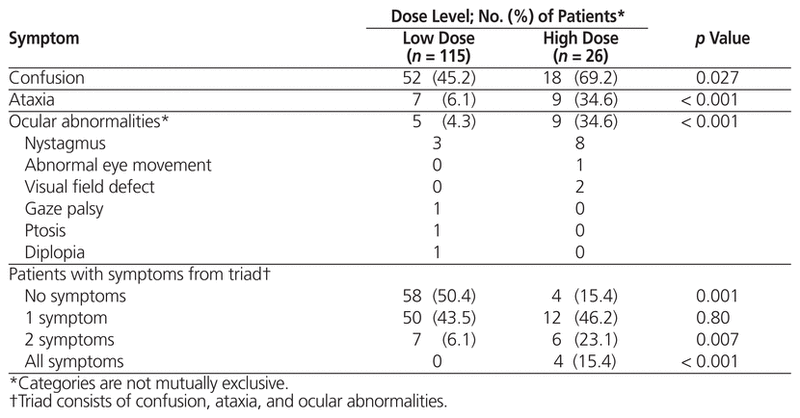
Table 5 Time to Resolution of Symptoms of Wernicke Encephalopathy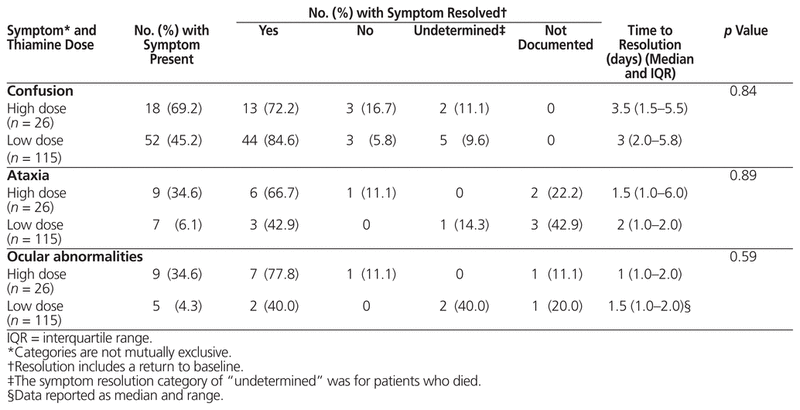
Thiamine is the mainstay of therapy in both prevention and treatment of WE; however, there is a lack of standardization of its optimal route of delivery and dosing.11 Thiamine has poor bioavailability. In healthy individuals, only 4.5 mg of thiamine is absorbed from a single 100-mg oral dose, and absorption is reduced to one-third or less in vulnerable patients.12 Parenteral administration of thiamine is recommended to avoid the problem of decreased gastrointestinal absorption in people with alcoholism and other disease states, and to maximize serum thiamine levels, promoting passive (concentration-dependent) movement of thiamine across the blood–brain barrier.1,2,12 In a small pharma-cokinetic study of 6 healthy patients who received an IV bolus of thiamine 50 mg, the half-life of thiamine was determined to be about 90 min.14 Thus, it is speculated that giving thiamine in multiple daily doses may aid in achieving better penetration of the central nervous system than giving a single daily dose.2,11
According to a Cochrane review published in 2013, evidence from randomized controlled trials concerning the optimal dose, route, duration, and frequency of IV thiamine for the treatment of WE are lacking.3 Only 2 trials met the inclusion criteria for the Cochrane review. The first trial was an unpublished double-blind study that compared the cognitive effects of 5 g/day of oral thiamine with placebo in 8 participants with memory impairment associated with chronic alcohol abuse.3 Participants in the thiamine group showed significant improvement in several cognitive measures (the Buschke Selective Reminding Test, the Halstead Category Test, the Medical College of Georgia Complex Figure Test, and the Controlled Oral Word Association Test) at 2 weeks relative to baseline.3 Although this study used a wide range of neuropsychological tests to assess the outcome, the data were considered insufficient for quantitative analysis because of the small number of participants and limited reporting of data.3 The second trial included in the Cochrane review was a randomized, double-blind, placebo-controlled trial of 107 patients who were alcohol-dependent but did not have symptoms of WE. The patients were randomly assigned to receive intramuscular (IM) thiamine (5, 20, 50, 100, or 200 mg) once per day for 2 consecutive days.15 The effect of thiamine was assessed on the third day by a single neuropsychological test, the delayed alternation task.15 Although the authors of the trial suggested that this test is sensitive to cognitive impairment,15 there is very little information about its reliability in humans.3 A significant difference was found favouring the 200 mg/day dosage over the 5 mg/day dosage; however, no significant differences were found among the other doses.3,15 This study had considerable methodological shortcomings in design (such as a high rate of noncompletion and a short duration of thiamine treatment) and incomplete documentation of results.3,15
Use of high-dose thiamine was also reported in a case series of 10 symptomatic patients with Wernicke–Korsakoff syndrome. All patients showed some degree of improvement with thiamine 500 mg IV every 8 h for 3 days, then 500 mg IV daily for 5 days, and continuation with at least 300 mg/day of oral thiamine.16 No specific information was provided about the time to clinical improvement of symptoms. High-dose thiamine for the treatment of WE led to either complete or partial symptom resolution in 2 case reports.17,18 A 51-year-old man presented with memory difficulties, confabulations, ataxic gait, and nystagmus and experienced almost complete resolution of symptoms after 1 week of treatment with high-dose thiamine.17 The regimen included thiamine 900 mg IM and 600 mg orally daily for the first 5 days and 900 mg IM daily the following week.17 The other case report involved a 52-year-old man with a 10-year history of alcohol abuse and symptoms of Wernicke–Korsakoff syndrome (confusion, ataxia, and nystagmus).18 After treatment with thiamine 100 mg IM daily, the patient had full resolution of ataxic and oculomotor symptoms, but there was no change in higher mental functioning.18 However, within 2 months of initiating high-dose thiamine (600 mg/day orally and 300 mg/day IM), the patient’s cognitive status retuned to baseline, and scores on all neuropsychological tests had substantially improved.18
Some clinicians have suggested that patients with confirmed WE may require up to 1 g of IV thiamine to achieve a clinical response in the first 12 h of hospitalization.19,20 In a review article, Cook21 stated that patients treated with a low dose of thiamine for 2 or 3 days with little response may subsequently show a dramatic response if they are then given 1 g once or twice daily.21 However, these statements are based on expert opinion. There have been reports suggesting that parenteral doses of 100 mg or 250 mg of thiamine per day may be inadequate to improve clinical symptoms or prevent death in patients with WE.1,8,21,22 The EFNS and RCP practice guidelines also justify their recommendations of high doses of parenteral thiamine for treatment of WE on the basis of anticipated treatment failure with lower doses.11,12
In the cohort of 141 patients in the current study, thiamine prescribing patterns were highly variable. A wide range of IV doses (from 100 to 1000 mg) and various frequencies (ranging from once daily to every 6 h) were prescribed. Most orders were for 100 mg once daily: 82.2% of total doses were ordered as 100 mg, and 65.3% of total doses were ordered as once daily. However, among the patients for whom a diagnosis of WE was being considered, only 40.5% of the doses were ordered as 100 mg, and only 43.2% of doses were ordered as once daily. Thus, this subset of patients was more likely to receive higher doses and greater frequencies of IV thiamine. The study institution also had an overall higher use of high-dose thiamine than was reported in a large multicentre retrospective review of thiamine prescribing practices within university-affiliated Canadian hospitals.1 In that study, a total of 48 806 prescriptions of parenteral and oral thiamine were provided to 32 213 patients.1 Overall, about 93% of the total prescriptions were for 100 mg of thiamine, and 75% of these 100-mg prescriptions were ordered as once daily.1 In a retrospective study of thiamine prescribing practices for 217 patients with alcohol-use disorder conducted in a large US teaching hospital,23 a regimen of thiamine 100 mg orally daily was prescribed for about 70% of the patients. Interestingly, for the subset of 124 patients (57%) identified as being at high risk because they presented with alcohol intoxication, withdrawal, or delirium tremens, no statistically significant association was revealed between risk status and receipt of high-dose thiamine (defined as doses ≥ 200 mg IV).23
There is no consensus on the appropriate duration of thiamine treatment for patients with suspected WE. Although no prospective studies have compared different durations of thiamine administration, the EFNS guidelines suggest that treatment be continued until symptoms resolve, whereas the RCP guidelines suggest completing 3 days of treatment and, if a response is noted, completing an additional 5 days of treatment or continuing treatment until clinical improvement ceases.11,12,23 In the study presented here, the median durations of IV thiamine were 3 days for those who received low-dose treatment and 5 days for those who received high-dose treatment. Treatment duration tended to be longer for the subset of patients in whom a diagnosis of WE was being considered, but this difference was not statistically significant. In addition, the choice of duration for thiamine may have been affected by the CIWA-AR order set in use at the study institution; this order set includes an order for thiamine 100 mg IV q24h for 3 days.
Although the current study included patients who received at least one dose of IV thiamine, 85 (60.3%) of the patients were initiated on oral thiamine after discontinuation of IV thiamine. The patients in whom WE was being considered were more likely to have a prescription for oral thiamine at discharge than the patients in whom this diagnosis was not being considered. The results of the current study and the retrospective review by Day and others1 signify that oral thiamine continues to be prescribed frequently for inpatients in Canadian academic hospitals.
No adverse effects of thiamine were noted in the current study. Parenteral thiamine has been shown to have an excellent safety profile.11 Minor adverse reactions such as irritation at the injection site may occur, and infusions should be given over 30 min.12,24 In a prospective study of 989 patients receiving 100 mg of thiamine as a single IV injection over 10 s or less, 1 patient experienced generalized pruritus and 11 patients had transient local irritation.25 In a retrospective survey, no cases of significant adverse reactions to thiamine were identified in more than 300 000 treatments.24 Sporadic anaphylactic reactions have been reported, but thiamine was not documented as the cause in all cases.11 Thus, the consequences of undertreatment with thiamine are considered to outweigh the risks of overtreatment, based on the safety and cost profile of parenteral thiamine.2,11,12,26
In the current study population, there was no statistically significant difference in the median time to resolution of WE symptoms in patients with a prescription for high-dose IV thiamine (> 100 mg IV daily) and those receiving low-dose IV thiamine (100 mg IV daily). To the best of our knowledge, no other studies have evaluated the effect of thiamine dosing on the time to resolution of symptoms of WE.
The strengths of this study include its relevance in a specific patient population where pharmacotherapy has a direct impact on patient care. We were able to address a gap in current knowledge by providing insight on the dosing, frequency, and duration of IV thiamine prescribed at the study institution. As previously reported in the literature, we documented a variety of prescribing patterns for IV thiamine.
Potential limitations of this study include its small sample size and retrospective design, which resulted in limited documentation in several cases. WE is a clinical diagnosis, and empiric treatment with thiamine is often given solely on the basis of a history of alcohol abuse and altered mental status. Also, there is no standardization in the assessment of WE symptoms. We relied on subjective assessments for the presence and resolution of symptoms, as documented in patient charts. It appeared that higher doses of thiamine may have been associated with a longer duration of WE symptoms; however, this finding could have been confounded by severity of confusion. The manifestation of confusion in hospital inpatients has a broad spectrum of severity and is associated with a wide variety of medical etiologies.
Given the limitations noted above, this study can be considered to be hypothesis-generating, as our findings suggest that there is no difference in the time to resolution of WE symptoms in patients receiving high-dose versus low-dose IV thiamine. This study revealed a wide variety of thiamine prescribing patterns and showed that patients in whom a diagnosis of WE is being considered may be more likely to be given higher doses of IV thiamine. These prescribing patterns signify the lack of consensus on how to appropriately treat WE and highlight the need for large-scale prospective trials to further investigate the optimal dosage, frequency, and duration of IV thiamine.
1 Day GS, Ladak S, Curley K, Farb NAS, Masiowski P, Pringsheim T, et al. Thiamine prescribing practices within university-affiliated hospitals: a multicenter retrospective review. J Hosp Med. 2015;10(4):246–53.
2 Donnino MW, Vega J, Miller J, Walsh M. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6):715–21.
3 Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke–Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033.
4 Victor M, Adams R, Collins G. The Wernicke–Korsakoff syndrome: a clinical and pathological study of 245 patients, 82 with post-mortem examinations. Philadelphia (PA): F A Davis Company; 1971.
5 Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911–5.
6 Alcohol-use disorders: diagnosis and clinical management of alcohol-related physical complications [clinical guideline]. London (UK): National Institute for Health and Care Excellence, National Clinical Guideline Centre; 2010.
7 Torvik A, Lindboe CF, Rogde S. Brain lesions in alcoholics: a neuropathological study with clinical correlations. J Neurol Sci. 1982;56(2–3):233–48.
8 Harper C, Giles M, Finlay-Jones R. Clinical signs in the Wernicke–Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341–5.

9 Harper C. Wernicke’s encephalopathy: a more common disease than realised. A neuropathological study of 51 cases. J Neurol Neurosurg Psychiatry. 1979; 42(3):226–31.

10 Cook CCH, Hallwood PM, Thomson AD. B vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 1998; 33(4):317–36.
11 Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408–18.
12 Thomson AD, Cook CCH, Touquet R, Henry JA. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and emergency department. Alcohol Alcohol. 2002;37(6):513–21.
13 Rethinking drinking: alcohol and your health. NIH Publ No 13-3770. Bethesda (MD): National Institute on Alcohol Abuse and Alcoholism; 2010 [cited 2016 May 17]. Available from: http://pubs.niaaa.nih.gov/publications/RethinkingDrinking/Rethinking_Drinking.pdf
14 Tallaksen CME, Sande A, Bøhmer T, Bell H, Karlsen J. Kinetics of thiamin and thiamin phosphate esters in human blood, plasma and urine after 50 mg intravenously or orally. Eur J Clin Pharmacol. 1993;44(1):73–8.
15 Ambrose ML, Bowden SC, Whelan G. Thiamin treatment and working memory function of alcohol-dependent people: preliminary findings. Alcohol Clin Exp Res. 2001;25(1):112–6.
16 Soler-González C, Sáez-Peñataro J, Balcells-Oliveró M, Gual-Solé A. Wernicke–Korsakoff’s syndrome: Waiting for Godot? [letter]. Alcohol Alcohol. 2014; 49(1):117–8.
17 Caso F, Fiorino A, Falautano M, Leocani L, Martinelli V, Minicucci F, et al. Treatment of Wernicke’s encephalopathy with high dose of thiamine in a patient with pyloric sub-stenosis: description of a case. Neurol Sci. 2010; 31(6):859–61.
18 Paparrigopoulos T, Tzavellas E, Karaiskos D, Kouzoupis A, Liappas I. Complete recovery from undertreated Wernicke–Korsakoff syndrome following aggressive thiamine treatment. In Vivo (Brooklyn). 2010;24(2):231–33.
19 Nakada T, Knight RT. Alcohol and the central nervous system. Med Clin North Am. 1984;68(1):121–31.
20 Lindberg MC, Oyler RA. Wernicke’s encephalopathy. Am Fam Physician. 1990;41(4):1205–9.
21 Cook CCH. Prevention and treatment of Wernicke–Korsakoff syndrome. Alcohol Alcohol. 2000;35(Suppl 1):19–20.
22 Tallaksen CME, Bell H, Bøhmer T. Thiamin and thiamin phosphate ester deficiency assessed by high performance liquid chromatography in four clinical cases of Wernicke encephalopathy. Alcohol Clin Exp Res. 1993;17(3):712–6.
23 Isenberg-Grzeda E, Chabon B, Nicolson SE. Prescribing thiamine to inpatients with alcohol use disorders: how well are we doing? J Addict Med. 2014;8(1):1–5.
24 Wrenn KD, Slovis CM. Is intravenous thiamine safe? Am J Emerg Med. 1992;10(2):165.
25 Wrenn KD, Murphy F, Slovis CM. A toxicity study of parenteral thiamine hydrochloride. Ann Emerg Med. 1989;18(8):867–70.
26 Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke–Korsakoff syndrome: under-recognized and under-treated. Psychosomatics. 2012; 53(6):507–16.
Funding: This study was funded by Pharmacy Services, Alberta Health Services. ( Return to Text )
The authors would like to thank Maurice Maslen, Data Analyst with Calgary Zone Clinical Services IT, Alberta Health Services, for assistance in collecting the data.
Canadian Journal of Hospital Pharmacy, VOLUME 70, NUMBER 3, May-June 2017