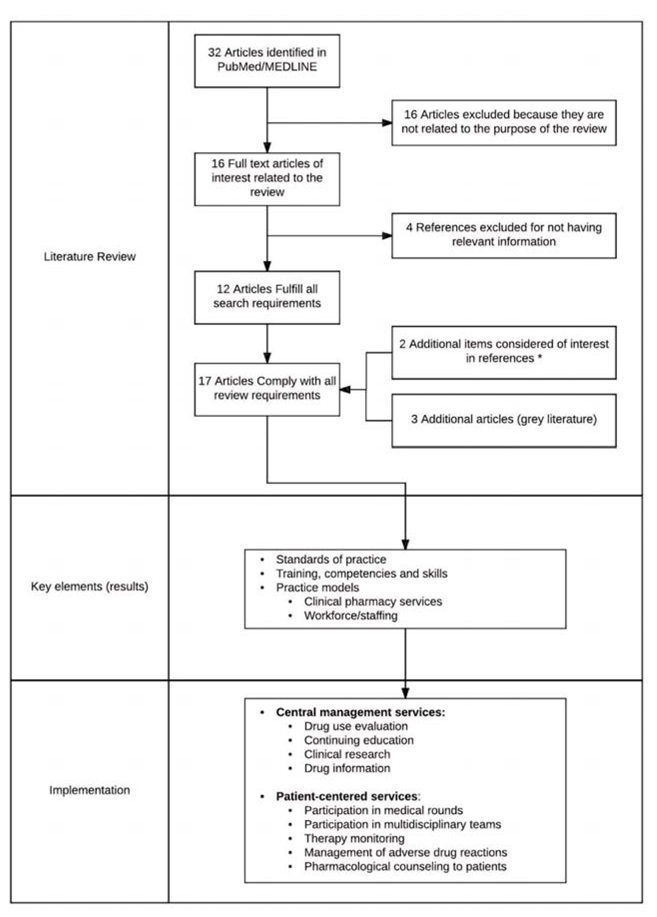
Figure 1 Steps for redesign of the clinical pharmacy practice model at Hospital Pablo Tobón Uribe, Medellín, Colombia.
Juan Pablo Botero Aguirre, Andrés Felipe Valencia Quintero, Elkyn Johan Granados Vega
The pharmacy function in hospitals is, by nature and history, associated with administrative activities, such as logistics and the purchase, storage, preparation, and distribution of drugs. This model, which focuses on medication as a product, has evolved over decades. Since the late 1960s and early 1970s, it has progressed rapidly, mainly in the United States and some European countries, toward a patient-centred model, in response to changes required by the health care system intended to ensure the effectiveness of medicines and their safe and cost-effective use.1 Currently, direct patient care by clinical pharmacists within multidisciplinary teams is recognized as one of the best pharmacy practice models because it minimizes risks, care costs, morbidity and mortality rates, as well as time spent in hospital; it also improves pharmacotherapy results.2–9
Several initiatives and standards of practice for clinical pharmacy have been proposed around the world. These proposals promote the transformation of clinical pharmacy services through participation of pharmacists as providers of direct patient care, counselling, and support to medical and nursing staff in their decision-making. This transformation results in the optimization of pharmacological therapy and facilitates the participation of pharmacists in multidisciplinary care teams.10–13
In Colombia, pharmacists’ professional practice has been regulated since 1962,14 yet it was only in 1995 that a legislative act entered into force specifying that pharmacists belong within the health care system,15 and 1996 when a decree complementing this law was published.16 This means that pharmacists were not legally recognized as health care professionals until the end of the 1990s. Later, in 2005, a decree regulating pharmacy processes and activities was issued,17 and in 2007 the pharmacy management model was defined, and a handbook on essential conditions and procedures was introduced18 (both applying to all health care settings). In this context, pharmacy practice within the health care system, especially in the hospital setting, is considered a relatively recent development in Colombia.
These regulations define the minimum programs that pharmacies should implement and introduce the concepts of “pharmaceutical care” and “medication-related problems”. However, the country does not have clinical pharmacy practice standards or initiatives that would allow pharmacy managers to have sufficient staff and to determine the appropriate clinical services to ensure consistent, continuous patient care. In addition, there are no residency, training, or certification programs to ensure that pharmacists are properly prepared and trained for direct patient care and to ensure they have the necessary experience for this role. Therefore, Colombia has no officially recognized pharmacy specialties.
Apart from pharmacists, practitioners in 2 other roles can perform their duties in a pharmacy: pharmacy regency technologists and pharmaceutical services technicians. Pharmacists are professionals with 5 years of university education and a scope of practice in hospitals that includes direction of high- and medium-level pharmacies, pharmaceutical care, and sterile/nonsterile compounding, with sterile compounding being restricted by law to this profession. Technologists study for 3 years, and their scope of practice in hospitals includes direction of low-level pharmacies, support to pharmacists in the clinical and administrative activities of high- and medium-level pharmacies, and nonsterile compounding. Technicians study for 1 year, and their scope of practice in hospitals is limited to activities such as reception, storage, distribution, and dispensing of drugs.
The Hospital Pablo Tobón Uribe (HPTU) is a tertiary university hospital located in Medellín, Colombia. The hospital currently has 490 beds, a number expected to increase to 681 by the end of 2017. At the beginning of 2015, the hospital did not have standardized clinical pharmacy services, and the number of pharmacists available to provide these services was insufficient to cover all inpatients. Until then, 4 pharmacists were available: 2 performing medical order review, 1 counselling patients receiving oral anticoagulants, and 1 managing adverse drug reactions (ADRs). The pharmacy regency technologists and pharmaceutical services technicians involved in distributive activities did not have defined roles distinguishing their responsibilities according to their level of training, and both groups were carrying out the same activities. In an attempt to improve the safety of inpatient care by standardizing clinical pharmacy services and defining the roles of the various parties at different phases of medication management (i.e., pharmacists, pharmacy regency technologists, and pharmaceutical services technicians), pharmacy managers proposed and the hospital adopted a new clinical pharmacy model. This report describes the implementation of this patient-centred clinical pharmacy model at the HPTU.
The design and implementation of the new model were carried out in several steps (Figure 1). First, a structured literature search was conducted in PubMed/MEDLINE (from inception to December 2015) for articles published in English or Spanish, freely available with full-text access, and addressing at least one of the following topics in the hospital setting: type of clinical pharmacy services offered by clinical pharmacists; workforce requirements/staffing; necessary skills of clinical pharmacists (training, certification, capabilities, competencies); economic evaluation of clinical pharmacy services; standards of practice in the United States, Canada, Australia, Spain, or other European countries; and role of technicians in the hospital pharmacy. Documents describing or evaluating clinical pharmacy services and those describing any of the topics listed above in health care settings other than a hospital were excluded.
|
|
||
|
Figure 1 Steps for redesign of the clinical pharmacy practice model at Hospital Pablo Tobón Uribe, Medellín, Colombia. |
||
The database search was complemented by searching the reference lists of selected articles (snowball search) for additional relevant materials and by searching the websites of the American College of Clinical Pharmacy, the American Society of Health-System Pharmacists, the European Society of Clinical Pharmacy, the Society of Hospital Pharmacists of Australia, the Canadian College of Clinical Pharmacy, and the Sociedad Española de Farmacia Hospitalaria. A grey literature search was conducted to identify unpublished literature in the above sources.
The second main step was to review the search results and identify the key elements to be taken into consideration for implementation of clinical pharmacy services in the hospital setting (general or critical care), especially workforce requirements/staffing and type of clinical pharmacy services. The third step was to formulate a model that could be adapted and implemented, considering that the greatest challenges would be recruitment of staff to cover all inpatients, definition of the clinical pharmacy services that would be offered, and specification of responsibilities to be assigned to each of the 3 roles available in the pharmacy (pharmacists, technologists, and technicians). The fourth step was to propose and explain the model to the HPTU board of directors, and the final step was implementation.
The literature search yielded publications related to the following key elements: standards of practice11,12,19–21; practice model experiences and considerations, in terms of services offered by the clinical pharmacy and workforce/staffing1,3,22–27; and training, competencies, and skills of clinical pharmacists.28–31
The pharmacy department’s organizational chart was subdivided into 2 sections: Clinical Pharmacy and Hospital Pharmacy. Both sections report to the director of pharmacy, and each has its own chief pharmacist and specific responsibilities. The Hospital Pharmacy section is in charge of activities such as purchasing, storage, sterile and nonsterile compounding, and distribution. The Clinical Pharmacy section is exclusively in charge of delivering clinical services.
The clinical services defined and offered to all inpatients were classified into 2 main categories: central management services and patient-centred services.
Central management services are those that do not involve direct patient care or regular contact with other members of the health care team (for duties related to patients’ health status or pharmacological treatment). Four services were defined: (1) medication-use evaluation, including evaluation of requests to update the therapeutic formulary with an evidence-based medicine approach, annual review of the therapeutic formulary, and monitoring of antibiotic consumption using the Anatomical Therapeutic Chemical/Defined Daily Dose system; (2) continuing education, which consists of training pharmacy staff and other health care professionals on a scheduled basis, at least 4 times a year; (3) clinical research conducted by pharmacy staff (as principal investigator or co-principal investigator), not including the compounding and dispensing of products used in research protocols; and (4) medication information, which consists of providing information on the proper use of medications to those who require it.
Patient-centred services involve the provision of direct patient care or direct contact with other members of the health care team for duties related to patients’ health status or pharmacological treatment. In this area, 5 services were defined: (1) participation in medical rounds (at least 3 days per week); (2) participation in multidisciplinary teams, which entails active and official membership in specific groups, with time allocated to attend meetings and carry out related tasks; (3) therapeutic monitoring, which consists of reviewing patients’ medical records and providing verbal or written follow-up concerning the clinical condition (involves repeated monitoring, with review of all medication orders and documentation of pharmaceutical interventions); (4) management of ADRs, which consists of detecting potential ADRs, providing and documenting appropriate follow-up until the ADR has resolved, and reporting ADRs to the national pharmacovigilance program; and (5) pharmacological counselling to patients, which involves provision of information about the proper use of medications to patients and/or family members during the hospital stay or after discharge.
One key element of the model redesign is that clinical pharmacy services are provided every day during the workday to all inpatients in each pharmacist’s assigned wards. Before implementation of the new model, each pharmacist was exclusively in charge of a single clinical pharmacy activity (medical order review, pharmacological counselling for patients receiving anticoagulants, or ADR management). In the new model, each pharmacist is responsible for providing all of the defined services, with no distributive or administrative activities. During the night shift, pharmacists are not present at the hospital; however, if they are called for a consultation, they can reach the hospital’s virtual private network to gain access to medical records and other required information.
The pharmacist–bed ratio required for provision of clinical pharmacy services under the new model had to be determined. It was defined as 1 pharmacist for every 60 (± 5) general hospitalization beds (including inpatients in the emergency department) or 1 pharmacist for every 20 (± 5) critical care beds, regardless of whether the beds are for adult or pediatric patients. Likewise, the wards were divided up among the pharmacists, with the goal of balancing complexity of care, inpatient profile, and expected demand for clinical pharmacy services. From early 2015 to November 2016, a total of 9 new pharmacists were hired, concurrent with the increase in beds to 490, bringing the total number of pharmacists to 13 (Table 1). In 2016, the hospital began opening new hospitalization services in a new tower block. It is expected that all services will be operational by the end of 2017, with the number of beds further increasing from 490 to 681. The defined pharmacist–bed ratio ensures the appropriate allocation and distribution of pharmacy staff for old and new hospital beds and ensures that existing clinical pharmacy services will be continued even as pharmacy services are expanded. The bed ratio for pharmacy regency technologists was defined as 1 technologist for every 40 (± 5) hospital beds for the day shift and 2 technologists for every 40 (± 5) hospital beds for the night shift. Thus, pharmacy regency technologists are present 24 hours/day, 7 days/week.
Table 1 Change in Pharmacist Staffing and Bed Coverage at the Hospital Pablo Tobón Uribe, Medellín, Colombia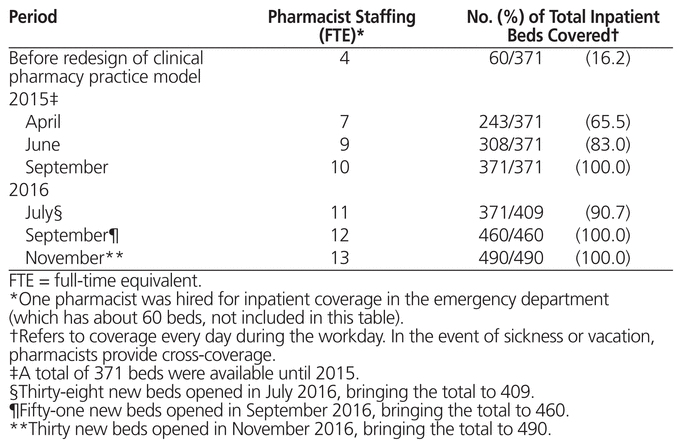
Before redesign of the clinical pharmacy services model, both pharmacy regency technologists and pharmaceutical service technicians fulfilled the same functions in the distribution system: preparation of dispensing carts and distribution of carts to the wards or dispensing directly from satellite pharmacies. With the recently implemented model, pharmacy regency technologists assist pharmacists in reviewing medication orders (allergy verification, therapeutic duplication, doses exceeding those recommended in the medical literature or exceeding the recommended infusion rate, and drug–drug interactions). Additionally, they develop the medication profile (a legal requirement), detailing each patient’s data, pharmacological treatment, and course of illness. Preparation of the medication profile is carried out in the pharmacy to ensure the safe, appropriate, and effective use of medications and to detect problems arising from pharmacotherapy (or the lack thereof ).
Activities related to dispensing and distribution of medications are now the exclusive responsibility of the pharmaceutical service technicians. These staff members are also available 24 hours/day, 7 days/week.
Every pharmacist working for the hospital, whether already on staff or newly hired, underwent training in the review of prescription appropriateness, therapeutic monitoring, and use of guidelines and institutional management protocols (for oral and IV anticoagulation, prophylaxis for venous thromboembolism, gastric prophylaxis, antibiotic prophylaxis for surgical patients, monitoring of laboratory tests, pharmacokinetics, and pain management). Additionally, protocols were developed for recording interventions in the pharmacy software. At the end of the training period, staff members took an examination (passing score 4.0 out of 5.0). Pharmacists’ participation in multidisciplinary teams also contributes to development of their competencies in specific health conditions and treatments. Pharmacy regency technologists have been trained by pharmacists in the verification of allergy history, therapeutic duplication, and use of clinical decision support tools.
A study group was formed for the promotion of continuing education on topics such as pharmacotherapy and the search for and critical review of scientific evidence. This group meets monthly, with each participating pharmacist being assigned a scientific paper or topic to be reviewed and then discussed with their coworkers. The initial topics for the study group were literature searching in scientific databases, use of software for bibliographic references, and formulation of PICO questions (patient, intervention, comparator/control, and outcome). The schedule of topics for the study group is now established annually.
A macro within Excel (2003 version; Microsoft Corporation, Redmond, Washington) was used to create a system for listing each patient’s medications and identifying drug–drug interactions of contraindicated, major, or moderate severity with excellent or good documentation, according to the Micromedex interaction checker (Micromedex Solutions, Truven Health Analytics, Inc). Another macro within Excel identifies doses that are higher than recommended by the medical literature for both adult and pediatric patients. Calculators to check dosage and rate of infusion for solutions containing potassium chloride and potassium phosphate were also developed. These tools were created in view of the fact that the HPTU has electronic health records and computerized physician order entry (CPOE), but does not have alerts incorporated in the prescription workflow. It was also necessary to take advantage of the pharmacy regency technologists’ knowledge and training, to assist pharmacists in medical order review during the creation of each patient’s medication profile. The development of these tools allows pharmacy regency technologists to identify prescriptions that deviate from the established appropriateness parameters and to alert the pharmacist to intervene with the physician. All interventions performed by the pharmacist or the pharmacy regency technologist are documented in an intervention-recording system.
The clinical pharmacy practice model implemented by the HPTU incorporates the 7 basic principles that have been described for this type of model1 and most of the issues covered by the revised Basel statements of the International Pharmaceutical Federation (FIP) Hospital Pharmacy Section.32–34 The defined ratios of staff (pharmacists and pharmacy regency technologists) to hospital beds allow all inpatients to be under a pharmacist’s responsibility and to receive clinical services while they are in hospital, even when there is a transition between services. The inclusion of pharmacy regency technologists in clinical activities and the reallocation of distribution activities to pharmaceutical services technicians release pharmacists from distribution and administrative activities and allow them to devote most of their time to patient care. These changes are expected to optimize pharmacotherapy results, reaffirming the value of pharmacists as direct patient care providers.35
The activities carried out by pharmacists before implementation of the new model were aimed at specific aspects of pharmacotherapy, and were not comprehensive in terms of either patients served or availability over time of the day or days of the week. Promoting the importance of pharmacists within health care services has become a major priority.28 With redesign of the HPTU model, each pharmacist is now engaged in direct observation and evaluation of a group of patients and their medication-related needs; the initiation, modification, or discontinuation of patient-specific pharmacotherapy; and ongoing monitoring and follow-up in collaboration with other health care professionals. In this way, the process of direct patient care has been made more consistent.19,28
Pharmaceutical care is generally applied in primary care practice but can be used in all health care settings. According to Hepler and Strand,36 it incorporates 4 essential elements, which have been described as the cornerstones of a pharmacist’s clinical care process: patient and medication therapy assessment, development of a care plan, implementation of the plan, and outcome evaluation. The definition of “pharmaceutical care” adopted by Colombian legislation is incomplete, because it includes only activities aimed at identification, resolution, and prevention of drug-related problems, excluding establishment of the therapeutic plan and evaluation of pharmacological outcomes. Nonetheless, despite this incomplete definition within the regulations, pharmacies are legally obliged to implement pharmaceutical care programs with a comprehensive approach to patient pharmacotherapy.
Clinical pharmacy practice models similar to the one implemented in the HPTU have been adopted elsewhere. Pickette and others2 described a patient-centred model in a multihospital system in the United States. Pharmaceutical interventions increased by 70% in one of the hospitals, and associated costs were reduced by about 134%. This model also highlighted the training of pharmacists and the importance of recording pharmaceutical interventions. Lorimer and others37 proposed redesign of the clinical pharmacy model in a 453-bed tertiary university hospital in Canada. In their model, orientation of pharmacists toward clinical practice and relevant training allowed devotion of up to 80% of pharmacists’ time to clinical activities on wards. Both of these models promoted the participation of clinical pharmacy specialists, recognizing it as a key element in the provision of direct patient care.29,36,37 In the case of HPTU, pharmaceutical interventions increased by 80%, classified mainly as risks that did not reach the patient (i.e., near misses). This result points to the work of pharmacists and technologists as highly preventive and important for patient safety, indirectly reducing the costs of patient care. Moreover, assignment of pharmacists to medical wards and determination of an appropriate pharmacist–bed ratio has allowed pharmacists to devote up to 90% of their time to clinical activities, with the remaining 10% of time invested in continuing education, attendance at academic meetings, and development of and participation in staff training activities for nurses and physicians. Similarly, the defined technologist–bed ratio has allowed technologists to devote up to 70% of their time to clinical activities, with the remaining 30% of time devoted to activities related to inventory control.
The clinical pharmacy practice model implemented at HPTU has some limitations. In Colombia, a pharmacist can start practising in a hospital or other health care setting after graduation from college and registration with the ministry of health, without any demonstration of knowledge. In other words, no accreditation or certification process is required to provide patient care, and the country does not have clinical residencies, training programs, or certified specialties for clinical pharmacists. As such, the model was implemented with pharmacists who did not have any certified specialty and who had achieved the required level of knowledge for their jobs through training and experience gained during provision of patient care in their assigned wards. Participation in patient care rounds 3 days a week is less than ideal. These situations represent opportunities to expand the recognition of pharmacists as members of the health team and of clinical pharmacy as a specialty.
Clinical services are covered by pharmacists only on the day shift, albeit on every day of the week. Access to the hospital’s virtual private network during the nighttime shift limits the provision of certain services, especially in wards where patients are admitted, discharged, and transferred at night. During holiday periods, sick leave, or other types of leave, patients are reassigned to the available pharmacists. In some of these cases, some important details of pharmacotherapy could be overlooked or receive less attention.
The organizational culture of the HPTU, which is focused on patient safety, and the confidence of the hospital’s board of directors in pharmacists’ contributions to this goal, have facilitated acceptance of the new model, the associated changes in the pharmacy’s organizational chart, and the hiring of new pharmacists required for implementation. During the transition from the previous model, the pharmacists already on staff were optimistic about the changes, which generated an environment without skepticism for the new staff. The experience gained from the old model also contributed to pharmacists’ rapid acceptance of the new model.
The model has had some challenges. For example, work-space is not always available for pharmacists, mainly in the wards of the old tower. This has hindered the full presence of pharmacists on the wards. Although in general terms the model has been accepted by physicians, nurses, and other health care professionals, some medical specialties have been resistant to changes and to accepting pharmacists as active members of the health care team.
Two studies are planned to evaluate the effects and performance of the model: an ambispective (both retrospective and prospective) cohort study to establish the association between pharmaceutical care and the incidence of medications errors (and related costs) for inpatients and a study to evaluate pharmacists’ concordance with the medical order review process.
The clinical pharmacy model implemented at the HPTU has allowed transition to a patient-centred approach in which each inpatient has a designated pharmacist and receives clinical pharmacy services according to the individual’s particular needs and health status. The roles of technologists and technicians have been redefined according to national laws, allowing pharmacists and technologists to devote more time to clinical activities. The new model incorporates elements found in the scientific literature and recommendations from recognized pharmacists’ associations around the world and allows the HPTU pharmacy to share in the current vision of clinical and hospital pharmacy.
Colombia has great challenges in the promotion of pharmaceutical care and the advancement of clinical pharmacy. Joint work by the Asociación Colombiana de Químicos Farmacéuticos Hospitalarios, the Colegio Nacional de Químicos Farmacéuticos, the Ministries of Health and Education, individual hospitals, and the country’s universities will be required to promote development of the profession to the level that has been achieved in other countries.
1 Breland BD. Believing what we know: pharmacy provides value. Am J Health Syst Pharm. 2007;64(12):1284–91.

2 Pickette SG, Muncey L, Wham D. Implementation of a standard pharmacy clinical practice model in a multihospital system. Am J Health Syst Pharm. 2010;67(9):751–6.

3 Bush PW, Ashby DM, Guharoy R, Knoer S, Rough S, Stevenson JG, et al. Pharmacy practice model for academic medical centers. Am J Health Syst Pharm. 2010;67(21):1856–61.

4 Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27(4):481–93.

5 Perez A, Doloresco F, Hoffman JM, Meek PD, Touchette DR, Vermeulen LC, et al. Economic evaluations of clinical pharmacy services: 2001–2005. Pharmacotherapy. 2009;29(1):128.
6 Nesbit TW, Shermock KM, Bobek MB, Capozzi DL, Flores PA, Leonard MC, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm. 2001;58(9):784–90.
7 Hatoum HT, Hutchinson RA, Witte KW, Newby GP. Evaluation of the contribution of clinical pharmacists: inpatient care and cost reduction. Drug Intell Clin Pharm. 1988;22(3):252–9.

8 Gallagher J, McCarthy S, Byrne S. Economic evaluations of clinical pharmacist interventions on hospital inpatients: a systematic review of recent literature. Int J Clin Pharm. 2014;36(6):1101–14.

9 Boyko WL, Yurkowski PJ, Ivey MF, Armitstead JA, Roberts BL. Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital. Am J Health Syst Pharm. 1997;54(14):1591–5.
10 Pharmacy practice model summit: executive summary. Am J Health Syst Pharm. 2011;68(12):1079–85.
11 Society of Hospital Pharmacists of Australia, Committee of Specialty Practice in Critical Care. SHPA standards of practice for critical care pharmacy practice. J Pharm Pract Res. 2008;38(1):58–60.
12 Society of Hospital Pharmacists of Australia (SHPA), Committee of Speciality Practice in Clinical Pharmacy. Overview: standards of practice for clinical pharmacy services. J Pharm Pract Res. 2013;43(2 Suppl):S2–S5.
13 Task Force on a Blueprint for Pharmacy. Blueprint for pharmacy: the vision for pharmacy. Ottawa (ON): Canadian Pharmacists Association; 2008.
14 Consejo Nacional de Seguridad Social en Salud (CNSSS). Ley 23 de 1962. Por la cual se reglamenta el ejercicio de la profesión de químico farmacéutico y se dictan otras disposiciones. [National Council of Social Security in Health. Law 23 of 1962. By which the regulation of the exercise of pharmacists’ profession and other provisions are dictated.]
15 Ley 212 de 1995. Por la cual se reglamenta la profesión de químico farmacéutico y se dictan otras disposiciones [Law 212 of 1995. By which the regulation of pharmacists’ profession and other provisions are dictated]. Bogotá (Colombia): Congreso de la República; 1995 Oct 26.
16 Ministerio de Salud y Protección Social. Decreto 1945 de 1996. Por el cual se reglamentan parcialmente las Leyes 23 de 1962 y 212 de 1995, y se dictan otras disposiciones. [Ministry of Health and Social Protection. Decree 1945 of 1996. By which the Laws 23 of 1962 and 212 of 1995 are partially regulated and other dispositions are dictated.]
17 Ministerio de la Protección Social. Decreto 2200 de 2005. Por el cual se reglamenta el servicio farmacéutico y se dictan otras disposiciones. [Ministry of Social Protection. Decree 2200 of 2005. By which the regulation of pharmaceutical services and other provisions are dictated.]
18 Ministerio de la Protección Social. Resolución 1403 de 2007. Por la cual se determina el Modelo de Gestión del Servicio Farmacéutico, se adopta el Manual de Condiciones Esenciales y Procedimientos y se dictan otras disposiciones. [Ministry of Social Protection. Resolution 1403 of 2007. By which the Model of Management of the Pharmaceutical Service is determined, the Manual of Essential Conditions and Procedures is adopted, and other dispositions are dictated.]
19 American College of Clinical Pharmacy; Harris IM, Phillips B, Boyce E, Griesbach S, Hope C, Sanoski C, et al. Clinical pharmacy should adopt a consistent process of direct patient care. Pharmacotherapy. 2014;34(8):e133–48.

20 American College of Clinical Pharmacy. Standards of practice for clinical pharmacists. Pharmacotherapy. 2014;34(8):794–7.

21 Yee GC, Haas CE. Standards of practice for clinical pharmacists: the time has come. Pharmacotherapy. 2014;34(8):769–70.

22 Smith MB, Gumpper KF, Riebandt G, Handel EM. Implementation of the Pharmacy Practice Model Initiative within comprehensive cancer centers. Am J Health Syst Pharm. 2014;71(19):1647–60.

23 O’Connor M, Weber RJ. Issues facing pharmacy leaders in 2013. Hosp Pharm. 2013;48(5):433–7.
24 Bickel RJ, Collins CD, Lucarotti RL, Stevenson JG, Pawlicki K, Baumann TJ, et al. Moving the Pharmacy Practice Model Initiative forward within a state affiliate. Am J Health Syst Pharm. 2014;71(19):1679–85.

25 Philip A, Gessner-Wharton M, Birney P, Blee J, Desai A, Gorbach C, et al. Pharmacy Practice Model Initiative task force report: improving participation in a survey on hospital pharmacy practices in Texas. Am J Health Syst Pharm. 2015;72(12):1053–7.

26 Pedersen CA, Schneider PJ, Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: prescribing and transcribing—2013. Am J Health Syst Pharm. 2014;71(11):924–42.

27 Shane R. Critical requirements for health-system pharmacy practice models that achieve optimal use of medicines. Am J Health Syst Pharm. 2011;68(12):1101–11.

28 American College of Clinical Pharmacy Board of Regents; Maddux MS. Board of Regents commentary. Qualifications of pharmacists who provide direct patient care: perspectives on the need for residency training and board certification. Pharmacotherapy. 2013;33(8):888–91.

29 American College of Clinical Pharmacy; Burke JM, Miller WA, Spencer AP, Crank CW, Adkins L, et al. Clinical pharmacist competencies. Pharmacotherapy. 2008;28(6):806–15.

30 White AM, Albertson B, Muncey L. Development and implementation of a clinical pharmacist training and assessment program. Am J Health Syst Pharm. 2012;69(4):278–81.

31 Ragucci KR, O’Bryant CL, Campbell KB, Buck ML, Dager WE, Donovan JL, et al. The need for PGY2-trained clinical pharmacy specialists. Pharmacotherapy. 2014;34(6):e65–73.

32 International Pharmaceutical Federation, Hospital Pharmacy Section. Revised FIP Basel Statements on the future of hospital pharmacy. The Hague (The Netherlands): The Federation; 2014 [cited 2016 Nov 6]. Available from: http://fip.org/files/fip/FIP_BASEL_STATEMENTS_ON_THE_FUTURE_OF_HOSPITAL_PHARMACY_2015.pdf
33 Vermeulen LC, Moles RJ, Collins JC, Gray A, Sheikh AL, Surugue J, et al. Revision of the International Pharmaceutical Federation’s Basel Statements on the future of hospital pharmacy: from Basel to Bangkok. Am J Health Syst Pharm. 2016;73(14):1077–86.

34 Penm J, Moles R. Global developments in hospital pharmacy: the revised Basel statements. J Pharm Pract Res. 2016;46(3):301–3.
35 Haas CE, Haase KK. Pharmacy Practice Model Initiative—it’s all about implementation. Pharmacotherapy. 2016;36(5):576–8.

36 Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533–43.
37 Lorimer HJ, Lalli SL, Spina SP. Redesign of the pharmacy practice model at a tertiary care teaching hospital. Can J Hosp Pharm. 2013;66(1):28–34.

Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors would like to thank members of the Hospital Pablo Tobón Uribe Direction Committee (Andrés Aguirre Martínez, María Victoria Restrepo, Antonio José Lopera, Piedad Cecilia Restrepo, and Gustavo Adolfo Gutierrez) and all members of the Department of Pharmacy for their support of and confidence in pharmacists.
Canadian Journal of Hospital Pharmacy, VOLUME 70, NUMBER 3, May-June 2017