Alexander D Smith, BSc, PhD, Postdoctoral Research Fellow, Gemma Rempel, BSc, Research Technician, Andras Szeitz, MPhil, Manager, Mass Spectrometry Facility, Tara L Klassen, BSc, PhD , Assistant Professor, Mary H H Ensom, BS(Pharm), PharmD, FASHP, FCCP, FCSHP, FCAHS, Professor
Although stability data are available for extemporaneously prepared vancomycin suspensions (25 mg/mL),1 a more concentrated suspension is preferable for many patients, especially those requiring larger doses. This study examined the physical characteristics and chemical stability of suspensions prepared from injectable liquid and powdered vancomycin (50 mg/mL) in a dye-free vehicle and stored in plastic bottles and oral syringes at 4°C or 25°C for up to 91 days.
Stock suspensions (50 mg/mL) of vancomycin (single lot from a single supplier of each formulation [liquid or powder]) were prepared in Oral Syrup (Medisca Pharmaceutique Inc, Saint-Laurent, Quebec; lot I184/A). Liquid vancomycin hydrochloride (Fresenius Kabi, Richmond Hill, Ontario; lot 6110902, expiry May 2016) was reconstituted with sterile water (Baxter, Mississauga, Ontario; lot WSL08AO) according to the manufacturer’s recommendations, and vancomycin hydrochloride powder (Medisca Pharmaceutique; lot 55824/H, expiry February 2017) was compounded to the desired concentration.
The suspensions were divided among 60-mL amber polyethylene terephthalate (PET) bottles (Medisca Pharmaceutique; product no. MBN91060AOB) and 3-mL amber oral syringes (Medisca Pharmaceutique; lot 605290/C). Three bottles were used for each combination of suspension type (prepared from liquid or power) and storage temperature (4°C or 25°C), for a total of 12 bottles. A total of 84 syringes were filled for each suspension type at each temperature tested.
All samples were examined for colour, odour, taste, and ease of resuspension on days 0 (baseline), 7, 14, 28, 42, 63, 77, and 91, when a 3-mL aliquot from each bottle and the contents of 3 syringes from each group were collected to determine pH (B10P benchtop pH meter, VWR, Radnor, Pennsylvania; product no. 89231-580). A 1.5-mL aliquot of each sample, obtained before drug expiry, was transferred to a threaded, tight-seal polypropylene vial (VWR; lot 98-5005) and frozen (−80°C) until batch analysis by a validated, stability-indicating high-performance liquid chromatography (HPLC) method.1
Stock solutions of vancomycin and the internal standard, metronidazole (Sigma-Aldrich, Oakville, Ontario; lot MKBW5062V), were prepared in Milli-Q purified water (EMD Millipore, Darmstadt, Germany), and a 7-point calibration curve was generated (vancomycin final concentrations 0.25, 0.5, 1, 1.5, 2, 3, and 4 mg/mL; internal standard 1 mg/mL). All samples were passed through a 0.45-μm filter (Pall Corporation, Ann Arbor, Michigan; lot 12643390), and diluted to 2.5 mg/mL concentration, before being combined 1:1 (v/v) with internal standard, followed by injection onto the HPLC column.
The HPLC instrumentation (Waters Alliance System model 2690, Waters Ltd, Mississauga, Ontario) consisted of a delivery pump, automatic 200-μL injector, Acquity dC18 4.6 × 150 mm column with a 1.7-μm pore size (Waters Ltd; lot 186001344), Nova-Pak C18 3.9 × 20 mm guard column (Waters Ltd; product no. WAT044380), and ultraviolet detector set at 274 nm. The mobile phase consisted of 22% methanol and 88% 0.01 mol/L ammonium acetate (EMD Millipore; lot 0165C513) at pH 2.5 and 25°C. All solvents were HPLC-grade and filtered before use. Flow rate was 1 mL/min.
For forced degradation studies, a 1 mg/mL solution prepared from vancomycin hydrochloride powder was mixed 1:1 with 0.1N sodium hydroxide and incubated at 100°C for 10 min, or was incubated without base at 100°C for 6 h. Samples were cooled to 25°C, centrifuged, diluted in mobile phase, combined 1:1 (v/v) with internal standard, filtered, and injected onto the column.
Regression analysis showed that the calibration curves were linear (r2 > 0.999, n = 4). Intra- and inter-day coefficients of variation were within acceptable limits (< 10%) across quality control ranges: 3.30% and 2.38%, respectively, for 0.31 mg/mL sample; 3.03% and 2.26%, respectively, for 0.62 mg/mL sample; 1.42% and 1.66%, respectively, for 1.25 mg/mL sample; and 2.96% and 1.77%, respectively, for 2.5 mg/mL sample. Forced degradation decreased the amount of vancomycin (with base, a 91.8% decrease; with heat, a 87.8% decrease) but no changes in peak shape or retention time. Small degradation peaks were observed1,2; however, there were no interfering peaks compared to controls.
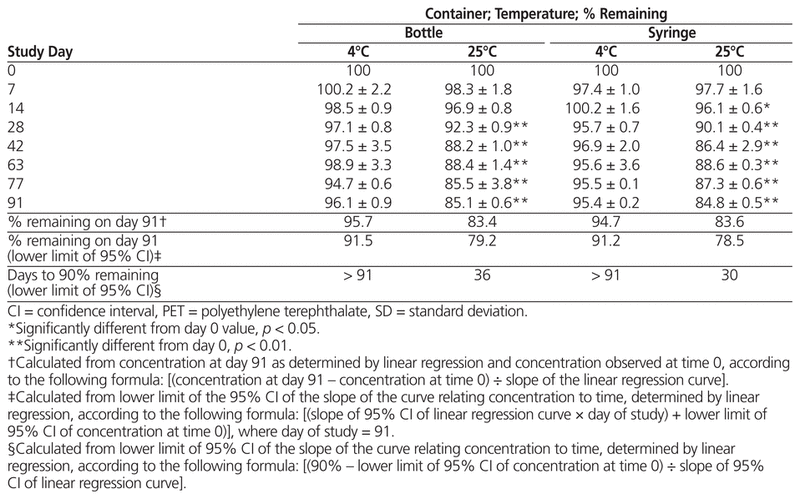
For samples prepared from liquid vancomycin, there was no statistically significant decline in concentration after storage at 4°C in bottles or syringes; however, samples stored at 25°C had a significant decrease in both types of container by day 91, dropping below 90% about 30 days after compounding (Table 1). For samples prepared from powder, vancomycin concentration dropped below 90% for most suspensions by day 7, with little difference between storage temperatures and vessel types by day 91 (Table 2).
Table 1 Percent Vancomycin Remaining (Mean ± SD) in Suspensions Prepared from Liquid Formulation and Stored for up to 91 Days in PET Bottles or Syringes
Table 2 Percent Vancomycin Remaining (Mean ± SD) in Suspensions Prepared from Powdered Formulation and Stored for up to 91 Days in PET Bottles or Syringes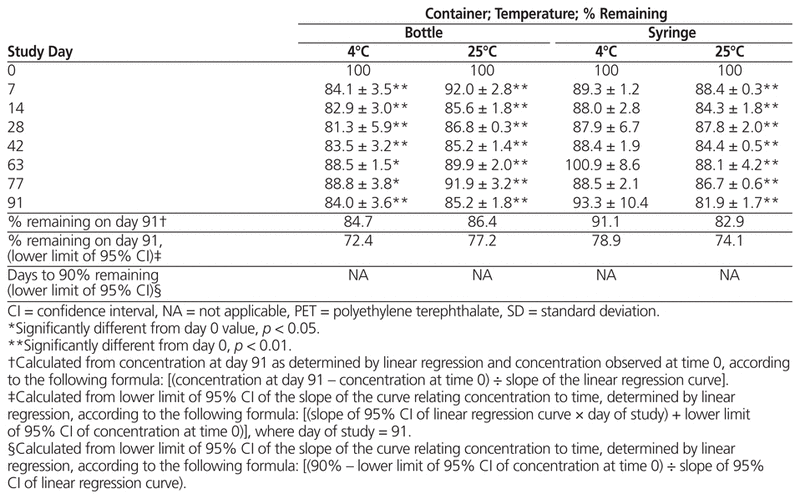
In contrast to a previous study using a lower concentration of vancomycin (25 mg/mL) in a different vehicle,1 there was a high degree of variability in taste, subjective palatability, viscosity, and miscibility of samples prepared from both liquid and powdered vancomycin. The mean pH (± standard deviation) for both types of preparation increased over time (for liquid, pH 3.29 ± 0.017 on day 0 to 3.85 ± 0.078 on day 91; for powder, pH 3.52 ± 0.021 on day 0 to 3.96 ± 0.032 on day 91), as has been observed previously.1,2 By day 7, samples had started to separate, with a thinner, clear liquid layer separating to the top of samples prepared from liquid vancomycin and to the bottom of samples prepared from powdered vancomycin. Suspensions prepared from powdered vancomycin became more viscous and difficult to resuspend over time, with those stored at 25°C being notably thicker than those stored at 4°C in both syringes and bottles.
In summary, suspensions of liquid vancomycin (50 mg/mL) prepared in Oral Syrup and stored in either syringes or PET bottles at 4°C were stable over a 91-day period, whereas those stored at 25°C can be expected to remain stable only up to 36 days (bottles) or 30 days (syringes). Suspensions of powdered vancomycin (50 mg/mL) in Oral Syrup fell to less than 90% of their initial concentrations after only 7 days. High intra-sample variability was observed across all storage vessels and study temperatures.
1 Ensom MHH, Decarie D, Lakhani A. Stability of vancomycin 25 mg/mL in Ora-Sweet and water in unit-dose cups and plastic bottles at 4°C and 25°C. Can J Hosp Pharm. 2010;63(5):366–72.

2 Mallet L, Sesin GP, Ericson J, Fraser DG. Storage of vancomycin oral solution. N Engl J Med. 1982;307(7):445.
Mary Ensom is also a Distinguished University Scholar with The University of British Columbia and a Clinical Pharmacy Specialist, Department of Pharmacy, Children’s & Women’s Health Centre of British Columbia, Vancouver, British Columbia. ( Return to Text )
Funding: This study was funded by an unrestricted educational grant from Medisca Pharmaceutique Inc. ( Return to Text )
Competing interests: Other than grant support, no competing interests were declared. ( Return to Text )
The authors thank Diane Décarie for her assistance with compounding and with establishing study protocols and parameters.
Canadian Journal of Hospital Pharmacy, VOLUME 70, NUMBER 3, May-June 2017