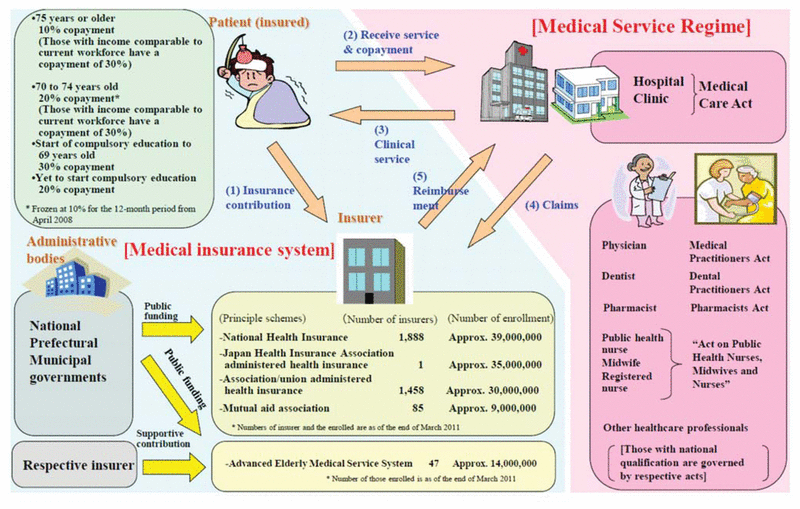
Figure 1 Overview of medical service regimen in Japan. Reproduced from “Health Insurance, an Outline of the Japanese Medical System”6 (Japanese Ministry of Health, Labour and Welfare).
Sari Nakagawa, Noriaki Kume
Japan is an island nation located in eastern Asia with a population of 127 043 413 (as of January 1, 2016).1 Most of the population (98.5%) is Japanese, with Koreans (0.5%), Chinese (0.4%), and others (0.6%) accounting for the remainder.2 Medical expenses were 4.1 trillion yen (about Can$50 billion) in 2013, an increase of 2.2% relative to 2012.3 The population and birth rate are declining rapidly, which has resulted in an unprecedented rate of demographic aging.4 Therefore, it is becoming financially difficult to maintain a health insurance system that keeps patients’ payments low. In this article, based upon the Health Systems Framework of the World Health Organization (WHO),5 we describe the current status of health care and the roles of pharmacists in Japan.
Since 1961, it has been mandatory for Japanese people to have public health insurance (known as “Kokumin Kaihoken”), which covers medical expenses for everyone. This health insurance system enables Japanese people to consult medical doctors without cost concerns. Health insurance resources cover 48.6% of national medical expenses; the remainder are covered by public resources (37.5%) and patients’ payments (13.9%)6 (Appendix 1*). The Japanese universal health insurance system allows choice of medical institution; provides high-quality, low-cost medical services; and uses a public subsidy to maintain universal coverage.6
Japanese patients pay only a certain percentage of medical expenses to hospitals, clinics, and pharmacies. Most patients pay 30% of their medical expenses, those under 7 years of age or between 70 and 74 years pay 20%, and those older than 74 years pay only 10%. In cases of extremely high medical expenses (e.g., for long-term hospitalization or innovative therapies), monthly costs are capped by patient age and income. An overview of the medical service regimen is shown in Figure 1.6
|
|
||
|
Figure 1 Overview of medical service regimen in Japan. Reproduced from “Health Insurance, an Outline of the Japanese Medical System”6 (Japanese Ministry of Health, Labour and Welfare). |
||
Japan’s universal health insurance, in conjunction with the current social insurance system, ensures the health and well-being of the country’s citizens.6 This universal health insurance system has allowed Japan to realize the world’s highest life expectancy and the highest level of health care standards. The universal system is made up of 2 main types of insurance: Employees’ Health Insurance, whereby employees of companies and organizations pay income-based premiums; and National Health Insurance, for people who are ineligible for an employment-based program. The National Health Insurance system has 3 types of premiums, based on annual income (details shown in Appendix 2).6
Trends in national health expenditure up to 2013 indicate that national medical expenditure increased yearly by almost 1 trillion yen (Appendix 3). National medical expenditure relative to gross national income increased to 11.2% in 2012, and national medical expenditure relative to gross domestic product (GDP) reached 10.3% in 2012.3
With the population aging at an unprecedented rate, it is becoming difficult to maintain the current health insurance system. In the near future, Japan will require revision of the welfare system (including health insurance) and development of a suitable taxation system, if the country is to be financially sustainable.
In fiscal year 2012, pharmacy dispensing accounted for 17.1% of Japan’s health expenditures, and drugs accounted for 22.5% of costs in medical institutions (Figure 2).3
|
|
||
|
Figure 2 Structure of national medical care expenditure (fiscal year 2012). Reproduced from Annual Health, Labour and Welfare Report 20153 (Japanese Ministry of Health, Labour and Welfare). |
||
The Organisation for Economic Co-operation and Development (OECD) Health Statistics Report for 2015 showed that health expenditures were expanding at a higher rate in Japan than in other OECD countries, dependent, at least in part, upon huge increases in drug expenditures. Since the world economic crisis in 2009, public drug expenditure has been expanding by 5% annually in Japan, while declining in other OECD countries. Currently, average drug expenditure per capita in Japan is the second highest in the world, after the United States, and is 45% higher than the mean of all OECD countries. Drug expenditure relative to GDP is 2.1% in Japan, the third highest in the world, after Greece and Hungary, and 50% higher than the OECD mean of 1.4%.7
The use of generic drugs in Japan is extremely low, less than half the OECD mean; therefore, it has been suggested that drug expenditures could be reduced by increasing the use of generic drugs. The Japanese Ministry of Health, Labour and Welfare launched a “promotion program for safe use of generic drugs” in 2007, which generated goals for a stable supply, quality assurance, and drug information system for generic drugs, and created a road map for the government and generic drug manufacturers to solve these problems.8 Current data (as of September 2015) show that generic drugs now constitute 56.2% of all prescription drugs. The national goal is for generic drug use to reach 70% in 2017 and 80% between 2018 and 2020,9 through aggressive promotion of this practice.
In 2014, elderly people (age ≥ 65 years) accounted for 26.0% (more than one-quarter) of the population, an increase from 23.0% in 2013. Because of declining birth rates, it is anticipated that elderly people will account for 39.9% (or 4 in 10) of the population by 2060 (Figure 3).4 The total fertility rate has decreased from more than 4.3 in the first-generation baby boom (i.e., 1947–1949) to 1.42 in 2014.5 Fetal and perinatal deaths continue to decline (Appendices 4 and 5).10
|
|
||
|
Figure 3 Population trends in Japan. Reproduced from Annual Health, Labour and Welfare Report 20154 (Japanese Ministry of Health, Labour and Welfare). |
||
In 1947, for the first time after World War II, life expectancy in Japan exceeded 50 years for both men and women. Since then, life expectancy has increased steadily (except in 2011, when the Great East Japan earthquake occurred) (Appendix 6).11 In 2014, life expectancy was 80.50 years for males and 86.83 years for females. Among men, life expectancy is increased by 0.07 year with malignant neoplasm and 0.05 year with pneumonia, relative to the life expectancy of the last year, whereas for women, it is increased by 0.06 year with both heart disease and cerebrovascular disease (Appendix 7).12
In 2015, Japan’s Ministry of Health, Labour and Welfare announced that cancer, heart disease, and pneumonia were the top 3 causes of death in Japan, accounting for 28.7%, 15.2%, and 9.4% of total deaths, respectively (Figure 4).13 Trends in cause-specific death rates from 1947 to 2015 are shown in Figure 5.10
|
|
||
|
Figure 4 Major leading causes of death in Japan in 2015. Reproduced from “Percentage distribution of causes of death in Japan”13 (Japanese Ministry of Health, Labour and Welfare). |
||
|
|
||
|
Figure 5 Trends in death rates for leading causes of death, from 1947 to 2015. Reproduced from “Vital Statistics”10 (Japanese Ministry of Health, Labour and Welfare). |
||
Although crude mortality rates for cancer, heart disease, and pneumonia appear to have increased recently, a decreasing trend is evident after adjustment for age (Appendix 8),10 which indicates that the increase in crude mortality rate may be age-related.
Rapid progress in medical technology and the development of new pharmaceuticals are leading to changes in the education and training of pharmacists and pharmaceutical researchers. Not only are these health care professionals required to develop advanced expertise and cope with rapidly expanding information and globalization, but they must also have humanity, common sense, high ethical standards, and good problem-solving skills. In response to these pressing needs, it was determined that pharmacy schools should provide a 6-year program, instead of the former 4-year program. Accordingly, since 2006, 2 different programs have been available in pharmacy schools: a 6-year program to educate pharmacists and a 4-year program to educate pharmaceutical scientists for drug development.14 Students enter pharmacy school after graduation from high school, at about age 18, which is equivalent to the age of students in Canadian grade 12. All students must pass an entrance examination set by the specific pharmacy school, and students who want to enroll in national and public pharmacy schools must also pass the National Center Test for University Admissions. Currently, there are 73 pharmacy schools in Japan (with a total enrolment of 11 550 students per year): 14 national schools, 3 schools managed by local governments, and 56 private schools. All 73 schools offer the 6-year program, and 30 schools also offer the 4-year program (14 national, 3 local government–managed, and 13 private schools, with total enrolment of 1489 students per year).15 Upon graduation from a 6-year program, students receive a bachelor-level pharmacy degree, not a doctorate-level degree. Since 2003, as many as 29 pharmacy schools have been newly established in Japan; therefore, new problems related to deteriorating quality of students have arisen, with some new pharmacy schools unable to accept sufficient numbers of qualified students.
The educational programs at the pharmaceutical schools consist of liberal arts and basic and advanced pharmacopedics (scientific study of drugs and medicinal preparations). The schools are continuously upgrading the quality of their courses in pharmacopedics. Students learn about a broad range of topics, including diseases, body systems, pharmacology, pharmacokinetics, pharmacotherapy, and patient communication. In addition, the knowledge-heavy curriculum of the past is evolving toward a more integrated curriculum that incorporates “skill and attitude” elements. The Conference for Studying the Pharmacopedical Curriculum was therefore inaugurated in 2001, with sponsorship from the Pharmaceutical Society of Japan. In April 2002, the draft of a new model curriculum was presented to all pharmaceutical schools and key organizations for input. The Conference then prepared the Model Core Curriculum for Pharmaceutical Education and the Practical On-site Training and Graduation Training Curriculum.
The Model Core Curriculum for Pharmaceutical Education (67 units) consists of humanism, an introduction to pharmacopedics, and specialized pharmacopedics (in the areas of physics-oriented pharmaceutical science, chemistry-oriented pharmaceutical science, biology-oriented pharmaceutical science, health and environment, drug and disease, pharmaceutical manufacturing, and pharmaceutical science and society). The Practical On-site Training and Graduation Training Curriculum (14 units) includes practical on-site training and final training before graduation.16 Students also learn about Kampo (Japan’s traditional herbal medicine), including its history, philosophy, herbal formulas, and mechanisms of action.
The basic principles of the Model Curriculum are (1) to educate students to become pharmacists and pharmaceutical researchers who meet the needs of society, (2) to let students specify their learning targets by themselves, (3) to objectively evaluate students’ progress, (4) to balance both basic and clinical pharmacopedical subjects, and (5) to provide more practical courses in both on-site and final training. The Core Curriculum has adopted plain language to make it more readily understandable, has integrated related areas into easy-to-follow courses, and has achieved a balance between knowledge versus skills and attitudes.16
Each school with the 6-year program follows a curriculum based on the Model Core Curriculum for Pharmaceutical Education, with the addition of unique components specific to the school and the use of suitable teaching methods to meet educational goals and assess achievement levels.17 In their fourth year, students must pass standardized national examinations: a computer-based test for knowledge and an objective structured clinical examination for practical skills, to confirm that they have sufficient knowledge and skills for pharmacy practice. The test standards of both examinations are based upon the Model Core Curriculum for Pharmaceutical Education. In the fifth year, students who have passed these national exams participate in experiential pharmacy practice training at hospitals and community pharmacies for 2.5 months each.16 Finally, after graduation from the 6-year program, students must pass a national licensing examination to become a pharmacist.
Residency training programs for pharmacists have recently been established in Japan. Unlike the situation in the United States and Canada, these residency training programs are not officially approved and are managed by individual institutions. The number of hospitals providing residency training programs has increased from fewer than 10 in 2011, before graduation of the first students in the 6-year pharmacy curriculum (which was established in 2006), to 23 in 2012 and 35 in 2013. Most of the hospitals with residency training programs are located in large cities, such as Tokyo and Osaka; the size of these hospitals ranges from fewer than 200 beds to more than 1000 beds, including university hospitals (Appendix 9).18
Residency training programs are usually 1 or 2 years long and are similar in organization and scope to residency training programs in the United States and Canada. Some institutions offer stepwise programs (similar to postgraduate years 1 and 2 in the United States), with general knowledge and practical skills for the first year and advanced clinical pharmacy for the second year. At most of the hospitals offering residency programs, the budget designated for part-time pharmacists is utilized for pharmacy residents, and most of the residents receive salaries and benefits similar to those of part-time pharmacists. Current problems of the residency programs include quality assurance, necessity of program approval by a third party (such as a professional society), and budget constraints. Further discussions appear to be needed if residency training programs are to be maintained.
Pharmacy practice has become specialized according to the development and innovation of medicine and pharmaceutical sciences. Therefore, pharmacists are now required to have specialized knowledge, advanced skills, and a range of experience. To test these requirements, a variety of board certification systems have been established, as approved by universities, health care organizations, and associations related to pharmaceutical sciences. As of June 30, 2016, a total of 43 868 board-certified pharmacists had been approved by the Japan Pharmacists Education Center,19 and 1351 board-certified pharmacists had been approved by the Japanese Society of Pharmaceutical Health Care and Sciences.20 In April 2015, the Japanese Society of Hospital Pharmacists established a new board certificate system,21 whereby pharmacists are required to have broad knowledge and experience (quantified as mandatory and elective units) in the pharmacy and at the bedside, for the following 5 areas: medical ethics and legal compliance, expand capability to basic practice, building a team-based medical practice, implementation of risk management, and the practice of pharmaceutical care.21 Generally, units are earned by participation in seminars (1 unit per 90 minutes) or through relevant e-learning; the seminars and e-learning are provided, organized, co-organized, or approved by the Japanese Society of Hospital Pharmacists. A pharmacist must earn 50 units over 3 years (with at least 10 units in each year) to be approved as a board-certified pharmacist.
This board certification requires, in each specialized area, experience in pharmacy practice, training for certain periods in hospitals where board-certified pharmacist specialists are teaching, development of advanced knowledge and skills after participation in seminars in a specialized area, and a passing mark on the examination for board-certified pharmacist specialists. Subsequently, the applicant’s research work in a specialized area is evaluated and approved. Board certification must be re-approved every 5 years. As of 2016, the most common areas for board-certified pharmacy specialists in Japan are infectious diseases (4253 pharmacists) and oncology (989 pharmacists) (Appendix 10).22,23
In Japanese law, the Pharmacists’ Act regulates pharmacists’ roles. This legislation states that “a pharmacist is to contribute to the improvement and promotion of public health by administering the dispensing of medicine, supply of medicine and other pharmaceutical health and sanitation services, thereby ensuring the healthy living of citizens”.24
In Japan, only medical doctors, dentists, and veterinarians have prescribing rights; pharmacists and other health care practitioners are not allowed to prescribe. Furthermore, drugs cannot be refilled in pharmacies; instead, patients must consult medical doctors and receive a new prescription every time they need a medication. Long-term prescriptions can be obtained for all medications except narcotics, psychotropic drugs, and new drugs (those within 1 year of their launch).
The numbers of pharmacists, doctors, and nurses are increasing every year (Figure 6, Appendices 11–13). As of December 31, 2012, there were 280 052 pharmacists, 303 268 doctors, and 1 571 600 nurses in Japan.3
|
|
||
|
Figure 6 Changes in numbers of pharmacists over time. Reproduced from Annual Health, Labour and Welfare Report 20153 (Japanese Ministry of Health, Labour and Welfare). |
||
The paragraphs below present an overview of the typical duties of pharmacists in the hospital setting in Japan.
Pharmacists confirm the content of doctors’ prescriptions and follow up with the prescriber if there are any problems. The pharmacist then correctly prepares the drug, with consideration of formulation, dosage, and route of administration as indicated by the prescription. If needed, the pharmacist simplifies dosage by using a tablet-packaging machine to make individual dose packages. A second pharmacist checks the prescription once it is prepared.
Pharmaceutical manufacturing refers to the preparation of medications by hospital pharmacists, for use exclusively in the same hospital. This process is necessary when drugs that are currently available on the market are not effective or usable in the available formulation. In this situation, medications must be prepared according to the pharmaceutical needs of each patient. Approval must first be given by the specific hospital’s institutional review board committees. Drugs are then prepared according to the “Guidelines for Pharmaceutical Manufacturing in the Hospital and Use within the Hospital”25 of the Japanese Society of Hospital Pharmacists. Preparation quality must be confirmed in the same way as for drugs directly available on the market, with attention to efficacy, safety, and stability. Before the drug is used, the patient must give informed consent.
The hospital pharmacy provides sterile rooms, clean benches, and safety cabinets for aseptic preparation of IV total parenteral nutrition, chemotherapeutic agents, and other products.
To use pharmaceutical agents appropriately and safely, pharmacists collect and evaluate data about routes of administration, dosages, adverse effects, drug interactions, and other aspects of medical therapy. Pharmacists then manage this information and provide it to other health care professionals or patients as necessary.
Clinical trials of new drugs are conducted in accordance with the “Ministerial Ordinance on Good Clinical Practice for Drugs (GCP)”26 and with the Pharmaceutical Affairs Law. As members of the medical teams responsible for conducting these trials, pharmacists are expected to assist doctors who have expertise in the field of the trial, in addition to storing and managing all trial drugs according to the trial protocol. The clinical research coordinators who assist doctors and patients in the course of clinical trials are usually hospital-employed pharmacists and/or pharmacists working for site management organizations.
Team-based medicine is driven by the desire to respect each patient’s unique perspective and to maintain or improve quality of life for both ambulatory patients and inpatients through collaborations among a variety of health care professionals, combining specialized skills from different professions. Pharmacists play important roles in every multidisciplinary team. Currently, hospitals have teams working on palliative care, control of infectious diseases, nutritional support, and prevention of bedsores.
Through the 1970s, the chief tasks of the hospital pharmacist were focused in the dispensary. However, since 1988, pharmacists’ jobs have moved from the pharmacy to the wards. At that time, medical facilities with more than 300 beds, a medication information room, and 2 drug information pharmacists (involved in the management of pharmaceutical information, as described above) were eligible to implement a reimbursement system for pharmacists for certain services—providing drug information to doctors or nurses, assisting with drug administration, preparing injectable drugs—on a “per inpatient per month” basis. In 1994, a new system of fees for inpatient drug management guidance was started, without the requirement for specific bed numbers; the main requirement was the presence of 2 full-time pharmacists, with one of these pharmacists working in drug information. In addition, medical remuneration points were increased, and the relevant tasks became critical parts of a hospital pharmacist’s duties.
Tasks related to inpatient drug management guidance include interviews with hospitalized patients to confirm their current medication use, use of over-the-counter drugs, supplements, or health foods, and checks for drug–drug interactions in reference works. Pharmacists also check for a history of allergies and adverse effects. Before drug administration, pharmacists review the patient’s illness, age, body weight, and renal and hepatic function to confirm that the dosage, rate of administration, and potential for drug interactions are appropriate. Pharmacists have a legal obligation to educate patients about how to take the drugs, as well as their effects and possible adverse effects. After the drugs are administered, pharmacists monitor effects and adverse effects through direct interaction with patients, sharing of information with other medical professionals, and, when necessary, provision of suggestions for further prescriptions. Pharmacists also instruct patients on how to continue taking their medications after discharge, in accordance with individual lifestyle.
Currently, the tasks of hospital pharmacists focus on the clinical pharmacy services described in the previous section. However, the tasks of hospital pharmacist teams and pharmacists working in hospital wards have recently been recognized, which resulted in the introduction of “ward pharmacist implementation addition fees” in 2012. New facility regulations include allowances for dedicated pharmacists for each ward (20 dedicated pharmacist-hours per week for each ward) or other systems.27
Because the profession of pharmacy technician does not exist in Japan, pharmacists are responsible for such varied tasks as drug dispensing and sterile preparation of IV total parenteral nutrition and chemotherapeutic agents. The tasks required of pharmacists keep expanding, and personnel numbers are often insufficient. To provide all necessary services sufficiently, the practical problems to be solved include recruitment of additional pharmacists.
Pharmacists working in community pharmacies mainly perform drug dispensing and clinical pharmacy services (as described above). They also manage patients’ drug administration history, including information about adverse effects. The medical remuneration points system was revised in 2016 and now includes newly established personal pharmacist guidance fees.28
The provision, by specialized pharmacists, of centralized drug management services for patients helps to prevent overprescription, duplication, and low patient adherence, thus contributing to drug safety, efficiency, and optimal medical expenses. It is also possible for patients to choose their own reliable pharmacists, called “primary care pharmacists”, who integrate and manage drug information and give appropriate advice and suggestions, if needed, at any time (24 h/day), even if the pharmacy is closed.
The primary care pharmacists also work with the prescribing doctor, monitoring both drug administration and changes in the patient’s health status, and consulting with the physician if needed. In addition, pharmacists visit patients’ homes to manage drugs as required. As the elderly population increases, pharmacists in community pharmacies will participate more in patients’ home care, visiting them in their personal residences or senior care facilities. In either case, pharmacists will dispense medications and offer guidance in drug administration after a drug has been prescribed by a physician. In the home setting, it is important for pharmacists to support patients’ mental and physical activities, as well as the families who take care of patients.
The use of electronic medical record systems is on the rise in Japan, particularly in hospitals with large numbers of beds (Appendix 14). The overall rate of electronic medical record use among hospitals was 34.2% in 2016. In larger hospitals (those with 400 or more beds [550 facilities]), the rate was as high as 77.5%. In medium-size hospitals (those with 200–400 beds [682 facilities]) the rate has been as low as 50.9%. In small hospitals (those with fewer than 200 beds [1310 facilities]), the rate has been 24.4%, and in general clinics (35 178 facilities), the rate has been 35.0%. The use of electronic medical record systems is expected to increase further among smaller hospitals and clinics (Appendix 15).29,30
To improve communication and interactions with local health care providers, community care systems, and home care health systems, increased use of electronic medical record systems is expected in the future. To create a society where patients can access high-quality medical care in comfortable and familiar regional settings near their hometowns, it is critical that local medical institutions be able to share patients’ medical information without hindrance.
By building networks that utilize information and communications technology, the Ministry of Health, Labour, and Welfare is facilitating effective information-sharing and is allowing local medical facilities to provide high-quality care. In particular, the ministry is putting in place guidelines for the safe management of medical information and is promoting medical data standardization to make information-sharing among facilities more seamless. It may become possible for community pharmacies to link their patients’ records to hospital medical records in the near future.
The classification of investigations for approval of drugs and other products is shown in Appendix 16.3
Determination of the quality, efficacy, and safety of new drugs requires an especially careful review. This review is conducted by a government advisory council composed of experts in medical, pharmaceutical, veterinary, and statistical sciences whose deliberations are based on data from basic and clinical studies. Good laboratory practices for the implementation of animal testing (to determine the toxicity of medications), among other nonclinical tests, and good clinical practices for the implementation of clinical tests are set forth by ministerial ordinances and are regulated to ensure appropriate testing (Appendix 17).
The Japanese Pharmacopoeia regulates the properties and quality of drugs. It is published by the Minister of Health, Labour and Welfare after hearing the opinion of the expert advisory council. Items selected for inclusion must be important to health care, according to need for the drug in medical practice, wide application, and experience of use. Since it was first published in June 1886, the Japanese Pharmacopoeia has been revised many times, with the latest (17th) edition coming into effect on April 1, 2016, with a total of 1962 drug entries.31
Only pharmaceuticals whose manufacture and sale have been approved by the government and that are listed in the Japanese Pharmacopoeia may be distributed.
Most prescription drugs and about half of the over-the-counter drugs are manufactured by pharmaceutical companies and then purchased by wholesalers who distribute them to individual medical institutions and pharmacies. Prescription drugs represent an 8.4 trillion yen market, and 97% of these products are purchased and distributed by wholesalers (2012 data).32
Health insurance pharmacies are dispensing community pharmacies that have a special insurance designation and can thus fill prescriptions using the public insurance system. Non-insurance pharmacies can sell over-the-counter drugs and dispense medicine, but their activities are not covered under health insurance.
Pharmaceutical wholesalers distribute more than 10 000 types of prescription drugs through a so-called “capillary distribution network”, supplying these items rapidly and reliably to about 230 000 hospitals, clinics, dental clinics, insurance pharmacies, and other facilities. As a result, this system maintains a service structure that enables patients at medical facilities all over the country to receive the same level of medical care. The successful distribution of necessary medical supplies in times of major disaster or emergency, such as the 2011 Great East Japan earthquake, demonstrates the resilience of the system. Pharmaceutical wholesalers also carry out additional functions, such as recalling defective products and gathering and disseminating opinions and information about adverse effects and other drug-related issues.32
Prescription drug prices are determined by the National Health Insurance Drug Price Standard, which also defines the scope of medical supplies that can be prescribed by physicians within the public health insurance system and the insurance billing price for prescribed items. There are currently about 16 000 entries in the drug price standard for prescription drugs, recognized officially by the Ministry of Health, Labour and Welfare, which can be used for health care services performed under health insurance at medical facilities.33
Diseases and persons subject to vaccination through the Preventable Disease Monitoring System in Japan are shown in Appendix 18.3
Mortality and morbidity for vaccine-preventable diseases are relatively high in Japan compared with Western countries. For example, 300 000 people had measles during the 2001 pandemic, and there were about 80 deaths.34,35 The reason appears to be a low rate of vaccine use because of problems with the current vaccine system. The safety of vaccines remains poorly recognized, relative to the situation in Europe and the United States. One event that may have raised concerns about vaccine safety was occurrence of meningitis as an adverse effect of a 3-antigen mixture vaccine that was administered from 1989 to 1993; this adverse effect resulted in discontinuation of the 3-antigen mixture in 1994, after which the vaccine was administered separately for each antigen. Vaccines for hepatitis B, mumps, and varicella, which are widely administered free of charge in most developed countries, have not yet become popular in Japan.36
There has been some recent progress in vaccine use and outcomes. In 2015, the WHO Western Pacific Regional Office reported that measles had been eliminated from Japan.37 Since October 2014, the pneumococcal vaccine has been administered routinely in the elderly population.38 In addition, varicella vaccine has been regularly administered to infants.39 The Japanese government is currently discussing whether hepatitis B vaccine should be regularly given to infants.40 Vaccine-related problems that need to be solved in the future include making hepatitis B, mumps, and varicella vaccines, which are standard around the world, free of charge; establishing systems to provide information about the safety and adverse effects of vaccines; and extensively educating medical staff and the public.
Demographic aging and a low birth rate are proceeding with unexpected acceleration. Therefore, it has become difficult to maintain a sustainable and stable medical welfare system. In the future, changes will be needed for the medical welfare system and the taxation system that financially supports it. On September 6, 2013, the Japanese government indicated that the combined revision of social welfare and taxation will occur at the time of the next revision of medical reward points (Appendix 19).41 In its statement, the government categorized hospital beds in detail, from acute care to chronic recovery care, and indicated the required number of hospital beds for each category for 2015. Furthermore, it clearly described the roles and functions of hospital beds for each category, and aimed to establish a seamless medical care system from admission to discharge and from hospital to home, designating those hospital beds (except for acute care beds) as community-based beds. Moreover, the government stated that medical information exchange between hospitals and local clinics should be promoted, along with the formation of medical teams (in outpatient clinics and in the home care setting), consisting of primary physicians, pharmacists, and nurses, who are connected and working locally.
When the medical reward system was revised in 2014, points were newly added for pharmacists’ work in hospital wards, to reduce physicians’ heavy tasks and to promote team medicine in hospital wards. Therefore, pharmacists are expected to communicate with patients and to become involved in optimal control of drug therapy as members of the medical team in hospital wards.
Furthermore, in communities, there is a need for pharmacists who can provide services for patients at home, because of increases in the elderly population. On October 23, 2016, the Japanese goverment released a report “Vision of Pharmacies for Patients”, describing 3 functions that pharmacists and pharmacies should have42: first, pharmaceutical management based on centralization of drug history information; second, patient care available 24 h/day, including at home; and third, close communication with primary physicians. Taking a long-term perspective, the Japanese government is trying to reform the medical system by working with various medical and welfare services to create a health care system that will be best for Japan in 2025. It is important that pharmacists understand these efforts and actively work with the government to be involved in developing their future role in patient care in the revised Japanese health care system.
1 Total population (final estimates). Tokyo (Japan): Ministry of International Affairs and Communications, Statistics Bureau; 2016 [cited 2016 Jul 29]. Available from: www.stat.go.jp/data/jinsui/pdf/201606.pdf
2 East and Southeast Asia: Japan. In: The world fact book. Washington (DC): Central Intelligence Agency; 2015 [cited 2016 Jul 29]. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/ja.html
3 Health and medical services. In: Annual health, labour and welfare report 2015. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2015 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/english/wp/wp-hw9/dl/02e.pdf
4 Overview of the system and the basic statistics. In: Annual health, labour and welfare report 2015. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2015 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/english/wp/wp-hw9/dl/01e.pdf
5 The WHO health systems framework. Geneva (Switzerland): World Health Organization; 2015 [cited 2016 Jul 29]. Available from: www.wpro.who.int/health_services/health_systems_framework/en/
6 Health insurance, an outline of the Japanese medical system. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2015 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/bunya/iryouhoken/iryouhoken01/dl/01_eng.pdf
7 OECD health statistics [2015]. Paris (France): Organisation for Economic Cooperation and Development; 2015 [cited 2016 Jul 29]. Available from: www.oecd-ilibrary.org/social-issues-migration-health/data/oecd-health-statistics_health-data-en
8 Promotion of the use of generic drugs. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2012 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/english/policy_report/2012/09/120921.html
9 [The share of generic drugs]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2016 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/file/06-Seisakujouhou-10800000-Iseikyoku/0000114903.pdf. In Japanese.
10 [Vital statistics]. Tokyo (Japan): Ministry of Health, Labour and Welfare, Statistics and Information Department; 2015 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/toukei/list/dl/81-1a2.pdf. In Japanese.
11 Life expectancies at specified ages. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2014 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/english/database/db-hw/lifetb14/dl/lifetb14-01.pdf
12 [Life expectances at specified ages]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2014 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/toukei/saikin/hw/life/life14/dl/life14-02.pdf. In Japanese.
13 [Percentage distribution of causes of death in Japan. Outline of the vital statistics]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2014 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai15/dl/kekka.pdf. In Japanese.
14 [Outline of pharmacy education system]. Tokyo (Japan): Ministry of Education, Culture, Sports, Science and Technology; 2013 [cited 2016 Jul 29]. Available from: www.mext.go.jp/a_menu/01_d/1329586.htm. In Japanese.
15 [List of pharmacy schools in Japan]. Tokyo (Japan): Ministry of Education, Culture, Sports, Science and Technology; 2016 [cited 2016 Jul 29]. Available from: www.mext.go.jp/component/a_menu/education/detail/__icsFiles/afieldfile/2016/07/26/1352588_01.pdf. In Japanese.
16 [Model core curriculum for pharmaceutical education]. Tokyo (Japan): Ministry of Education, Culture, Sports, Science and Technology; [cited 2016 Jul 29]. Available from: www.mext.go.jp/a_menu/01_d/08091815.htm. In Japanese.
17 Pharmaceutical education model core curriculum (draft). Tokyo (Japan): Pharmaceutical Society of Japan; [cited 2016 Jul 29]. Available from: www.pharm.or.jp/eng/curriculum.html
18 Hashida T. [Residency training program in Japan: current situation]. J Jpn Soc Hosp Pharm. 2013;49(1):39–41. In Japanese.
19 [List of board-certified pharmacists]. Tokyo (Japan): Japan Pharmacists Education Center; 2016 [cited 2016 Jul 29]. Available from: www.jpec.or.jp/nintei/kenshunintei/certified_list.html. In Japanese.
20 [List of board-certified pharmacists]. Tokyo (Japan): Japanese Society of Pharmaceutical Health Care and Sciences; 2016 [cited 2016 Jul 29]. Available from: www.jsphcs.jp/nintei/n-yakuzaishi.pdf. In Japanese.
21 Masato H. [New Japanese Society of Hospital Pharmacists certified pharmacists system]. J Jpn Soc Hosp Pharm. 2015;51(5):492–6. In Japanese.
22 [Board-certified pharmacists and board-certified pharmacy specialists in Japan]. Tokyo (Japan): Japanese Society of Pharmaceutical Health Care and Sciences; [cited 2016 Jul 29]. Available from: www.jsphcs.jp/nintei/01.php. In Japanese.
23 [Board-certified pharmacists and board-certified pharmacy specialists in Japan]. Tokyo (Japan): Japanese Society of Hospital Pharmacists; [cited 2016 Jul 29]. Available from: www.jshp.or.jp/senmon/senmon.html. In Japanese.
24 Pharmacists Act. Act No. 146. Tokyo (Japan): Ministry of Justice, Japanese Law Translation; 1960 [cited 2016 Jul 29]. Available from: www.japaneselawtranslation.go.jp/law/detail_main?re=01&vm=04&id=2596. In Japanese and English.
25 [Guidelines for pharmaceutical manufacturing in the hospital and use within the hospital]. Version 1.0. Tokyo (Japan): Japanese Society of Hospital Pharmacists; 2014 [cited 2016 Jul 29]. Available from: www.jshp.or.jp/cont/12/0621-2.pdf. In Japanese.
26 Ministerial ordinance on good clinical practice for drugs. Tokyo (Japan): Ministry of Health, Labour and Welfare; 1997 Mar 27 [amended 2012 Dec 28; cited 2017 May 19]. Available from: https://www.pmda.go.jp/files/000152996.pdf
27 [Health insurance: an outline of the Japanese medical system]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2015 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/bunya/iryouhoken/iryouhoken15/dl/h24_01-06-3.pdf. In Japanese.
28 [Revision of the medical remuneration points system]. Tokyo (Japan): Ministry of Health, Labour and Welfare; [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/file/06-Seisakujouhou-12400000-Hokenkyoku/0000116338.pdf. In Japanese.
29 [Utilization of the ICT in the health, medical and care field]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2015 [cited 2017 May 12]. Available from: www.mhlw.go.jp/file/06-Seisakujouhou-12600000-Seisakutoukatsukan/0000075101.pdf. In Japanese.
30 [Current situation of the informatization in the medical field]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2015 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryou/johoka/. In Japanese.
31 Japanese pharmacopoeia. 17th ed (electronic version). Tokyo (Japan): Ministry of Health, Labour and Welfare; 2016 [cited 2016 Jul 29]. Available from: http://jpdb.nihs.go.jp/jp17e/. In Japanese and English.
32 [Guidebook for the Federation of Japan Pharmaceutical Wholesalers Association 2014–2015]. Tokyo (Japan): Federation of Japan Pharmaceutical Wholesalers Association; 2014 [cited 2016 Jul 29]. Available from: www.jpwa.or.jp/jpwa/pdf/guide_2014-2015.pdf. In Japanese.
33 [Information about pahrmaceuticals listed in the JP and generic drugs]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2016 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/topics/2016/04/tp20160401-01.html. In Japanese.
34 [Regular vaccination status]. Tokyo (Japan): National Institute of Infectious Diseases; 2016 [cited 2016 Jul 29]. Available from: www.nih.go.jp/niid/ja/y-graphs/5508-measles-yosoku-vaccine2014.html. In Japanese.
35 [Number of regular vaccinations]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2013 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/topics/bcg/other/5.html. In Japanese.
36 [Current sitation and problems of vaccination in the world and in Japan]. Tokyo (Japan): Japan Institute for Global Health; 2014 [cited 2016 Jul 29]. Available from: http://jigh.org/wp/wp-content/uploads/2014/04/World-Immunization-Week_with-pics-SET.pdf. In Japanese.
37 Brunei Darussalam, Cambodia, Japan verified as achieving measles elimination [news release]. Geneva (Switzerland): World Health Organization; 2015 [cited 2016 Jul 29]. Available from: www.wpro.who.int/mediacentre/releases/2015/20150327/en/
38 [Pneumococcal infection (elderly people)]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2014 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/haienkyukin/index_1.html. In Japanese.
39 [Chickenpox]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2014 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/varicella/. In Japanese.
40 [Regular vaccination for B hepatitis]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2014 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-Kouseikagakuka/0000113332.pdf. In Japanese.
41 [The combined renovation of social welfare and taxation at the time of the next revision of medical reward points]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2013 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/file/05-Shingikai-12601000-Seisakutoukatsukan-Sanjikanshitsu_Shakaihoshoutantou/0000022009.pdf. In Japanese.
42 [Vision of pharmacies for patients]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2016 [cited 2016 Jul 29]. Available from: www.mhlw.go.jp/file/06-Seisakujouhou-12400000-Hokenkyoku/0000039891.pdf. In Japanese.
Competing interests: None declared. ( Return to Text )
*All appendices for this article are available from www.cjhp-online.ca/index.php/cjhp/issue/view/121/showToc. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 70, NUMBER 3, May-June 2017