Abdullah Aboukarr, Mirella GiudiceABSTRACT
Background
Monoamine oxidase B (MAO-B) inhibitors are used to treat the motor symptoms of Parkinson disease. Depression is commonly associated with Parkinson disease, and selective serotonin reuptake inhibitors (SSRIs) are often used for its management. Tertiary sources warn that the combination of MAO-B inhibitors and SSRIs can result in increased serotonergic effects, leading to serotonin syndrome.
Objective
To explore the mechanism, clinical significance, and management of this potential drug interaction through a review of the supporting evidence.
Data Sources
PubMed, MEDLINE (1946 forward), Embase (1947 forward), PsycINFO (1806 forward), and International Pharmaceutical Abstracts (1970 forward) were searched on February 4, 2017.
Study Selection and Data Extraction
Studies and case reports describing aspects of the potential interaction between MAO-B inhibitors and SSRIs in patients with Parkinson disease and published in English were identified by both title and abstract.
Data Synthesis
The search identified 8 studies evaluating the potential interaction between SSRIs and the MAO-B inhibitors selegiline and rasagiline. The largest, a retrospective cohort study of 1504 patients with Parkinson disease, found no cases of serotonin syndrome with coadministration of rasagiline and an SSRI. A survey of 63 investigators in the Parkinson Study Group identified 11 potential cases of serotonin syndrome among 4568 patients treated with the combination of selegiline and antidepressants (including SSRIs). In addition, 17 case reports describing the onset of serotonin syndrome with coadministration of an SSRI and either selegiline or rasagiline were identified. Following discontinuation or dose reduction of one or both of the agents, the symptoms of serotonin syndrome gradually resolved in most cases, with none being fatal.
Conclusions
According to the literature, serotonin syndrome occurs rarely, and the combination of SSRI and MAO-B inhibitor is well tolerated. Therefore, SSRIs and MAO-B inhibitors can be coadministered, provided that their recommended doses are not exceeded and the SSRI dose is kept at the lower end of the therapeutic range. Among the SSRIs, citalopram and sertraline may be preferred.
KEYWORDS: Parkinson disease, serotonin syndrome, selegiline, rasagiline, selective serotonin reuptake inhibitor, drug interactions
RÉSUMÉ
Contexte
Les inhibiteurs de la monoamine oxydase B (MAO-B) sont employés dans le traitement des symptômes moteurs de la maladie de Parkinson, maladie à laquelle la dépression est souvent associée et fréquemment traitée à l’aide d’inhibiteurs sélectifs de la recapture de la sérotonine (ISRS). Des sources tertiaires mettent en garde contre la combinaison d’inhibiteurs de la MAO-B et d’ISRS car elle peut mener à une augmentation des effets sérotoninergiques, dégénérant en un syndrome sérotoninergique.
Objectif
Chercher à connaître le mécanisme, la signification clinique et la prise en charge de cette potentielle interaction médicamenteuse en procédant à une revue des preuves à l’appui.
Sources des données
Les bases de données PubMed, MEDLINE (depuis 1946), Embase (depuis 1947), PscyINFO (depuis 1806), et International Pharmaceutical Abstracts (depuis 1970) ont été interrogées le 4 février 2017.
Sélection des études et extraction des données
Des études et des observations cliniques, publiées en anglais, portant sur des aspects de la potentielle interaction entre les inhibiteurs de la MAO-B et les ISRS chez les patients atteints de la maladie de Parkinson ont été repérées par une recherche ciblant les titres et les résumés.
Synthèse des données
La recherche a permis de trouver 8 études analysant la potentielle interaction entre les ISRS et deux inhibiteurs de la MAO-B : la sélégiline et la rasagiline. La plus importante d’entre elles, une étude de cohorte rétrospective sur 1504 patients atteints de la maladie de Parkinson, n’a relevé aucun cas de syndrome sérotoninergique en présence d’une prise concomitante de rasagiline et d’un ISRS. Une enquête auprès de 63 chercheurs dans le Parkinson Study Group a permis de relever 11 potentiels cas de syndrome sérotoninergique chez 4568 patients traités avec une combinaison de sélégiline et d’antidépresseurs (notamment des ISRS). De plus, 17 observations cliniques qui décrivaient un début de syndrome sérotoninergique en présence d’une prise concomitante d’un ISRS et de sélégiline ou de rasagiline ont été recensées. Suivant la réduction de la posologie ou l’interruption d’un ou des deux médicaments, les symptômes du syndrome sérotoninergique se sont graduellement résolus dans la plupart des cas, et il n’y a eu aucune mortalité.
Conclusions
Selon la documentation, le syndrome sérotoninergique est rare et la combinaison d’ISRS et d’inhibiteurs de la MAO-B est bien tolérée. Ainsi, les deux types d’inhibiteurs peuvent être administrés conjointement pourvu que l’on ne dépasse pas la posologie recommandée et que la dose d’ISRS demeure dans le bas de l’intervalle thérapeutique. Parmi les ISRS, il peut être préférable d’employer le citalopram ou la sertraline.
MOTS CLÉS: maladie de Parkinson, syndrome sérotoninergique, sélégiline, rasagiline, inhibiteur sélectif de la recapture de la sérotonine, interaction médicamenteuse
The monoamine oxidase B (MAO-B) inhibitors selegiline and rasagiline are among the agents used to treat the motor symptoms of Parkinson disease.1 Depression is commonly associated with Parkinson disease, with up to 50% of patients being affected.1,2 The treatment of depression in patients with Parkinson disease should be individualized, with particular emphasis on concomitant therapy.1,3 Although selective serotonin reuptake inhibitors (SSRIs) are often used to manage the symptoms of depression associated with Parkinson disease,2 these drugs have the potential to worsen the motor symptoms of the disease (tremor, restless legs, and periodic limb movement).3–5 Furthermore, a potential interaction between SSRIs and MAO-B inhibitors can lead to serotonin syndrome.6–8 This review explores the mechanism of, supporting evidence for, clinical significance of, and management of potential serotonin syndrome with concomitant use of MAO-B inhibitors and SSRIs.
Serotonin syndrome is a measure of central nervous system (CNS) hyperexcitability in relation to an excess of serotonin. This hyperexcitability can manifest in multiple ways, and ultimately there is no true “gold standard” for the diagnosis of serotonin syndrome. Published diagnostic criteria have attempted to identify symptoms or symptom combinations that best capture the nature of CNS hyperexcitability related to an excess of serotonin.9
Serotonin syndrome can manifest as symptoms that range from mild to life-threatening. Initially, the patient may present with akathisia and tremor, followed by mental status changes and inducible clonus. The clonus can become sustained, and may then evolve to muscular rigidity or hypertonicity. Hyperthermia is a symptom that manifests later; it can be life-threatening.10 Most cases of serotonin syndrome present within 24 h of a dose increase or initiation of a new serotonergic agent.11
Sternbach12 first suggested diagnostic criteria for serotonin syndrome in 1991. The Sternbach criteria require at least 3 of the following clinical features, coincident with adding or increasing the dose of a serotonergic agent: mental status changes, agitation, myoclonus, hyperreflexia, diaphoresis, shivering, tremor, diarrhea, incoordination, and fever.12 Despite their widespread use, the Sternbach criteria have some limitations. They can be nonspecific, because of heavy reliance on mental status changes. Furthermore, the inclusion of incoordination (ataxia), a cerebellar feature, is controversial, because serotonin toxicity is not known to affect the cerebellum. Any patient who is agitated or confused may also appear ataxic or uncoordinated. Lastly, the Sternbach criteria were developed on the basis of a series of published cases; therefore, any clinical features not identified as features of serotonin syndrome by the authors of the original cases may have been missed.9,13
In 2003, Dunkley and others13 undertook to improve the Sternbach criteria, and developed the Hunter serotonin toxicity criteria (HSTC). According to these criteria, a diagnosis of serotonin syndrome requires the presence of a serotonergic agent and one of the following conditions:
clonus
inducible clonus AND agitation or diaphoresis
ocular clonus AND agitation or diaphoresis
tremor AND hyperreflexia
hypertonicity AND temperature > 38°C AND ocular clonus or inducible clonus
The HSTC are currently the most accurate criteria for diagnosing serotonin syndrome, being both more sensitive and more specific than the Sternbach criteria.12–14 Nonetheless, the HSTC have their shortcomings. They are based solely on cases of SSRI overdose, making them potentially nongeneralizable to cases of serotonin syndrome involving therapeutic doses. In addition, a subset of cases used to derive the criteria were also used for validation, leading to a potential overestimate of validity.9 Lastly, clonus or hyperreflexia may not be elicited in patients with severe serotonin syndrome who have peripheral neuropathy or muscle rigidity, which can cloud the clinical diagnosis of serotonin syndrome.9,10
MAO exists as 2 isoforms, MAO-A and MAO-B. Inhibition of MAO-A reduces the metabolism of both serotonin and noradrenaline, whereas inhibition of MAO-B does not affect the metabolism of these neurotransmitters, unless sufficient doses (as described below) are used.15 Inhibition of MAO-B, the major isoform in the human brain, prevents the breakdown of extracellular levels of dopamine in the striatum. The resulting increase in dopaminergic activity in the striatum may explain the mechanism by which MAO-B inhibitors exert their beneficial effects in Parkinson disease.16,17 Inhibition of MAO can also be reversible or irreversible; irreversible inhibitors can lead to longer-lasting toxic reactions caused by MAO inhibition, including serotonin syndrome.15 Selegiline and rasagiline, both irreversible MAO-B inhibitors, are selective for MAO-B at therapeutic doses of 10 mg daily and 1 mg daily, respectively, for patients with Parkinson disease. At higher doses, they lose selectivity and inhibit both MAO-B and MAO-A.15 Higher doses of MAO-B inhibitors alone have resulted in serotonin syndrome.17,18
Serotonin syndrome results from increased serotonergic activity in the CNS. The SSRIs increase serotonin activity by blocking the reuptake of serotonin from synapses.5 Serotonin receptors are classified into 7 families, which are designated 5-HT1 to 5-HT7, with specific families having multiple subtypes. The development of serotonin syndrome has not been attributed to one specific receptor; however, evidence suggests that agonism of the 5-HT2A subtype may play a considerable role. The 5-HT1A subtype may also be implicated in the development of serotonin syndrome through a pharmacodynamic interaction in which increased synaptic concentrations of serotonin can saturate all receptor subtypes.10
MAO-A inhibitors can augment the serotonergic effects of SSRIs by preventing the breakdown of serotonin.6,19 Consequently, serotonin syndrome has been reported with concomitant use of SSRIs and nonselective MAO inhibitors (e.g., phenelzine, tranylcypromine), as well as with selective MAO-A inhibitors (e.g., moclobemide).6,20 According to references reporting these interactions, patients have experienced symptoms such as agitation, confusion, myoclonus, rigidity, nausea, and insomnia; some cases were fatal.6,20 MAO-B inhibitors have also been implicated in the development of serotonin syndrome, as discussed below (see “Summary of Evidence”).
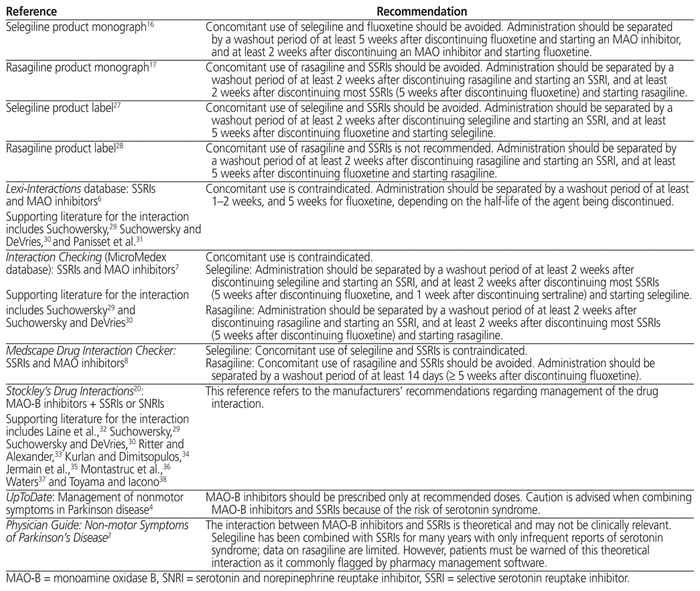
The product monographs for escitalopram, paroxetine, fluoxetine, sertraline, citalopram, and fluvoxamine all recommend avoiding their concurrent use with selective and nonselective MAO inhibitors and separating administration of these drugs by a washout period ranging from 2 to 5 weeks.21–26 The product monograph for sertraline reports serious, sometimes fatal reactions with concomitant use of selegiline.24 Various drug interaction references and the product monographs and labels for selegiline and rasagiline (Table 1) reiterate these recommendations.2,4,6–8,16,17,20,27,28
Table 1 Recommendations for Management of the Interaction between MAO-B Inhibitors and SSRIs from Tertiary Sources
Based on the Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder,39 SSRIs vary in their overall potential for drug–drug interactions: citalopram and escitalopram have minimal or low potential; sertraline has moderate potential; and fluoxetine, fluvoxamine, and paroxetine have high potential. Selegiline is also classified as having a higher potential for drug–drug interactions relative to other antidepressants; rasagiline is not classified.39 Selegiline is metabolized primarily via the cytochrome P450 isozymes CYP2B6, CYP2C9, CYP3A4, and CYP2A6, whereas rasagiline is metabolized via CYP1A2.40,41
A search of PubMed, MEDLINE (1946 forward), Embase (1947 forward), PsycINFO (1806 forward), and International Pharmaceutical Abstracts (1970 forward) was conducted on February 4, 2017, using the MeSH (Medical Subject Heading) term “drug interaction” and combined keywords “selegiline and SSRI,” “rasagiline and SSRI”, “fluoxetine and selegiline”, “fluvoxamine and selegiline”, “sertraline and selegiline”, “paroxetine and selegiline”, citalopram and selegiline”, “escitalopram and selegiline”, “fluoxetine and rasagiline”, “fluvoxamine and rasagiline”, “sertraline and rasagiline”, “paroxetine and rasagiline”, citalopram and rasagiline”, and “escitalopram and rasagiline”. Studies and case reports were identified by both title and abstract. Duplicate citations, identified by author, journal, and date of publication, were excluded. Only studies and case reports published in English were considered.
Eight relevant studies were identified (3 retrospective studies, 1 observational study, 1 open sequential-setting study, 1 case series, 1 survey study, and 1 randomized controlled trial) that evaluated the potential for an interaction between SSRIs and the MAO-B inhibitors selegiline and rasagiline (Table 2).31–33,37,38,42–44 Most of the studies had a small sample size, and most evaluated selegiline; this finding was unsurprising, given that selegiline was approved by the US Food and Drug Administration (FDA) in 1989 and has therefore been available much longer than rasagiline, which was approved by the FDA in 2006.45
Table 2 Studies Examining the Interaction between Selegiline or Rasagiline and SSRIs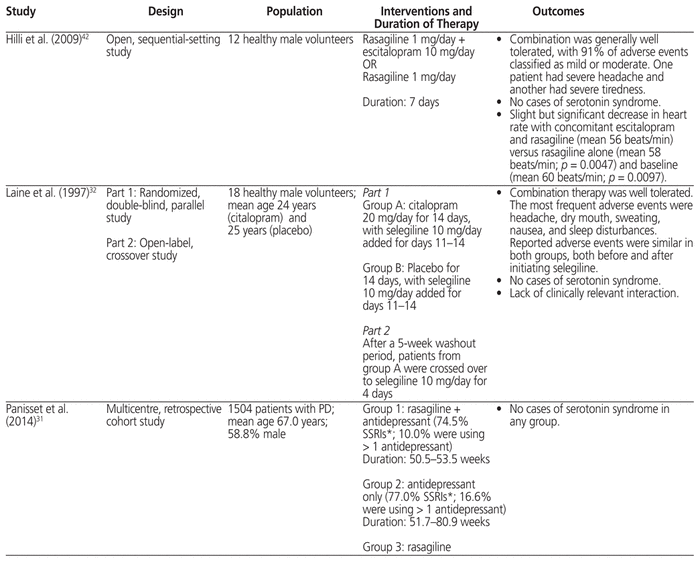
Two studies involved healthy patients, so their results may not be applicable to patients with Parkinson disease.32,42 In one of these studies,42 which involved rasagiline and escitalopram, the area under the curve (AUC) for rasagiline increased by 42% (p < 0.0001) and oral clearance decreased by 35% (p < 0.001) after 7 days of combination therapy, relative to rasagiline treatment alone. The elimination half-life, peak plasma concentration (Cmax), and time from drug intake to peak concentration (tmax) of rasagiline were not significantly affected by escitalopram.42 In the other study,32 which involved selegiline and citalopram, the AUC of selegiline decreased by 29% with concomitant citalopram, relative to selegiline alone; Cmax and tmax were not significantly affected. Citalopram pharmacokinetics were unaffected, and the authors reported a lack of clinically relevant pharmacokinetic interaction.32
The largest study, involving rasagiline,31 was a multicentre, retrospective cohort study, in which the authors systematically evaluated the incidence of serotonin syndrome among patients taking rasagiline plus an antidepressant. Study centres were selected from individual neurology practices with medical records for 50 or more patients with Parkinson disease who had received rasagiline, 50 or more patients with Parkinson disease who had received an antidepressant, and 50 or more patients with Parkinson disease who had received the combination of rasagiline and an antidepressant. Serotonin syndrome was defined using the HSTC, which to date are the most sensitive and specific criteria for diagnosing serotonin syndrome.31 Out of 1507 patients initially considered, all with Parkinson disease, 471 were taking rasagiline in combination with an antidepressant (351 or 74.5% using SSRIs), 511 were taking rasagiline without an antidepressant (3 of whom did not meet the eligibility criteria and were later excluded from analysis), and 525 were taking antidepressants (404 or 77.0% using SSRIs) without rasagiline. The mean SSRI doses in the antidepressant + rasagiline group and the antidepressant-only group were, respectively, citalopram 23.7 and 26.0 mg, escitalopram 13.8 and 14.2 mg, fluoxetine 24.1 and 27.2 mg, paroxetine 22.3 and 22.9 mg, and sertraline 78.0 and 85.0 mg, which fall mainly at the lower end of the therapeutic ranges of these drugs. Of the 1419 patients (94.3%) with known outcomes, none experienced serotonin syndrome. The authors stated that the lack of serotonin syndrome cases suggests a rarer-than-expected incidence of the syndrome, which was below the study’s detection threshold.31 They further stated that future studies should increase the sample size to assess the true incidence of serotonin syndrome with concomitant use of rasagiline and antidepressants.31 This study had both strengths and limitations. One major strength was the independent, systematic review of each case according to the HSTC, which ensured that cases of serotonin syndrome that might not have been properly recognized during a medical encounter were reassessed against robust criteria for this syndrome. Conversely, a major limitation was the retrospective study design, which meant that roughly 20% of medical records were unavailable for ascertainment of serotonin syndrome. Furthermore, there was limited access to the medical records of deceased patients, because of the requirement for informed consent at several study centres, which potentially prevented the capture of further cases of serotonin syndrome. A final limitation was the potential dismissal of symptoms of serotonin syndrome as features of the underlying disease. As such, practitioners might not have documented unusual findings as symptoms of serotonin syndrome, giving rise to false negatives.31
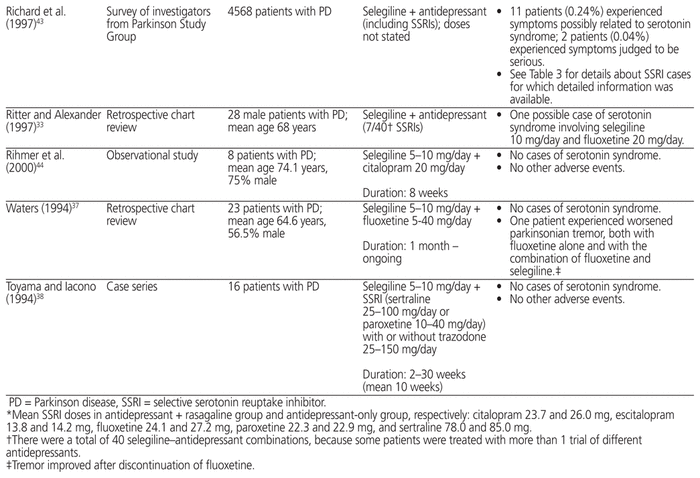
A survey of 63 investigators in the Parkinson Study Group utilized a standardized questionnaire to identify patients treated with the combination of selegiline and antidepressants.43 Forty-seven investigators responded, which allowed identification of a total of 4568 patients who were taking this combination. Of these patients, 11 (0.24%) experienced symptoms “possibly consistent” with serotonin syndrome, with 2 patients having symptoms that were considered serious. Details were provided for only 5 patients, and one of these cases was already published (in 2 reports).29,30 All 5 patients had used an SSRI (see “PSG cases” in Table 3 for further details).29,30,43 Because details were unavailable for the remaining 6 patients, it is unknown whether they were taking an SSRI or another antidepressant.43 In addition to their survey of the Parkinson Study Group investigators, the authors obtained a summary of all possible cases of drug interactions with concomitant use of selegiline and an antidepressant that had been submitted to the FDA between 1989 and 1996. Fifty-seven cases were identified, of which 27 involved an SSRI. From these 27 cases, only 2 were stated as having possibly fulfilled the Sternbach criteria for serotonin syndrome (see “FDA cases” in Table 3 for further details).43 Lastly, the authors conducted a MEDLINE search and reviewed bibliographies for published cases of adverse events associated with concomitant use of selegiline and an antidepressant. Six cases were identified, 5 of which (including one of the cases identified by the Parkinson Study Group survey) involved an SSRI (see “published cases” in Table 3 for further details).43
Table 3 Case Reports of Possible Serotonin Syndrome Induced by Concomitant Use of Selegiline or Rasagiline with SSRI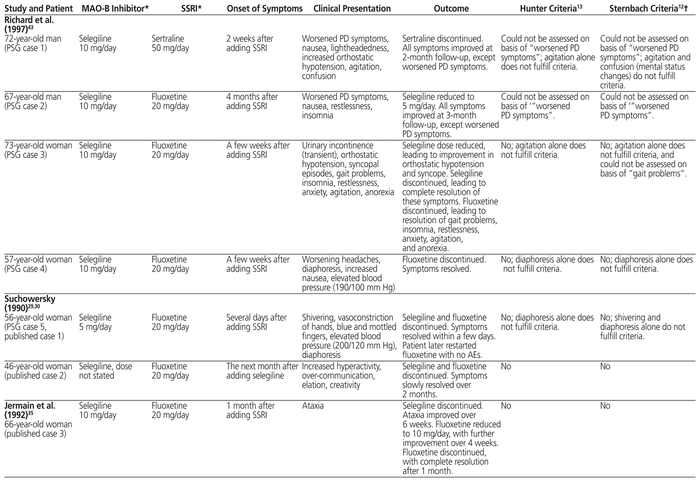
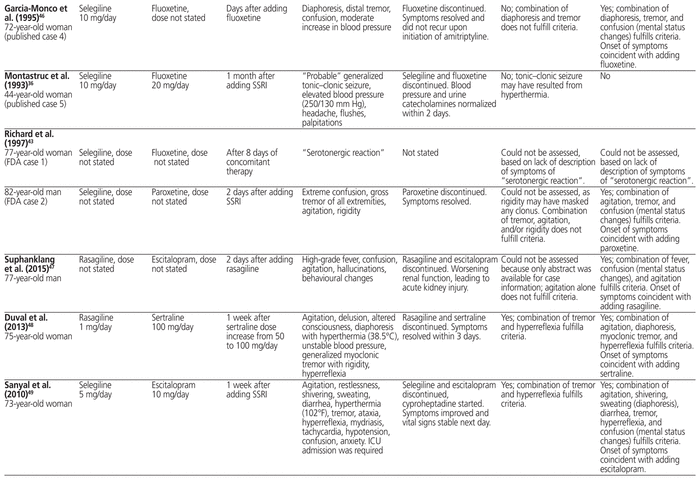
Apart from these 11 potential cases of serotonin syndrome (4 cases from the survey [excluding the published case], 2 cases submitted to the FDA, and 5 cases from the MEDLINE search [including the case identified in the survey]), a retrospective chart review of 28 patients with Parkinson disease identified 1 possible case of serotonin syndrome.33 The patient in question experienced increased nervousness, anxiety, tremor, and confusion less than 1 week after starting fluoxetine 20 mg/day (in addition to selegiline 10 mg/day). Although the authors stated that the reaction was consistent with serotonin syndrome, it was not possible to establish a firm diagnosis. Soon after stopping fluoxetine, the patient’s symptoms improved, but they had not completely resolved 3 weeks later.33
Overall, the combination of SSRI and MAO-B inhibitor was well tolerated in these studies, with 12 possible cases of serotonin syndrome (1 additional case from the retrospective chart review). One additional patient experienced worsening of a parkinsonian tremor, which was attributed to fluoxetine.37 The evaluated doses of selegiline and rasagiline were both within the recommended range for Parkinson disease, and the doses of SSRIs were generally at the lower end of the therapeutic range for depression. From these studies, it appears that selegiline or rasagiline can be used with an SSRI, provided that the recommended doses of both agents are not exceeded and, ideally, the SSRI dose is kept at the lower end of the therapeutic range. However, further studies using larger sample sizes would be welcome to determine the true incidence of this drug interaction.
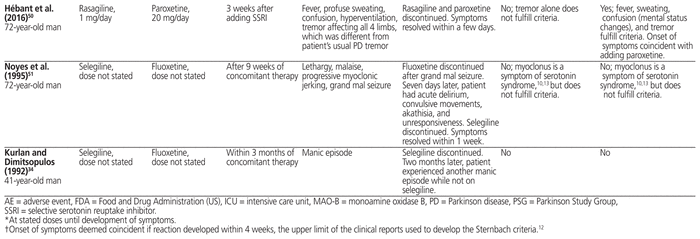
Although the largest study found no evidence of a clinically relevant interaction between MAO-B inhibitors and SSRIs,31 and only 12 possible cases of serotonin syndrome were identified in other studies, 6 further case reports have described the onset of possible serotonin syndrome with coadministration of an SSRI and either selegiline or rasagiline.34,47–51Table 3 describes these cases, in addition to the 11 cases identified by the Parkinson Study Group (including the survey, literature search, and review of FDA-reported cases).29,30,34–36,43,46–51 For one published case identified by the Parkinson Study Group, we noted discrepancies between their description43 and the reports of the original authors.29,30 As such, the authors’ original description29,30 has been included in Table 3.
The onset of serotonin syndrome varied from days to weeks following a dose increase (1 case only) or initiation of the new serotonergic agent (most reports). This finding is inconsistent with the statement by Mason and others11 that most cases of serotonin syndrome manifest within 24 h after a dose change or initiation of a serotonergic agent; however, the observations of those authors were based on only 41 patients. Although most of these patients had underlying diseases, none had Parkinson disease.11 In addition, selegiline was not implicated in any of the cases, rasagiline had not yet been approved,45 and a substantial proportion of the patients (26%) experienced serotonin syndrome after more than 24 h, with the longest period of symptom onset being 36 days.11 Therefore, serotonin syndrome cannot be ruled out on the basis of timeframe alone, and a specific timeframe is not a requirement to fulfill the HSTC.13
The most commonly implicated MAO-B inhibitor and SSRI were selegiline and fluoxetine, respectively, likely because of their lengthier market approval and the relatively longer half-life of fluoxetine. This longer half-life is important because changes in plasma concentration will not be fully observed for several weeks. Similarly, when fluoxetine is discontinued, plasma concentrations drop slowly, and the drug remains in the body for several weeks.23 In these case reports, the doses of the MAO-B inhibitor were within the recommended range for Parkinson disease, and the doses of the SSRI were at the lower end of the therapeutic range for depression.16,17,21–24
Following discontinuation or dose reduction of one or both serotonergic agents, symptoms of serotonin syndrome gradually resolved in most cases; no cases were fatal. In 2 cases, the patients also experienced worsened symptoms of Parkinson disease. In the first case, worsening of tremor persisted despite sertraline discontinuation.43 In the second case, worsening of tremor also persisted, but no mention was made of whether the SSRI was discontinued; notably, however, the dose of selegiline had been reduced.43 It is interesting to note the similarities between the motor symptoms of Parkinson disease, specifically resting tremor and rigidity, and the motor symptoms of serotonin syndrome.1,2 Both of these cases were published before development of the HSTC, which include both tremor and hypertonicity as clinical features of serotonin syndrome.13 Therefore, a mild form of serotonin syndrome might have been considered, had the HSTC been available at the time of the event in these 2 cases.
In the case presented by Montastruc and others,36 the patient experienced a tonic–clonic seizure. Seizures are more severe complications of serotonin syndrome, often associated with hyperthermia.10,14 The authors did not report the patient’s temperature. For this reason, it was not possible to confirm the cause of the seizure as serotonin syndrome.
Cyproheptadine has been proposed as an off-label treatment for serotonin syndrome. It is a histamine-1 receptor antagonist with additional nonspecific binding at the 5-HT1A and 5-HT2A receptors. It is recommended as an antidote for serotonin syndrome,10 despite a lack of evidence for its efficacy. The suggested dosage is 12 mg initially, followed by 2 mg every 2 h, or 4–8 mg every 6 h until symptoms are controlled. Because of its anticholinergic activity, it causes sedation.10,14,52 In only one of the identified cases was cyproheptadine reported as having been used to manage serotonin syndrome.49 The dosing in this case differed somewhat from the previously proposed dosing: it was prescribed as 4 mg orally every 2–4 h, with up to 30 mg per day. The patient’s vital signs stabilized, and her symptoms had improved by the next day.49
Four cases of serotonin syndrome47–50 were reported after publication of the HSTC in 2003, and we determined that 2 of them met these criteria.48,49 Fulfillment of the HSTC in these 2 cases is noteworthy because it may indicate that the HSTC are emerging as a new standard for diagnosis. It may also suggest that the HSTC can be applied to cases of serotonin syndrome occurring in patients who receive therapeutic doses, despite the criteria being based solely on cases of SSRI overdose. Of these 2 cases, Duval and others48 used the HSTC for their diagnosis of serotonin syndrome, whereas Sanyal and others49 used the Sternbach criteria. Conversely, we determined that all 4 of the cases published after 2003 met the Sternbach criteria,47–50 which would suggest that these criteria are still being used to diagnose serotonin syndrome. Of the 2 cases that we deemed as not meeting the HSTC criteria, the case reported by Suphanklang and others47 would not be classified as serotonin syndrome, on the basis of information available in the abstract; the full article could not be obtained for examination. In the other case, Hébant and others50 diagnosed serotonin syndrome with the Sternbach criteria and made no mention of the HSTC.
Three further case reports (describing 2 individual patients) did not explicitly mention serotonin syndrome, but described the occurrence of mania with concomitant use of selegiline and fluoxetine. In the first case (described in 2 separate articles),29,30 the authors thought the prolonged mania resulted from concomitant use of fluoxetine and selegiline, because both medications are known to induce mania when used alone. In the other case, the patient experienced a second manic episode 2 months after discontinuing selegiline. At that point, the authors did not further clarify the patient’s psychiatric diagnosis.34 Mania alone does not fit the Sternbach criteria12 or the HSTC.13
The benefits of MAO-B inhibitor and SSRI in combination in the treatment of depression related to Parkinson disease generally outweigh the risks; therefore, either selegiline or rasagiline can be used cautiously with an SSRI, if their recommended doses are not exceeded (i.e., total daily doses of up to 10 mg and 1 mg for selegiline and rasagiline, respectively) and doses of SSRI are kept at the lower end of the therapeutic range. When adding an SSRI to either selegiline or rasagiline, the SSRI should be initiated at the lowest possible dose and titrated slowly. The use of other serotonergic agents should be avoided; drugs that can decrease the metabolism of either MAO-B inhibitors or SSRIs should also be avoided. Among the SSRIs, citalopram and sertraline may be preferred because of their demonstrated efficacy and tolerability as antidepressants in Parkinson disease.2,4,5 Sertraline is the SSRI that appears to have the least potential for inducing parkinsonism,4 whereas citalopram has an overall low potential for drug interactions.39 Proper patient monitoring is imperative. According to case reports, the onset of the interaction is variable, ranging from a few days to weeks after initiation of the new agent. Therefore, clinicians must remain vigilant for this interaction at all times. As a cautionary measure, the patient and/or caregiver should be advised of the signs and symptoms of serotonin syndrome, despite the low risk.
To date, the only report to estimate the incidence of serotonin syndrome with the coadministration of an MAO-B inhibitor and antidepressants (including SSRIs) is the Parkinson Study Group survey.43 In that survey, the authors found an incidence of 0.24%, although these were not cases of serotonin syndrome retroactively assessed using the Sternbach criteria, and the survey report predated the development of the HSTC. Furthermore, the large retrospective study that used a preset definition of serotonin syndrome based on the HSTC did not identify any cases of serotonin syndrome.31 The clinical data supporting this potential interaction are therefore based on case reports alone. Given that the benefits of this combination in treating depression related to Parkinson disease generally outweigh the risks, either selegiline or rasagiline can be used cautiously with an SSRI, provided that their recommended doses are not exceeded and the doses of SSRIs are kept at the lower end of the therapeutic range.
1 Grimes D, Gordon J, Snelgrove B, Lim-Carter I, Fon E, Martin W, et al. Canadian guidelines on Parkinson’s disease. Can J Neurol Sci. 2012;39 (4 Suppl 4):S1–30.

2 Postuma R, Rios Romenets S, Rakheja R. Physician guide: non-motor symptoms of Parkinson’s disease. Montréal (QC): McGill University Health Centre; 2012.
3 Goodarzi Z, Mele B, Guo S, Hanson H, Jette N, Patten S, et al. Guidelines for dementia or Parkinson’s disease with depression or anxiety: a systematic review. BMC Neurol. 2016;16(1):244.


4 Tarsy D. Management of nonmotor symptoms in Parkinson disease. In: Post TW, editor. UpToDate. Waltham (MA): UpToDate; 2014.
5 Chen JJ, Marsh L. Depression in Parkinson’s disease: identification and management. Pharmacotherapy. 2013;33(9):972–83.

6 Selective serotonin reuptake inhibitors/MAO inhibitors. In: Lexi-Interactions [database on internet]. Hudson (OH): Lexi-Comp, Inc; 2007 [cited 2017 Feb 4]. Available from: http://online.lexi.com/lco/action/home/switch. Subscription required to access content.
7 Selective serotonin reuptake inhibitors/MAO inhibitors. In: Interaction checking [database on internet]. Ann Arbor (MI): Truven Health Analytics; 2016 [cited 2017 Jan 28]. Available from: www.micromedexsolutions.com. Subscription required to access content.
8 Selective serotonin reuptake inhibitors/MAO inhibitors. In: Medscape drug interaction checker [database on internet]. New York (NY): Medscape, LLC; 1994 [cited 2017 Feb 4]. Available from: http://reference.medscape.com/drug-interactionchecker
9 Werneke U, Jamshidi F, Taylor DM, Ott M. Conundrums in neurology: diagnosing serotonin syndrome—a meta-analysis of cases. BMC Neurol. 2016;16(1):97.
10 Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005; 352(11):1112–20.

11 Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome presentation of 2 cases and review of the literature. Medicine. 2000;79(4):201–9.

12 Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705–13.

13 Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. Q J Med. 2003;96(9):635–42.
14 Boyer EW. Serotonin syndrome (serotonin toxicity). In: Post TW, editor. UpToDate. Waltham (MA): UpToDate; 2014.
15 Finberg JPM. Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: focus on modulation of CNS monoamine neurotransmitter release. Pharmacol Ther. 2014;143(2):133–52.

16 Teva-Selegiline [product monograph]. In: Drug product database. Ottawa (ON): Health Canada; 2017 [updated 2015 Jul 14; cited 2017 Oct 22]. Available from: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp. Also available in paper copy from the publisher.
17 Azilect [product monograph]. In: Drug product database. Ottawa (ON): Health Canada; 2017 [updated 2014 Nov 20; cited 2017 Oct 22]. Available from: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp. Also available in paper copy from the publisher.
18 Fernandes C, Reddy P, Kessel B. Rasagiline-induced serotonin syndrome [letter]. Mov Disord. 2011;26(4):766–7.

19 McKie J. What is serotonin syndrome and which medicines cause it? NHS Med Q&As. 2016;:219.4. Also available from: https://www.sps.nhs.uk/articles/what-is-serotonin-syndrome-and-which-medicines-cause-it-2/
20 Preston CL, editor. Stockley’s drug interactions. London (UK): Pharmaceutical Press; 2015.
21 Cipralex [product monograph]. In: CPS. Ottawa (ON): Canadian Pharmacists Association; [updated 2016 Jun 9; cited 2017 Feb 3]. Available from: www.e-therapeutics.ca. Subscription required to access content; also available in paper copy from the publisher.
22 Paxil [product monograph]. In: CPS. Ottawa (ON): Canadian Pharmacists Association; [updated 2014 Nov 13; cited 2017 Feb 3]. Available from: www.e-therapeutics.ca. Subscription required to access content; also available in paper copy from the publisher.
23 Prozac [product monograph]. In: CPS. Ottawa (ON): Canadian Pharmacists Association; [updated 2016 Jul 7; cited 2017 Feb 3]. Available from: www.e-therapeutics.ca. Subscription required to access content; also available in paper copy from the publisher.
24 Zoloft [product monograph]. In: CPS. Ottawa (ON): Canadian Pharmacists Association; [updated 2016 Jul 8; cited 2017 Feb 3]. Available from: www.e-therapeutics.ca. Subscription required to access content; also available in paper copy from the publisher.
25 Celexa [product monograph]. In: CPS. Ottawa (ON): Canadian Pharmacists Association; [updated 2016 Jun 9; cited 2017 Feb 3]. Available from: www.e-therapeutics.ca. Subscription required to access content; also available in paper copy from the publisher.
26 Luvox [product monograph]. In: CPS. Ottawa (ON): Canadian Pharmacists Association; [updated 2016 Jul 6; cited 2017 Feb 3]. Available from: www.e-therapeutics.ca. Subscription required to access content; also available in paper copy from the publisher.
27 Eldepryl® [label]. Tampa (FL): Somerset Pharmaceuticals, Inc; 2008 Jan [cited 2017 Oct 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020647s006s007lbl.pdf
28 Azilect® [label]. Overland Park (KS) Teva Neuroscience, Inc; 2014 May [cited 2017 Oct 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021641s016s017lbl.pdf
29 Suchowersky O. Possible interactions between deprenyl and prozac. Can J Neurol Sci. 1990;17(3):352–3.

30 Suchowersky O, DeVries JD. Interaction of fluoxetine and selegiline [letter]. Can J Psychiatry. 1990;35(6):571–2.

31 Panisset M, Chen JJ, Rhyee SH, Conner J, Mathena J; STACCATO Study Investigators. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250–8.

32 Laine K, Anttila M, Heinonen E, Helminen A, Huupponen R, Mäki-Ikola O, et al. Lack of adverse interactions between concomitantly administered selegiline and citalopram. Clin Neuropharmacol. 1997;20(5):419–33.

33 Ritter JL, Alexander B. Retrospective study of selegiline-antidepressant drug interactions and a review of the literature. Ann Clin Psychiatry. 1997;9(1):7–13.

34 Kurlan R, Dimitsopulos T. Selegiline and manic behavior in Parkinson’s disease [letter]. Arch Neurol. 1992;49(12):1231.

35 Jermain DM, Hughes PL, Follender AB. Potential fluoxetine–selegiline interaction [letter]. Ann Pharmacother. 1992;26(10):1300.

36 Montastruc JL, Chamontin B, Senard JM, Tran MA, Rascol O, Llau ME, et al. Pseudophaeochromocytoma in parkinsonian patient treated with fluoxetine plus selegiline [letter]. Lancet. 1993;341(8844):555.

37 Waters CH. Fluoxetine and selegiline—lack of significant interaction. Can J Neurol Sci. 1994;21(3):259–61.

38 Toyama SC, Iacono RP. Is it safe to combine a selective serotonin reuptake inhibitor with selegiline? Ann Pharmacother. 1994;28(3):405–6.

39 Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al.; CANMAT Depression Working Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540–60.


40 Selegiline. In: Lexi-drugs online [database on internet]. Hudson (OH): Lexicomp, Inc; 2016 [updated 2017 Apr 4; cited 2017 Apr 12]. Available from: http://online.lexi.com. Subscription required to access content.
41 Rasagiline. In: Lexi-drugs online [database on internet]. Hudson (OH): Lexicomp, Inc; 2016 [updated 2017 Apr 4; cited 2017 Apr 12]. Available from: http://online.lexi.com. Subscription required to access content.
42 Hilli J, Korhonen T, Laine K. Lack of clinically significant interactions between concomitantly administered rasagiline and escitalopram. Progr Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1526–32.
43 Richard IH, Kurlan R, Tanner C, Factor S, Hubble J, Suchowersky O, et al.; Parkinson Study Group. Serotonin syndrome and the combined use of deprenyl and an antidepressant in Parkinson’s disease. Neurology. 1997; 48(4):1070–6.

44 Rihmer A, Satori M, Pestality P. Selegiline-citalopram combination in patients with Parkinson’s disease and major depression. Int J Psychiatry Clin Pract. 2000;4(2):123–5.
45 Orange book: approved drug products with therapeutic equivalence evaluations. Silver Spring (MD): US Food and Drug Administration; 2018 [cited 2018 May 19]. Available from: https://www.accessdata.fda.gov/scripts/cder/ob/
46 Garcia–Monco JC, Padierna A, Beldarrain MG. Selegiline, fluoxetine, and depression in Parkinson’s disease. Mov Disord. 1995;10(3):352.
47 Suphanklang J, Santimaleeworagun W, Supasyndh O. Combination of escitalopram and rasagiline induced serotonin syndrome: a case report and review literature. J Med Assoc Thai. 2015;98(12):1254–7.
48 Duval F, Flabeau O, Razafimahefa J, Spampinato U, Tison F. Encephalophaty associated with rasagiline and sertraline in Parkinson’s disease: possible serotonin syndrome [letter]. Mov Disord. 2013;28(10):1464.

49 Sanyal D, Chakraborty S, Bhattacharyya R. An interesting case of serotonin syndrome precipitated by escitalopram. Indian J Pharmacol. 2010;42(6):418–9.


50 Hébant B, Guillaume M, Desbordes M, Gaillon G, Maltête D, Lefaucheur R. Combination of paroxetine and rasagiline induces serotonin syndrome in a parkinsonian patient [letter]. Revue Neurol. 2016;172(12):788–9.
51 Noyes MA, Rhymes JA, Chandler SW. Case report of drug induced serotonin syndrome in an elderly man [abstract]. Int Pharm Abstracts. 1995 Dec;30: P-268(D) [accession no. 32-13090].
52 Cyproheptadine. In: Drug information [database on internet]. Hudson (OH): Lexi-Comp, Inc; 2007 [cited 2017 Oct 28]. Available from: http://online.lexi.com/lco/action/home/switch. Subscription required to access content.
Competing interests: This manuscript was undertaken when Abdullah Aboukarr was a fourth-year PharmD student with a placement at the Ottawa Valley Regional Drug Information Service, for which Mirella Giudice was the preceptor. Dr Aboukarr now works as a medical information/pharmacovigilance officer with Purdue Pharma (Canada), which does not market or manufacture any of the products mentioned in this article, or any competing products. No other competing interests were declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 71, NUMBER 3, May-June 2018