Stability of Dapsone in Extemporaneously Compounded Oral Suspensions
Hugo Alarie, BSc, MSc candidate, M Mihaela Friciu, MSc, Research Associate, Grégoire Leclair, BPharm, PhD, Associate Professor
Dapsone is a sulphone antibiotic with anti-inflammatory properties.1 It is used as a first-line treatment for multibacillary and paucibacillary leprosy, and its mechanism of action is to reduce the synthesis of dihydrofolic acid.2,3 Its structure is illustrated in Appendix 1 (available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/126/showToc). It has a solubility of 0.284 mg/mL in water and a pKa of 2.39.4,5 The very low solubility of dapsone over the acceptable range of pH for an oral preparation does not allow formulation of a solution. In the absence of commercial alternatives, compounded liquid suspensions are required for children and when administration of solid dosage forms is not suitable. Liquid preparations in Ora-Blend (Paddock Laboratories; constituents sucrose, glycerin, sorbitol, flavouring, microcrystalline cellulose, carboxymethylcellulose sodium, xantham gum, carrageenan, calcium sulphate, trisodium phosphate, citric acid, sodium phosphate, dimethicone, methylparaben, and potassium sorbate), SyrSpend (Fagron Inc; constituents modified food starch, sodium citrate, citric acid, malic acid, sodium benzoate, and simethicone), and other noncommercial aqueous vehicles have been reported, with stability of at least 3 months under refrigerated conditions and between 1 and 3 months at room temperature.6–8
SyrSpend is not readily available in North America, and there may in future be shortages of (or a patient may be intolerant of ) Ora-Blend. Therefore, this study was conducted to assess the stability of dapsone in Oral Mix and Oral Mix SF vehicles, which are suitable as alternative dye-free formulations for children. These agents are easy to work with and are globally available.
Suspensions were compounded on March 29, 2016, from dapsone tablets (5 × 100 mg, Jacobus Pharmaceutical, Princeton, New Jersey; lot 16387, expiry November 2017), which were pulverized with mortar and pestle. Oral Mix (Medisca Pharmaceutique Inc, Montréal, Quebec; lot I185/A, expiry January 2018; constituents glycerin, sorbitol, flavouring, microcrystalline cellulose, carboxymethylcellulose sodium, sodium saccharin, xantham gum, carrageenan, sodium citrate, citric acid, methylparaben, propylparaben, potassium sorbate, and simethicone) or Oral Mix SF (Medisca Pharmaceutique Inc; lot H1136, expiry October 2017; constituents sucrose, glycerin, sorbitol, flavouring, microcrystalline cellulose, carboxymethylcellulose sodium, xantham gum, carrageenan, sodium citrate, citric acid, methylparaben, potassium sorbate, and simethicone) was then geometrically incorporated to a final volume of 250 mL.
Each preparation was packaged in 50-mL amber plastic bottles (6 bottles containing 30 mL per preparation; polyethylene terephthalate [PET] with black phenolic cap, Medisca Pharmaceutique Inc) and 3-mL amber polypropylene syringes (48 syringes containing 1 mL per preparation; PreciseDose syringes with tip cap, Medisca Pharmaceutique Inc). Three bottles of each preparation and 3 syringes for each time point were stored at a mean temperature of 5°C (standard deviation [SD] 2°C) or 25°C (SD 2°C), with relative humidity 60% (SD 5%). At predetermined time points (0, 7, 14, 30, 45, 60, 75, and 90 days), a 1-mL aliquot was retrieved from each bottle and 3 syringes were retrieved from each temperature condition. The bottles and syringes were vigorously shaken until complete resuspension before sampling.
On each study day, the appearance of each test sample was inspected. The pH was evaluated (pH 211 model pH meter, Hanna Instruments, Montréal, Quebec) and concentration was assayed using a stability-indicating high-performance liquid chroma tography (HPLC) method with ultraviolet detection.
For the HPLC analysis, each test sample (50 μL) was diluted with methanol (950 μL) in a 1.5-mL centrifuge tube, vortexed (20 s) and then centrifuged (10 000g for 10 min). The supernatant (150 μL) was further diluted using a mixture of acetonitrile and water (20:80, 150 μL) and vortexed (20 s). These solutions for injection (nominal 0.05 mg/mL) were analyzed immediately after preparation using an HPLC system (Prominence UFLC, Shimadzu, Laval, Quebec) equipped with an LC-20AD binary pump, a DGU-20A5 solvent degasser, an SPD-M20A multiple-wavelength photodiode array detector set at 290 nm, an SIL-20AC HT refrigerated autosampler set at 5°C, a CTO-20AC column oven set at 40°C, and a Kinetex XB-C18 column (4.6 × 100 mm, 5 μm, Phenomenex Inc, Torrance, California). An isocratic method (acetonitrile–aqueous KH2PO4 10 mmol/L, 12:88, 1 mL/min) was used. The dapsone peak eluted at approximately 7.1 min; the peak area was used to perform the quantification. Injections (10 μL) were performed in duplicate for test samples and in triplicate for standard samples.
To perform the calibration, standard suspensions of dapsone bulk powder (AK Scientific, Union City, California; lot 90411H, expiry May 2019) in Oral Mix and Oral Mix SF were prepared as described above, diluted to concentrations of 1.6, 1.8, 2.0, 2.2, and 2.4 mg/mL (80% to 120% of the target concentration), and analyzed by HPLC (r2 not less than 0.999). The 2 mg/mL standard was also injected after every 24 injections of test solution to ensure system stability. The highest intraday coefficient of variation observed was 1.65% (n = 3), and the highest interday coefficient of variation was 1.82% (over 3 days; n = 3 × 3) at the target concentration.
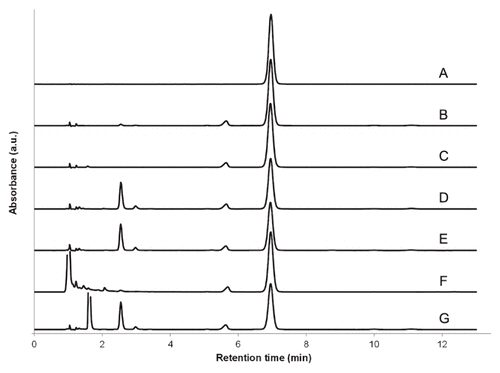
Forced degradation at 60°C for 3 h in one volume each of purified water, hydrogen peroxide 3%, sodium hydroxide 1 mol/L, and hydrochloric acid 1 mol/L resulted in recoveries of 76%, 73%, 91%, and 69%, respectively. No peak overlap of dapsone with excipients, impurities, or degradation products was observed. All non-dapsone peaks eluted between 1 and 5 min. Dapsone peak purity index calculated between 260 and 320 nm was not less than 0.9999 in all cases.3 Representative chromatograms are presented in Figure 1.
| |
|
|
|
Figure 1 Representative chromatograms of (A) dapsone standard solution in acetonitrile–water (1:4 v/v), (B) dapsone suspension prepared from tablets using Oral Mix vehicle, and (C) dapsone suspension prepared from tablets using Oral Mix SF vehicle. Also shown are representative chromatograms of dapsone suspension in Oral Mix submitted to forced degradation at 60°C for 4 h in (D) water, (E) 3.0% H2O2, (F) 1 mol/L NaOH, and (G) 1 mol/L HCl. All sample preparations for injection resulted in nominal dapsone concentrations of 50 μg/mL.
|
For all suspensions, no notable changes in odour or colour were observed after storage under different conditions for 90 days. The suspensions remained opaque beige with a sweet aroma, but settling was observed after a few days. As shown in Table 1, the concentration of dapsone was not less than 90% of the initial concentration for all preparations at each tested condition, except suspensions in Oral Mix stored at 25°C for 7 days or longer. The difference in pH relative to the initial pH was not more than 0.5 unit for all preparations at all tested conditions (mean initial pH 4.18 [SD 0.03] for suspensions in Oral Mix and 4.43 [SD 0.03] for suspensions in Oral Mix SF).
Table 1 Chemical Stability of Dapsone Suspension Prepared from Tablets in Oral Mix and Oral Mix SF*
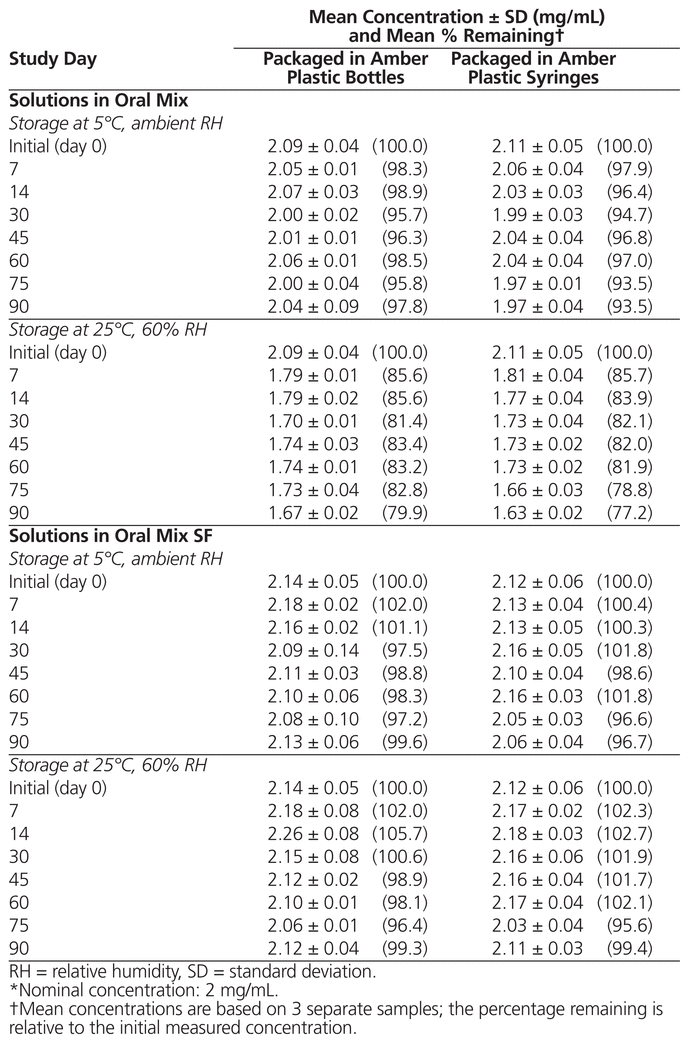
The instability of dapsone suspensions prepared in Oral Mix and stored at 25°C may be explained by a Maillard reaction between the sucrose in the vehicle and the amino groups of the dapsone.9 Storage under refrigeration prevented this reaction. Therefore, to avoid the Maillard reaction, Oral Mix SF should be preferred over other sugar-containing vehicles.
The results of this study have demonstrated the stability, for up to 90 days, of dapsone suspensions (2 mg/mL) prepared from commercial tablets in Oral Mix SF and stored at 5°C and 25°C or prepared in Oral Mix and stored at 5°C, in amber plastic bottles and amber plastic syringes. These suspensions should be shaken before use.
References
1 Wozel G, Blasum C. Dapsone in dermatology and beyond. Arch Dermatol Res. 2014;306(2):103–24.


2 Coleman MD. Dapsone: modes of action, toxicity and possible strategies for increasing patient tolerance. Br J Dermatol. 1993;129(5):507–13.

3 Kar HK, Gupta R. Treatment of leprosy. Clin Dermatol. 2015;33(1):55–65.

4 Yalkowsky SH, He Y, Jain P. Handbook of aqueous solubility data. 2nd ed. Boca Raton (FL); CRC Press; 2016. p. 873.
5 Perrin DD. Dissociation constants of organic bases in aqueous solution. IUPAC Chemical Data Series. London (UK): Butterworths; 1965.
6 Nahata MC, Morosco RS, Trowbridge JM. Stability of dapsone in two oral liquid dosage forms. Ann Pharmacother. 2000;34(7–8):848–50.

7 Ferreiraa AO, Polonini HC, Silva SL, Patrício FB, Brandão MAF, Raposo NRB. Feasibility of amlodipine besylate, chloroquine phosphate, dapsone, phenytoin, pyridoxine hydrochloride, sulfadiazine, sulfasalazine, tetracycline hydrochloride, trimethoprim and zonisamide in SyrSpend® SF PH4 oral suspensions. J Pharm Biomed Anal. 2016;118:105–12.

8 Kaila N, El-Ries M, Riga A, Alexander K, Dollimore D. Formulation development and stability testing of extemporaneous suspension prepared from dapsone tablets. Int J Pharm Compounding. 2013;7(3):233–9.
9 Waterman KC, Adami RC. Accelerated aging: prediction of chemical stability of pharmaceuticals. Int J Pharm. 2005;293(1–2):101–25.

Faculty of Pharmacy, Université de Montréal, Montréal, Quebec
Competing interests: Grégoire Leclair has received additional grants from Medisca Pharmaceutique Inc for research outside the work reported here. No other competing interests were declared. (
Return to Text
)
Funding: This study was funded by an unrestricted grant from Medisca Pharmaceutique Inc. The company was not involved in the design or conduct of the study; the collection, management, or interpretation of the data; or the preparation, review, or approval of the manuscript. (
Return to Text
)
Acknowledgements
The authors acknowledge Gaëlle Roullin and Katia Bichar for critical reading of this manuscript in advance of submission.
Canadian Journal of Hospital Pharmacy, VOLUME 71, NUMBER 2, March-April 2018