Jack T Seki, Andrea Bozovic, Roy Lee, Rita Kwong, Eshetu G Atenafu, Anna Xu, Jin-Hyeun HuhABSTRACT
Background
The addition of the immunostimulant plerixafor to the current standard-of-care regimens of granulocyte colony-stimulating growth factor with or without chemotherapy has improved clinical results in terms of successful stem cell mobilization and the outcomes of stem cell transplant in various settings. With this medical innovation has come an added financial cost for institutions where stem cell transplants are routinely performed, and there may be a further financial burden when the contents of partial vials of the drug are wasted, given that plerixafor vials (Mozobil, Sanofi-Aventis Canada Inc) are currently deemed suitable only for single use.
Objective
To determine whether the portion of plerixafor remaining in an opened vial of the Mozobil product after administration of a single dose is chemically stable, by comparison with the original product.
Methods
Stability testing of partial drug contents of an opened vial, stored at room temperature or under refrigeration (4°C), was conducted using liquid chromatography–tandem mass spectrometry analysis. The mean concentration of plerixafor (μmol/L), standard deviation, coefficient of variation, and bias were determined on days 2, 3, 11, 17, 24, and 31. Method validation included determination of precision, sensitivity, recovery, dilution linearity, and carryover.
Results
Throughout the 4-week testing period, measured plerixafor concentration in aliquots stored at room temperature and under refrigeration, tested in series over time, appeared similar. The mean residual drug concentration after initial opening was slightly, but not significantly, higher for the sample designated for storage at room temperature than the one designated for refrigerated storage (40.4 versus 39.9 μmol/L; p = 0.37).
Conclusions
Residual plerixafor after initial opening of a vial of the Mozobil product remained chemically stable for at least 2 weeks both at room temperature and under refrigeration. The results of this study provide in vitro evidence to support multiple uses, instead of single use, of vials of this drug in an aseptic, controlled environment.
KEYWORDS: plerixafor, stem cell transplant, drug stability
RÉSUMÉ
Contexte
L’ajout de l’immunostimulant plérixafor aux traitements reconnus comme la norme de soins actuelle quant au facteur de stimulation des colonies de granulocytes, accompagné ou non de chimiothérapie, a amélioré les résultats cliniques de mobilisation des cellules souches et les résultats de greffe de cellules souches dans différents contextes. Cela dit, avec cette innovation médicale vient un poids financier supplémentaire pour les établissements où l’on exécute couramment des greffes de cellules souches. En outre, comme les fioles de plérixafor (Mozobil, Sanofi-Aventis Canada Inc.) sont présentement jugées adéquates pour un usage unique seulement, l’excédent de médicament gaspillé peut représenter une dépense additionnelle.
Objectif
Déterminer si ce qui reste de Mozobil dans une fiole ouverte après l’administration d’une dose unique est chimiquement stable comparativement au produit de départ.
Méthodes
Une étude de stabilité du contenu partiel d’une fiole de médicament ouverte, entreposé à température ambiante ou conservé au réfrigérateur (4°C), a été réalisée par chromatographie en phase liquide couplée à la spectrométrie de masse en tandem. La concentration moyenne de plérixafor (μmol/L), l’écart-type, le coefficient de variation et le biais ont été établis aux jours 2, 3, 11, 17, 24 et 31. La méthode de validation comprenait la détermination de : la précision, la sensibilité, la récupération, la limite de linéarité et la contamination inter-échantillons.
Résultats :Tout au long des quatre semaines d’analyse, les concentrations mesurées des aliquotes de plérixafor, entreposées à température ambiante ou conservées au réfrigérateur et analysées en séries chronologiques, semblaient similaires. Les concentrations moyennes de médicament restant après l’ouverture initiale étaient légèrement plus élevées lorsqu’entreposées à température ambiante (40,4 μmol/L) que lorsque réfrigérées (39,9 μmol/L) (p = 0,37), mais pas de façon significative.
Conclusions
Le plérixafor résiduel, après l’ouverture initiale des fioles de Mozobil, demeurait chimiquement stable pendant au moins deux semaines, qu’il soit entreposé à température ambiante ou conservé au réfrigérateur. Les résultats de la présente étude offrent des données in vitro qui soutiennent une utilisation multiple, plutôt qu’un usage unique, des fioles de ce médicament en milieu aseptique contrôlé.
KEYWORDS: plérixafor, greffe de cellules souches, stabilité des médicaments
The immunostimulant plerixafor, in combination with the current standard-of-care regimens of granulocyte colony-stimulating growth factor (G-CSF) with or without chemotherapy, has improved the success of stem cell mobilization and stem cell transplant outcomes in various settings.1,2 In an efficacy and safety study, a median 3.9-fold increase in CD34+ counts and a high success rate for stem cell mobilization (97% [82/85]) were achieved when plerixafor was used in immediate rescue attempts.2 In a phase III prospective randomized trial comparing G-CSF plus plerixafor with G-CSF alone in patients with non-Hodgkin lymphoma, a significantly higher proportion of patients using the combination achieved ideal CD34+ counts.3 The result is an increase in transplant efficacy. In contrast, patients with poor stem cell mobilization results after G-CSF with or without chemotherapy were deemed to be at high risk for incurring additional costs for transplant resources and increased morbidity and mortality.4 Not only is the process of repeated stem cell mobilization and harvest in patients with previous transplant failure very costly and labour intensive, but the economic implications of failed attempts can also be felt through the domino effects of treatment complications: prolonged hospital stay, expected morbidity, and poor survival.5,6 As such, Kymes and others,6 using Markov modelling, found that the increased cost of providing plerixafor in combination with G-CSF could be justified.
With continuing demand for stem cell transplant, use of plerixafor in the current vial size (Mozobil, Sanofi-Aventis Canada Inc, Laval, Quebec) may not represent the most efficient drug usage, because of the short expiry date for the drug that remains after opening the vial and using one dose (within 24 h after opening).7 Although a single-use policy is specified by the manufacturer, discarding the unused portion of an expensive drug is counterintuitive and economically prohibitive. In 2015, the autologous stem cell program at the Princess Margaret Cancer Centre in Toronto, Ontario, began using plerixafor (Mozobil) in the context of the reactive setting for stem cell mobilization, whereby plerixafor may be considered as an add-on medication if initial stem cell mobilization is unsuccessful (contrasting with practices in other jurisdictions, where plerixafor is used for initial or “up-front” therapy1). On average, the cost of drug wastage for plerixafor at the study hospital amounted to $240 000 in the previous fiscal year.
Within the publicly funded health care system, cost-effectiveness has become a key consideration, alongside improvement in clinical outcomes. This dual goal served as the impetus to investigate of how best to preserve plerixafor in its original container after the vial was opened. The primary objective of this study was to determine whether the portion of plerixafor remaining after opening of the vial remains chemically stable, relative to the original product.
Plerixafor (lot 5-YFD-88-1) and the internal standard plerixafor-D4 (lot 1-ZNL-71-1) were purchased from Toronto Research Chemicals (Toronto, Ontario). Optima purity-grade methanol was obtained from Fisher Scientific (Fair Lawn, New Jersey). Ultra-pure water was prepared in house using an Elga Purelab Ultra water purification system (18.2-M•Ω cm resistivity). Trifluoroacetic acid of at least 99% purity was purchased from Sigma-Aldrich (St Louis, Missouri).
Stock standards were prepared by adding 1 mL methanol to each vial of compound and calculating the concentration from the exact mass (where exact weight was provided by the vendor). More specifically, a working calibration standard of plerixafor was prepared at a concentration of 400 μmol/L in methanol. A semi-stock standard of plerixafor-D4 was prepared at the same concentration in the same solvent, and the working internal standard was prepared at a concentration of 40 μmol/L in methanol. Calibration standards were prepared in methanol at concentrations of 10, 20, 40, 80, and 160 μmol/L. A second quantity of plerixafor (lot 5-YFD-88-1) was purchased and used for preparation of quality control samples. Quality control material was prepared, as described below, at 3 concentrations (low, medium, and high), which covered the calibration range.
Before liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis, 50 μL of the Mozobil product diluted 1000-fold in methanol was mixed with 50 μL of internal standard. The samples were vortex-mixed and analyzed.
The quantitative analysis of plerixafor in the Mozobil product was performed by LC-MS/MS. The equipment consisted of an Agilent Technologies 1200 system (binary pump, autosampler, and column oven) coupled to an API5000 triple quadrupole (Applied Biosystems/Sciex). Chromatographic separation was achieved on a reverse-phase Kinetex C18 100 × 3.0 mm, 2.6-μm column (Phenomenex, Torrance, California) using a mobile phase consisting of methanol (0.3% trifluoroacetic acid) and water (0.3% trifluoroacetic acid) (90:10, v/v) in isocratic mode with flow rate 0.7 mL/min. Each chromatographic run was 1 min long, with mean retention time for the analyte and internal standard of 0.58 min. The injection volume was 1 μL. The oven temperature was kept at 20°C. The mass spectrometer was operated in the positive electrospray ionization mode. Ion spray potential of 4500 V was employed. Declustering potential, entrance potential, and cell exit potential were kept at 80, 5, and 15 V, respectively, for both plerixafor and the internal standard. Data acquisition was performed in the high-resolution (both quadrupole Q1 and Q3) multiple reaction monitoring mode. Two selective reaction monitoring transitions were monitored for plerixafor (quantifier ion 503.5 > 105.1 m/z; qualifier ion 503.5 > 84.1 m/z) and one for the internal standard, plerixafor-D4 (507.5 > 109.1 m/z). Instrument control, data acquisition, and data analysis were performed with Analyst 1.6.2 software (Sciex, Concord, Ontario).
The method was validated by evaluating precision, sensitivity, recovery, dilution linearity, and carryover. These assessments were conducted in accordance with guidelines of the Clinical and Laboratory Standards Institute.8
Three quality control samples, with nominal concentrations of 8, 44, and 139 μmol/L, were prepared. Each of these samples was assayed 10 times within a single batch to evaluate the within-run precision. Between-run precision was assessed by assaying the quality control samples over 5 days (5 replicates each). Precision was deemed acceptable if the coefficient of variation was less than 10%.
The sensitivity of the assay was evaluated by assessing the limit of quantification, the lowest concentration with a coefficient of variation less than 20%; this limit of quantification was determined by measuring several low concentrations of plerixafor (8.6, 6.6, 4.5, 2.2, 0.6 μmol/L) in triplicate and calculating the following values: mean, standard deviation (SD), and coefficient of variation.
Analyte recovery was assessed by comparing the plerixafor concentration of a sample containing 10 μmol/L of the analyte before and after the known amount of plerixafor was added (10, 20, or 40 μmol/L). A third quantity of plerixafor (lot 5-YFD-88-1) was used for this experiment. After adding the known amount of plerixafor, each sample was assayed in triplicate. The recovery was calculated using the following formula: (measured concentration/expected concentration) × 100.
The Mozobil product is highly concentrated, so the determination of assay linearity required serial dilution to enable analysis of samples by the LC-MS/MS equipment. An aliquot of the Mozobil product was first diluted to 100 μmol/L with methanol; this initial solution was designated “10:0”. This sample was further diluted with the same solvent, according to the following ratios: 8:2 (i.e., 8 parts of the initial solution + 2 parts methanol), 6:4, 4:6, 2:8, and 1:9. Each of these samples was analyzed in triplicate. The measured plerixafor value was then plotted as a function of the expected value. If the correlation coefficient for the curve was greater than 0.99, as calculated by linear regression analysis, it could be concluded that the assay was linear.
Sample carryover was evaluated by analyzing 3 pairs of high–low plerixafor aliquots in methanol (in triplicate). Carryover, k, was calculated using the following formula: k = (Low1 – Low3) / (High3 – Low3), where “Low” refers to a sample with low plerixafor concentration, and “High” refers to a sample with high plerixafor concentration.
The manufacturer states that the concentration of plerixafor in the Mozobil product is 20 mg/mL,7 which is equivalent to 40 μmol/L.
One vial of plerixafor was opened for the purpose of patient care. Once the amount of drug required for patient treatment had been removed, the remainder was set aside for the stability study, as follows: a 100-μL aliquot of the drug was transferred into each of 2 separate 2-mL vials with inserts. One vial was stored at room temperature, and the other was stored in the refrigerator (4°C). Analysis by LC-MS/MS began 24 h later.
On each day of LC-MS/MS analysis, the 2 vials containing the drug samples were brought to the laboratory, where an aliquot of each sample was first diluted, then mixed with internal standard and analyzed. Five replicates of each diluted sample were assayed. The concentration of plerixafor was measured on days 2, 3, 11, 17, 24, and 31 after the vial was initially opened, with storage at room temperature or under refrigeration between analysis days.
In addition to the typical storage conditions of room temperature and refrigeration, concentration of drug in a room temperature aliquot was measured after either freezing the drug overnight at −20°C or heating at 60°C for 18 h on day 24.
Summary statistics for the concentration of residual drug after initial opening are presented as means, standard deviations, coefficients of variation, medians, and/or ranges for each group. Normality of the data was assessed with the Kolmogorov–Smirnov test, and the Student t test or Kruskal–Wallis test was then used as appropriate, according to the test of normality. All p values were 2-sided, and p < 0.05 was considered to indicate a statistically significant result. Statistical analyses were performed with the SAS system for Windows, version 9.4 (2002–2012) (SAS Institute, Inc, Cary, North Carolina).
Between-run precision of the assay was calculated at 3 quality control levels. The mean values for the low-, medium-, and high-concentration quality control samples were 8.1, 45.9, and 138.8 μmol/L, with coefficients of variation of 6.4%, 7.6%, and 6.5%, respectively. Within-run coefficients of variation were all less than 5% (3.3%, 4.0%, and 3.1% for the low-, medium-, and high-concentration quality control samples, respectively). The limit of quantification (the lowest concentration with a coefficient of variation less than 20%) was 2.2 μmol/L. The method had a mean recovery of 101.3% (coefficient of variation 0.3%). The Mozobil sample was diluted in the range of 100 to 10 μmol/L and measured in triplicate. The measured concentration was compared with the expected value. Data were plotted, and linear regression analysis was applied. The resulting line was described by the equation y = 0.9538x – 0.1777, with R2 = 0.9966. We concluded that the method yielded linear dilution. For assessment of carryover, 3 pairs of high–low samples (160–14 μmol/L, 120–0 μmol/L, and 53–10 μmol/L) were each assessed in triplicate. Calculated carryover values were 0.48%, 0.08%, and 1.35%, respectively, and were found to be nonsignificant.
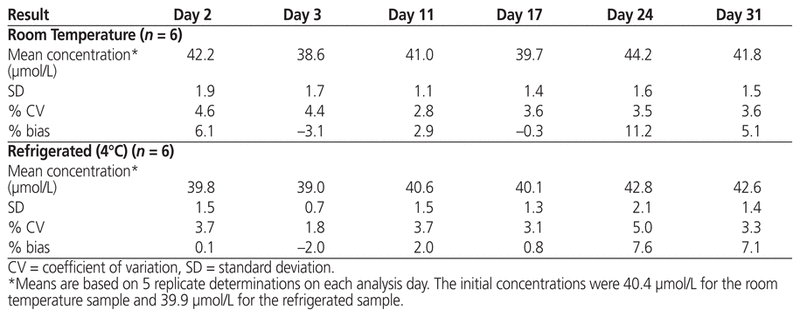
The samples for this study were obtained from a single vial and were tested on 6 separate dates over the course of the study period. Over the 4-week study period, no visible precipitate or change in colour was observed. According to the Kolmogorov–Smirnov test, the assumption of normality for concentrations at both storage temperatures was not violated, and hence the Student t test was used for statistical comparisons. Over the study period, the measured concentrations showed little variation. The mean residual concentration of drug after initial opening of the vial was slightly, but not significantly, higher for the sample designated for storage at room temperature than the one designated for refrigerated storage (40.4 versus 39.9 μmol/L; p = 0.37). The measured concentrations of plerixafor under conditions of room temperature and refrigerated storage are presented in Table 1. The standard deviation ranged from 1.1 to 1.9 for the room temperature sample and from 0.7 to 2.1 for the refrigerated sample. The measured concentration ranged from 38.6 to 44.2 μmol/L with storage at room temperature and from 39.0 to 42.8 μmol/L with storage under refrigeration.
Table 1 Analytical Results after Storage for up to 31 Days, by Storage Temperature
The measured concentrations of plerixafor subjected to stress conditions of either freezing overnight at −20°C or heating at 60°C for 18 h are presented in Table 2. Under these stress conditions, the mean concentration ± standard deviation was 40.7 ± 1.6 μmol/L after freezing and 40.6 ± 0.9 μmol/L after heating.
Table 2 Analytical Results after Stress Conditions, by Temperature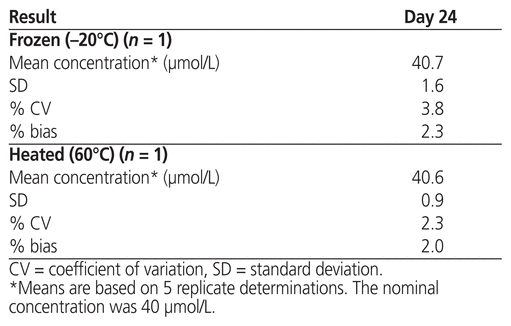
This rigorous study generated stability data that are consistent with little variability in plerixafor concentration throughout a 4-week testing period. The LC-MS/MS method used here was developed specifically for quantification of plerixafor in the Mozobil product, such that the measured concentration could serve as an indicator of the product’s chemical stability. The results presented in Table 1 indicate that the study parameters, obtained in series over time, for aliquots stored with refrigeration and at room temperature appeared similar. We would, however, favour storage under refrigeration (at 4°C) to preserve the unused portion of plerixafor in its original container over a 2-week period, at which point it should be discarded. Out of curiosity, we took the liberty of testing the product in extreme temperatures. Surprisingly, the product was stable with both heating and freezing, within the context of drug concentration (Table 2). These findings suggest that plerixafor has the potential to remain chemically stable under a wide range of storage conditions.
According to General Chapter <797> of the United States Pharmacopeia (USP), sterile products in single-use vials may be used within 6 h after opening if maintained in an ISO 5 environment.9 USP General Chapter <797> suggests that in the context of compounded sterile preparations, for which sterility testing is not performed in low-risk conditions, the duration of storage and the assigned beyond-use date is 14 days with refrigerated temperatures (2°C to 8°C), provided the preparations are manipulated aseptically.9,10
We have elected to also use this 14-day benchmark for leftover plerixafor, on the basis of other studies suggesting that beyond-use dates may be extended in low-risk environments, provided that the sterility of drugs in single-use vials can be maintained with closed-system transfer devices.11 The appropriateness of storage under refrigeration for up to 14 days is further supported by the results of the current study: concentrations of leftover plerixafor stored under refrigeration remained similar to the baseline concentration (nominally 40 μmol/L; measured value 39.9 μmol/L) on days 11 and 17 (40.6 and 40.1 μmol/L, respectively; Table 1). Kim and others12 used high-performance liquid chromatography to evaluate the chemical stability of plerixafor in opened vials over 84 days. Although they found that the drug remained stable under both refrigerated and room temperature conditions, they recommended refrigeration over room temperature storage to decrease the likelihood of antimicrobial contamination.12
One limitation of the current study was that the LC-MS/MS method was not developed as a stability-indicating assay. Rather, the LC-MS/MS analysis was developed to allow us to accurately identify and quantify plerixafor in the Mozobil product, which provided evidence for chemical stability and indicated that extension of the beyond-use date may be possible after opening of the vial, provided that storage conditions are appropriate and sterility is maintained. That being said, another limitation is that sterility was not tested in this study. Sterility is an important consideration that influences the feasibility and safety of reusing leftover product, since extension of the beyond-use date coincides with a risk of antimicrobial contamination.11,12 However, the objective of this study was to test whether leftover plerixafor could remain chemically stable and to ascertain which storage conditions might facilitate chemical stability. Finally, it was not feasible for this study to have access to more than one opened vial of the Mozobil product, because of the high cost of the drug itself and the financial constraints of the study institution.
Currently, there are 2 practices leading to stem cell collection and transplant. Ogunniyi and others1 reported an “up-front method” involving the use of plerixafor plus G-CSF for stem cell mobilization.1 The success rate of 92.8% was defined by the ability to collect at least 5 × 106 CD34+ cells/kg by apheresis, enabling 2 transplants in the process. Alternatively, the Cancer Care Ontario practice guidelines outline specific criteria indicating when plerixafor is recommended as an add-on treatment to G-CSF in mobilizing and harvesting stem cells before autologous stem cell transplant.13
The reported failure rate of stem cell mobilization has been quite high, in global terms, with the number of patients experiencing failure estimated in the range of 5000 to 10 000 annually.4 Mobilization failure rates were documented as high with the use of G-CSF and chemotherapy, with an estimated range from 5% to 30% in both allogeneic and autologous stem cell transplantation.3,6 Age, hemoglobin level, and bone marrow involvement are other factors said to be correlated with the success of mobilization.7
Although a full pharmacoeconomic analysis is not available, avoidance of this high failure rate could provide strong economic justification for introducing plerixafor into the stem cell mobilization regimen. This drug is clinically appropriate in its ability to augment stem cell mobilization by improving CD34+ cell counts at harvest and by improving overall engraftment outcome, thereby offsetting the health care burden of failed mobilization attempts. In a smaller-scale evaluation, Kymes and others6 put forward such an economic justification, although their study may have had a large sampling error because of the small number of participants (n = 20). Further cost savings from drug wastage could be alleviated if we were able to re-access residual drug in the partial vial after initial opening in the clinical setting. Because plerixafor has only been in use at the study institution since 2015, further drug-use evaluation studies are needed to assess its long-term financial impact. Estimating the frequency of plerixafor use may be more difficult for institutions that use this drug in “reactive settings” than for those that use plerixafor “up front”. Thus, the type of clinical approach would affect the extent of potential cost savings. In either case, there is an opportunity to reduce drug wastage and drug costs if the beyond-use date can be extended.
The results of stability testing strongly supported the possibility of reusing partial vials of plerixafor (Mozobil). Although the mean residual drug concentration after initial opening was a little higher at room temperature than under refrigeration, this difference was not statistically significant (40.4 versus 39.9 μmol/L; p = 0.37).
This study showed, via LC-MS/MS analysis, the chemical stability of plerixafor under regular storage temperatures and extreme conditions, in terms of concentration (μmol/L), over a study period of 4 weeks. For the purpose of more stringent accountability and patient safety, we have elected to use a 2-week expiry date for partial vials of this product stored under refrigeration. Future studies are planned to evaluate the stability and sterility of opened vials, to further explore the possibility of extended clinical use. We look forward to a new phase of research in this area, which will not only focus on the safety and effectiveness of using partial vials of plerixafor in the stem cell transplant setting, but will also address fiscal savings for the institution.
1 Ogunniyi A, Rodriguez M, Devlin S, Adel N, Landau H, Chung DJ, et al. Upfront use of plerixafor and granulocyte-colony stimulating factor (GCSF) for stem cell mobilization in patients with multiple myeloma: efficacy and analysis of risk factors associated with poor stem cell collection efficiency. Leuk Lymphoma. 2016;58(5):1123–9.
2 Worel N, Fritsch G, Agis H, Böhm A, Engelich G, Leitner GC, et al. Plerixafor as preemptive strategy results in high success rates in autologous stem cell mobilization failure. J Clin Apher. 2017;32(4):224–34.
3 DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(28):4767–73.
4 To LB, Levesque JP, Herbert KE. How I treat patients who mobilize hematopoietic stem cells poorly. Blood. 2011;118(17):4530–40.
5 Meehan KR, Hill JM, Patchett L, Webber SM, Wu J, Ely P, et al. Implementation of peripheral blood CD34 analyses to initiate leukapheresis: marked reduction in resource utilization. Transfusion. 2006;46(4):523–9.
6 Kymes SM, Pusic I, Lambert DL, Gregory M, Carson KR, DiPersio JF. Economic evaluation of plerixafor for stem cell mobilization. Am J Manag Care. 2012;18(1):33–41.

7 Mozobil® plerixafor injection 24 mg/1.2 mL (20 mg/mL), single use vial. Subcutaneous use only. Hematopoietic agent [product monograph]. Version 2.0. Laval (QC): Sanofi-Aventis Canada Inc; 2014 Oct 8 [cited 2016 Nov 18]. Available from: http://products.sanofi.ca/en/mozobil.pdf
8 C62-A: Liquid chromotography-mass spectrometry methods; approved guideline. Wayne (PA): Clinical and Laboratory Standards Institute; 2014.
9 General chapter <797>: Pharmaceutical compounding—sterile preparations. In: The United States Pharmacopeia [34th rev] – The National Formulary [29th ed]. Rockville (MD): United States Pharmacopeial Convention; 2011. p. 336–73.
10 Donnelly RF. An overview of United States Pharmacopeia sterility testing. Sci Technol Hosp Pharm. 2013 [cited 2016 Oct 4];3(4). Available from: http://compoundingtoday.com/newsletter/Science_and_Tech_1304.cfm
11 Edwards MS, Solimando DA, Grollman FR, Pang JL, Chasick AH, Hightman CM, et al. Cost savings realized by use of the PhaSeal® closed-system transfer device for preparation of antineoplastic agents. J Oncol Pharm Pract. 2013;19(4):338–47.
12 Kim SH, Thiesen J, Krämer I. Physicochemical stability of Mozobil® (plerixafor) solution for injection in glass vials and plastic syringes over a three-month storage period. Pharm Technol Hosp Pharm. 2016;1(2):73–81.
13 Kouroukis CT, Varela NP, Bredeson C, Kuruvilla J, Xenocostas A. Plerixafor for autologous stem-cell mobilization and transplantation for patients in Ontario. Curr Oncol. 2016;23(4):e409–30.

Competing interests: None declared. ( Return to Text )
Funding: This was an internally funded study. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 70, NUMBER 4, July-August 2017