Renaud Roy, Janice MaABSTRACT
Background
Spontaneous reports of adverse drug reactions (ADRs) form an essential component of both drug safety monitoring and patient safety initiatives. Pharmacists are well positioned to report ADRs, but many barriers exist to their doing so. Over the past decade, substantial changes have occurred with regard to drug regulations and medication safety initiatives, and it is possible that knowledge-based interventions may be needed to enhance ADR reporting by pharmacists.
Objective
To determine whether ADR reporting behaviours of pharmacists improved after release of a revised policy on the reporting of medication incidents.
Methods
A telephone survey was administered to pharmacists practising in the Canadian Forces Health Services Group. Self-reported behaviours and perceived barriers related to ADR reporting were compared before and 3 months after the updated policy was released. Accuracy in participants’ self-assessed ADR reporting was evaluated using independently derived workload statistics.
Results
During the second survey phase (after release of the revised policy), a greater proportion of respondents reported awareness of institutional policies on ADR reporting and declared that they were able to complete all necessary ADR reports during their assigned work hours. However, the number of ADR reports submitted did not increase. Participants’ recall of their ADR reporting behaviour was corroborated by workload data. During the second survey phase, there was a noticeable reduction in the number of free-form comments mentioning lack of staff as a barrier to ADR reporting.
Conclusions
Release of a more comprehensive policy was not associated with an increase in the number of ADR reports generated by pharmacists in the study setting. Interventions to strengthen the organization’s work processes for detection of ADRs and submission of individual ADR reports should be strongly considered, to reinforce and enhance existing ADR reporting behaviours among pharmacists.
KEYWORDS: adverse reactions, pharmacists, drug monitoring, organization and administration
RÉSUMÉ
Contexte
Les déclarations spontanées des réactions indésirables aux médicaments (RIM) sont essentielles à la pharmacovigilance et aux initiatives au profit de la sécurité des patients. Les pharmaciens sont bien placés pour déclarer des RIM, mais divers éléments y font obstacle. Au cours de la dernière décennie, d’importants changements ont eu lieu en ce qui touche aux règlements sur les médicaments et aux initiatives en sécurité des médicaments, et il est possible que des interventions fondées sur le savoir soient nécessaires pour améliorer dans l’ensemble les déclarations des RIM par les pharmaciens.
Objectif
Déterminer si les habitudes des pharmaciens relatives à la déclaration des RIM se sont améliorées après la mise à jour d’une politique portant sur la déclaration des incidents liés aux médicaments.
Méthodes
Les pharmaciens qui exerçaient dans le Groupe des Services de santé des Forces canadiennes ont été sondés par téléphone. On a comparé les réponses des pharmaciens quant à leurs propres habitudes de déclaration et aux éléments perçus comme des obstacles à la déclaration des RIM, avant la mise à jour de la politique et trois mois après sa mise à jour. L’exactitude des réponses des participants à propos de leurs propres habitudes de déclaration des RIM a été vérifiée à l’aide de statistiques sur la charge de travail obtenues indépendamment.
Résultats
Pendant la deuxième phase de l’enquête (après la mise à jour de la politique), une plus grande proportion de répondants ont indiqué être conscients des politiques institutionnelles sur la déclaration des RIM et ils ont soutenu qu’ils étaient en mesure de remplir tous les rapports de déclaration des RIM nécessaires pendant leurs heures normales de travail. Cependant, le nombre de déclarations de RIM soumises n’a pas crû. Les habitudes de déclaration de RIM que les participants ont affirmé avoir ont été corroborées par les données sur la charge de travail. Dans la deuxième phase de l’enquête, il y a eu une baisse notable du nombre de commentaires libres indiquant le manque de personnel comme obstacle à la déclaration des RIM.
Conclusions
La mise en place d’une politique plus détaillée n’a pas été associée à une augmentation du nombre de déclarations de RIM produites par des pharmaciens dans le contexte de cette étude. Des interventions visant à améliorer, au sein de l’organisme, les méthodes de travail pour la détection des RIM et le dépôt de déclarations de RIM individuelles doivent être fortement envisagées afin de consolider et d’améliorer les habitudes de déclaration des RIM chez les pharmaciens.
MOTS CLÉS: réactions indésirables, pharmaciens, suivi pharmacologique, organisation et administration
Over the past 2 decades, there have been calls for greater action to reduce the harms arising from inappropriate medication use. The US Institute of Medicine’s landmark report in 1999 was the first to draw widespread attention to the impact of medication errors and adverse drug events,1 and its findings have been corroborated elsewhere.2–4 Other publications have further emphasized the extent to which these harms are preventable.5–7 As a result, several guidance documents now exist that outline practices to prevent harm from medication use. However, pharmacists may encounter challenges and conflicts as they strive to implement these recommendations.8,9
Adverse drug reactions (ADRs) are an important subset of adverse drug events. Interest in ADRs—which are considered to reflect the innate safety profile of specific chemical compounds (drug safety)—predates more recent efforts to address the safety of drugs in clinical use (patient safety). Following the thalidomide disaster in 1961, regulatory bodies adopted an international approach to addressing drug safety issues, and the resulting activities were regrouped under the term “pharmacovigilance”.10 ADR monitoring is a key component of the pharmacovigilance activities that are performed both by national drug regulators11,12 and by the pharmaceutical industry,13 and it is recognized that spontaneously generated ADR reports play a key role in this regard.10,14,15 Surveillance of ADRs in medication users outside the hospital setting may be especially helpful, as such individuals may have fewer confounding factors to complicate the assessment of causality. Surveillance in outpatients may also detect ADRs in different drug categories,16 such as herbal and natural health products.17,18 ADR reports obtained directly from patients may also provide earlier signals of adverse effects and can capture humanistic outcomes that may be overlooked or downplayed by health professionals.19 As a result, many drug regulatory bodies now encourage direct reporting of ADRs by consumers.20
Pharmacists are clearly well positioned to contribute meaningfully to drug safety through ADR reporting,21 particularly in hospitals and other organized health care settings.22–24 Canadian pharmacists have led a number of initiatives to enhance reporting of ADRs, including efforts to investigate natural health products used in community settings.17,18 to encourage completion of ADR reports when nonformulary drugs are required,25 and to establish networks for ADR monitoring in high-risk patient populations.26 The importance of ADR investigation and reporting is also incorporated into the professional practice standards for pharmacy in Canada,27 and the practice is variably mandated in different Canadian provinces.28–30 Health Canada is also implementing legislative changes to mandate the reporting of serious ADRs (as well as medical device incidents) through hospitals.31 Nonetheless, underreporting of ADRs remains common, with pharmacists’ reports accounting for just 10.4% of all ADR reports submitted to Health Canada in 2012.32 Many barriers have been known to contribute to underreporting of ADRs (and adverse drug events more broadly) among pharmacists and other health care professionals,33 including factual and skill-based knowledge deficits,33–37 personally held beliefs and attitudes,33–35,38 and social or environmental pressures.33,36,37
The Canadian Forces Health Services Group (CFHSG) currently maintains over 20 distinct outpatient treatment clinics, which have differing levels of pharmacy support for both clinical services and dispensing of medications. In 2015, the existing organizational policy on ADR reporting was revised to reiterate the importance of reporting adverse reactions to all health products. This new version of the policy streamlined the number of references that had to be consulted for reporting purposes, and also enabled the organization to better address requirements for formal accreditation as a health care institution. Under this revised policy, an adverse reaction is defined as any undesirable effect that arises in a patient and is suspected to be associated with the use of a specific health product. Five categories of health products— aligned with the regulatory regime for pharmaceuticals in Canada—are named in the policy. The policy also clearly identifies when reports must be submitted to a monitoring department within the organization, in addition to designated departments of Health Canada.
This study was conducted primarily to determine whether the newly introduced policy was associated with changes in the ADR reporting behaviours of pharmacists working in the outpatient clinics of the CFHSG. Secondary objectives involved verifying the accuracy of pharmacists’ recall of their ADR reporting behaviour using workload-based records and assessing perceived barriers to ADR reporting.
This study involved administration of a telephone survey to individual pharmacists and review of administrative workload records for clinical teams. Institutional approval of the study concept was first obtained through the Surgeon General’s Health Research Program, whereas the study protocol itself was approved independently by the Human Research Ethics Committee of Defence Research and Development Canada on April 3, 2014 (Protocol Number 2014–012). This research was conducted in accordance with the ethical standards of these organizations and the Helsinki Declaration.
Individuals who were provincially licensed and directly employed as pharmacists within the CFHSG (i.e., occupying a designated position, either on a short-term contractual basis or as an ongoing member of staff ) were eligible to participate in the study. Persons who were not registered as pharmacists—including pharmacy students, pharmacy assistants, and pharmacy technicians— were excluded from the survey. Similarly, any licensed pharmacists working in positions that were not officially classified as requiring licensure as a pharmacist (e.g., health care administrators, project officers) were not eligible to participate. All eligible personnel were advised of the study via email before being contacted by the research nurse. Informed consent was sought verbally from individual participants at the beginning of each telephone survey.
A standardized telephone survey (Appendix 1, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/177/showToc) was administered to all eligible personnel (in English or French, as appropriate) by a single research nurse at 2 separate time points: once before the revised policy was formally introduced (“pre”) and once 3 months afterward (“post”). The survey contained a total of 25 questions, divided across 5 separate domains: respondent characteristics, awareness of current policy, technical expertise related to ADR reporting, personal ADR reporting behaviours, and perceived barriers to ADR reporting. Seventeen of the survey questions were formulated to generate yes/no responses, and the remaining 8 questions were open-ended. During each sampling period (i.e., pre– and post–policy change), 3 attempts were made to contact each eligible individual. At any point in the survey, participants could decline to answer any specific survey questions without further elaboration. All submitted responses were analyzed.
The McNemar test was applied to determine whether there were any significant changes in the proportion of respondents answering yes/no questions in the affirmative in the post–policy change survey. For determining whether changes in pharmacists’ ADR reporting behaviour occurred after release of the revised policy (based on the numbers of ADRs reported), the analysis was restricted to individuals who reported providing patient care during at least 15% of their work time in the previous 3 months. (This proportion is consistent with practice requirements established for direct patient care in one Canadian province,39 and ensured that full-time clinical pharmacists who were absent due to extended leave or work assignments during the preceding 3 months would be appropriately distinguished from those in nonclinical positions.) All responses to open-ended questions were further collated, anonymized, and reviewed to identify recurring themes.
To assess the accuracy of pharmacists’ recall of their ADR reporting behaviours (a secondary objective), 2 different measures of ADR reports were generated and compared to determine the level of agreement. Pharmacists were first grouped according to the clinic to which they were assigned, and their individual responses to question 14 of the survey—asking whether the pharmacist had reported an ADR during the preceding 3 months—were pooled. This allowed each clinic to be categorized as either having reported an ADR or not. A separate categorization was then made of the same clinics (i.e., as either reporting or nonreporting) using counts of ADR reports previously logged in the organization’s pharmacy workload measurement system. This particular workload measurement system forms an integral part of the software that patient care pharmacists use regularly throughout their work day, and enables key clinical interventions, including ADR reports, to be recorded in real time, e.g., immediately before or after making a change to a patient’s drug therapy. The kappa statistic was then used to assess the level of agreement between these 2 categorizations.
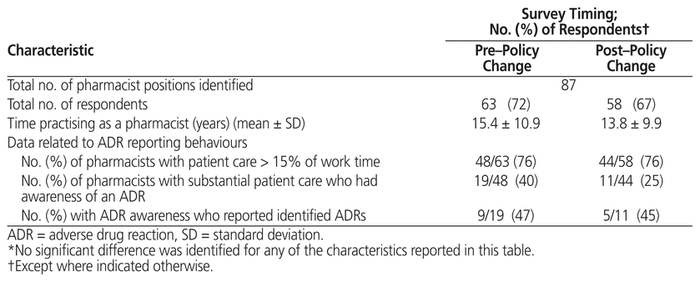
According to records in the CFHSG central database, a total of 87 discrete positions for study-eligible pharmacists were identified across the organization for each of the study’s sampling periods. Attempts were made to contact the individuals who officially occupied each of these positions during the 2 sampling periods (June 2014 for the pre–policy change survey and November 2014 for the post–policy change survey). Because of staff absences and rotation/cross-coverage between clinics, not all individuals who responded in the first survey period were available to reply during the second survey period. In total, 63 individuals completed the survey in the period before the policy change (72% response rate) and 58 after (67% response rate). Completion rates for individual survey questions were generally high, with only 4 questions that were not answered by all respondents. Further description of the respondents is provided in Table 1.
Table 1 Characteristics of Survey Respondents and ADR Reporting Behaviours*
ADR reporting behaviour was assessed for those individuals who reported spending at least 15% of their work time providing patient care. This restriction limited the responses to 48 (76%) of the 63 respondents to the pre–policy change survey, and 44 (76%) of the 58 respondents to the post–policy change survey (Table 1). The absolute number of these “patient care” pharmacists who were aware of an ADR was lower in the period following introduction of the new policy (19 in the pre–policy change survey versus 11 in the post–policy change survey), but the proportion of pharmacists who reported the ADRs they identified did not change (9 of 19 [47%] versus 5 of 11 [45%], respectively).
For this part of the analysis, ADR reporting metrics were generated for all sites that provided a response to the survey during either the pre– or post–policy change sampling period. This yielded a total of 31 observation periods for comparison (17 and 14, respectively). When the pooled survey responses and submitted workload reports were compared, there was agreement in terms of reporting and nonreporting status for all but 4 of the observation periods, which resulted in good agreement overall (kappa = 0.7647). In 3 of the discordant cases, the survey respondent(s) did not recall submitting an ADR report, although such a report had been recorded for their clinic within the workload measurement system.
There was a significant change from baseline for only 2 questions (Table 2). Overall, participants who responded after the policy change were significantly more likely to indicate that they were familiar with current organizational policies on ADR reporting (54 of 58 [93%] post–policy change versus 48 of 63 [76%] pre–policy change; p = 0.013). The second question asked respondents whether they felt they could complete all necessary ADR reports during their assigned work hours; for this question, fewer survey participants declined to respond in the survey period following introduction of the revised policy (i.e., 55 of 63 participants responded at baseline, compared with 57 of 58 participants after the revised policy was released). This improved response rate was associated with a significant improvement in this measure of ADR reporting capability (53 of 57 [93%] responding in the affirmative post–policy change versus 41 of 55 [75%] pre–policy change; p = 0.006).
Table 2 Responses to Other Survey Questions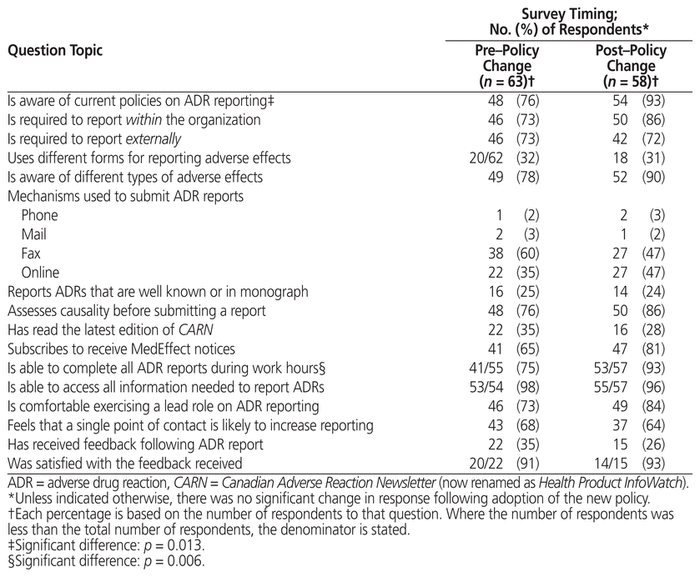
Responses to other survey questions did not differ significantly between the surveys done before and after the policy change (Table 2). Nonsignificant increases were noted in the proportions of respondents attesting to awareness of different types of ADRS, assessing causality before submitting an ADR report, and subscribing to receive notifications from the MedEffect Canada program. A majority of respondents to both surveys stated that they would be comfortable exercising a lead role in the reporting of ADRs (46 of 63 respondents [73%] in the first versus 49 of 58 respondents [84%] in the second survey period). In the associated free-form comments, many respondents stated that they were “already doing this”, with several noting that it was considered a “duty” or employment requirement. Smaller proportions of respondents (68% and 64%) agreed that creating a single point of contact for all drug-related adverse effects would increase the likelihood that they would report ADRs specifically.
With regard to barriers to ADR reporting, comments provided voluntarily before the policy change repeatedly cited the need for more staff (9 of 15 responses). Fewer comments were made about the need for dedicated time (n = 4) and tangible resources (n = 3), such as more computers in the pharmacy, to support ADR reporting. In contrast, after the policy change, comments on the need for additional staff were not predominant (i.e., cited in only 2 of 6 comments submitted).
Following release of a comprehensive revised policy on medication incident reporting, pharmacists in the CFHSG reported both greater awareness of ADR-related policies and an enhanced ability to report ADRs during their assigned work hours. Enhanced policy awareness was to be expected, as additional communications related to this study may have prompted participants to familiarize themselves with existing policies in preparation for the survey. However, the detected increase in self-reported ability to report ADRs—a finding supported by dramatically fewer free-form comments regarding a need for additional staff—was surprising to us. Because no direct changes were made in the practice environment to address barriers cited in the initial survey responses (such as increasing the number of work hours, staff, or computers for the pharmacy), it appears that the revised policy altered the perception of “necessary” ADR reports, such that these now appeared to be eminently do-able in respondents’ existing practice sites.
Unfortunately, despite the observed improvement in pharmacists’ confidence in reporting ADRs, there was no detectable increase in the actual number of ADR reports generated. Following release of the new policy, both a lower number of identified ADRs and an unchanged rate of reporting for identified ADRs were noted. There is no reason to believe that the incidence of ADRs would have changed substantially during the study’s timeframe; therefore, the lack of an observable effect on the primary outcome measure can best be attributed to a lower rate of ADR detection by pharmacists. Previous studies have noted that altering the working definition of an ADR, either alone or in concert with modifications to reporting infrastructures, can significantly change the rates at which ADRs are both detected and subsequently reported.16,40–42
The pharmacists’ self-identified ADR reporting rate remained consistent at about 45% in both survey periods, and accuracy of respondents’ recall was supported by independently generated workload data. Given the substantial number of considerations that must be taken into account when deciding to report suspected ADRs,43 this rate appears reasonable. Therefore, if a greater number of ADR reports is desired (i.e., to increase the power to detect safety issues affecting this patient population), new mechanisms will be needed to make ADR detection more sensitive and ADR reporting less cumbersome. Such system modifications should be carefully designed to capture data against the full range of medication-related monitoring that needs to occur, with recognition that the number of reports required may vary depending on whether the system aims to investigate drug safety or patient safety.
It must also be recognized that systems designed to detect ADRs in other settings may not be ideally suited for implementation in this specific outpatient environment. As an example, although the presence of dedicated ADR personnel (supplemental to the existing pharmacy teams) can increase the detection of ADRs,17,18,44 adoption of a single point of contact for incident reporting does not appear to be strongly supported by the out - patient pharmacists surveyed in this study, many of whom clearly felt compelled, professionally, to exercise a leading role in this area. Instead, given the encouraging improvements reported here (following an extremely low-intensity educational intervention), more formalized training interventions should be investigated preferentially for pharmacists in these practice sites.
It is clear that training interventions should incorporate mechanisms to provide meaningful feedback that can reinforce health professionals’ learned behaviours over time.45 In particular, standardized procedures to electronically acknowledge receipt of ADR-related information are likely be well received among CFHSG pharmacists, most of whom already subscribe to receive electronic notifications from Health Canada’s MedEffect Canada program. Standardization of procedures to transmit ADR reports is also expected to be highly appreciated, particularly among military pharmacists, who are highly mobile (Pharmacy Officers can expect to be posted to a different base or work unit every 2–3 years.). Electronic modes of communication could also be used to address persistent knowledge deficits, which may lower the numbers or the quality of submitted reports.
The study design unfortunately did not allow us to conclusively determine the degree to which the observed increase in self-reported ADR reporting ability was directly attributable to the policy change itself. The study population may have evolved in 2 key respects over the course of the study period, either of which would independently alter collective confidence in ability to report ADRs during work time. First, pharmacy managers may have made staffing decisions (either consciously or unconsciously) that preferentially assigned pharmacists with greater ADR experience and training to patient care positions during the later survey period. However, if that were the case, these “higher-capability” pharmacists would have had multiple opportunities to detect new and existing ADRs, and both the proportion of pharmacists detecting ADRs and the overall number of ADR reports ought to have increased over time. Alternatively, this finding could be explained if hiring processes over the study period introduced a greater number of recent graduates into the population of outpatient pharmacists. In at least one previous North American report, younger pharmacists were more likely to hold attitudes conducive to ADR reporting,38 and certainly pharmacists licensed more recently could be assumed to be more familiar with current ADR reporting requirements and drug categorizations established by the federal regulator over the past decade. While not a statistically significant difference, the average number of years worked as a pharmacist was lower among those who responded after the policy change (15.4 versus 13.8 years, p = 0.70; see Table 1), which supports the second theory. The latter explanation is also consistent with the finding of a greater awareness of existing policies after the policy change, since review of such policies would normally be completed during “onboarding” processes for new hires. Despite this limitation, it remains reasonable to assume that additional work to enhance ADR reporting would be appropriate, particularly to create mechanisms and tools that would make completion of ADR reports less time consuming and ADR detection more thorough.
1 Institute of Medicine, Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, editors. To err is human: building a safer health system. Washington (DC): National Academies Press; 2000. Available for purchase from: https://doi.org/10.17226/9728
2 Reducing and preventing adverse drug events to decrease hospital costs. Research in Action, Issue 1. Publ 01-0020. Rockville (MD): Agency for Healthcare Research and Quality; 2001 [cited 2016 Oct 27]. Available from: https://archive.ahrq.gov/research/findings/factsheets/errors-safety/aderia/ade.html
3 Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38(5):437–53.


4 Adverse drug reaction–related hospitalizations among seniors, 2006 to 2011. Ottawa (ON): Canadian Institute for Health Information; 2013 Mar [cited 2017 Jul 31]. Available from: http://publications.gc.ca/collections/collection_2013/icis-cihi/H117-5-25-2013-eng.pdf
5 Hakkarainen KM, Gyllensten H, Jönsson AK, Andersson Sundell K, Petzold M, Hägg S. Prevalence, nature and potential preventability of adverse drug events – a population-based medical record study of 4970 adults. Br J Clin Pharmacol. 2014;78(1):170–83.


6 Boeker EB, de Boer M, Kiewiet JJS, Lie-A-Huen L, Dijkgraaf MGW, Boermeester MA. Occurrence and preventability of adverse drug events in surgical patients: a systematic review of literature. BMC Health Serv Res. 2013;13:364.


7 Onakpoya IJ, Heneghan CJ, Aronson JK. Delays in the post-marketing withdrawal of drugs to which deaths have been attributed: a systematic investigation and analysis. BMC Med. 2015;13:26.


8 Thomas CEL, Phipps DL, Ashcroft DM. When procedures meet practice in community pharmacies: qualitative insights from pharmacists and pharmacy support staff. BMJ Open. 2016;6(6):e010851.


9 Egan G, Law E, Mailman J, Sunderland M, Dalen D. Should Accreditation Canada’s required organizational practices and standards lead to prioritization of clinical pharmacy services over distribution-related medication safety strategies? The “pro” side. Can J Hosp Pharm. 2013;66(3):194–5.
10 The importance of pharmacovigilance: safety monitoring of medicinal products. Geneva (Switzerland): World Health Organization; 2002 [cited 2017 Jul 31]. Available from: http://apps.who.int/medicinedocs/en/d/Js4893e/
11 Guidance for industry: good pharmacovigilance practices and pharmacoepidemiologic assessment. Rockville (MD): US Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research, Centre for Biologics Evaluation and Research; 2005 Mar [cited 2018 Jul 23]. Available from: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071696.pdf
12 Good pharmacovigilance practices (GVP) guidelines GUI-0102. Ottawa (ON): Health Canada, Health Products and Food Branch Inspectorate; 2013 [cited 2017 Jul 31]. Available from: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/compli-conform/gmp-bpf/docs/gui-0102_gvp-eng.pdf
13 Talbot JCC, Nilsson BS. Pharmacovigilance in the pharmaceutical industry. Br J Clin Pharmacol. 1998;45(5):427–31.


14 Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med. 2005;165(12):1363–9.

15 Aagaard L, Hansen EH. Information about ADRs explored by pharmaco-vigilance approaches: a qualitative review of studies on antibiotics, SSRIs and NSAIDs. BMC Clin Pharmacol. 2009;9:4.
16 Egberts AC, de Koning FH, Meyboom RH, Leufkens HG. ADR related questions received by a telephone medicines information service and ADRs received by a spontaneous ADR reporting system: a comparison regarding patients and drugs. Pharmacoepidemiol Drug Saf. 1997;6(4):269–76.

17 Vohra S, Cvijovic K, Boon H, Foster BC, Jaeger W, LeGatt D, et al. Study of natural health product adverse reactions (SONAR): active surveillance of adverse events following concurrent natural health product and prescription drug use in community pharmacies. PLoS One. 2012;7(9):e45196.


18 Necyk C, Tsuyuki RT, Boon H, Foster BC, LeGatt D, Cembrowski G, et al. Pharmacy study of natural health product adverse reactions (SONAR): a cross-sectional study using active surveillance in community pharmacies to detect adverse events associated with natural health products and assess causality. BMJ Open. 2014;4(3):e003431.


19 Banerjee AK, Okun S, Edwards IR, Wicks P, Smith MY, Mayall SJ, et al. Patient-reported outcome measures in safety event reporting: PROSPER Consortium guidance. Drug Saf. 2013;36(12):112–49.
20 van Hunsel F, Härmark L, Pal S, Olsson S, van Grootheest K. Experiences with adverse drug reaction reporting by patients: an 11-country survey. Drug Saf. 2012;35(1):45–60.
21 van Grootheest K, Olsson S, Couper M, de Jon-van den Berg L. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf. 2004;13(7):457–64.

22 Jones KJ, Cochran GL, Xu L, Skinner A, Knudson A, Hicks RW. The association between pharmacist support and voluntary reporting of medication errors: an analysis of MEDMARX® data. In: Henriksen K, Battles JB, Keyes MA, Grady ML, editors. Advances in patient safety: new directions and alternative approaches. Vol 1: Assessment. Rockville (MD): Agency for Healthcare Research and Quality; 2008 [cited 2018 Jul 3]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK43627/
23 Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals. Pharmacotherapy. 2006; 26(6):735–47.

24 Taras-Zasowski KM, Einarson TR. Review of Canadian pharmacist involvement in adverse drug reaction reporting. Can J Hosp Pharm. 1989; 42(3):105–8,133.
25 Vaillancourt R, Gervais A, Gauthier C. Mandatory reporting of adverse drug reactions [abstract]. International Pharmaceutical Federation Congress; 2005 Sep 3–8; Cairo (Egypt). Available from: www.fip.org/?page=abstracts&action=generatePdf&item=682 [cited 2017 Aug 1].
26 Carleton B, Poole R, Smith M, Leeder J, Ghannadan R, Ross C, et al. Adverse drug reaction active surveillance: developing a national network in Canada’s children’s hospitals. Pharmacoepidemiol Drug Saf. 2009; 18(8):713–21.

27 Model standards of practice for Canadian pharmacists (March 2009). Ottawa (ON): National Association of Pharmacy Regulatory Authorities; 2009 [cited 2017 Jul 31] http://napra.ca/pharmacists/model-standards-practicecanadian-pharmacists
28 Item 12(7). In: Health Professions Act – bylaws. Schedule F, Part 1: Community pharmacy standards of practice. Vancouver (BC): College of Pharmacists of British Columbia; 2017 Jan 20 [revised 2017 Mar 3; cited 2017 Aug 1]. Available from: http://library.bcpharmacists.org/6_Resources/6-1_Provincial_Legislation/5078-HPA_Bylaws_Community.pdf
29 Item 2.4.9. In: Practice direction – ensuring patient safety. Winnipeg (MB): College of Pharmacists of Manitoba; 2014 Jan 1 [revised 2015 Feb 9; cited 2017 Aug 1]. Available from: www.cphm.ca/uploaded/web/Legislation/Ensuring%20Patient%20Safety%20(effective%20March%201,%202015).pdf
30 Standards of practice for pharmacists and pharmacy technicians. Edmonton (AB): Alberta College of Pharmacists; 2014 [cited 2017 Aug 1]. Available from: https://abpharmacy.ca/sites/default/files/StandardsOfPractice_May2014_v2.pdf
31 Regulations amending the Food and Drug Regulations (serious adverse drug reaction reporting — hospitals). Canada Gazette, Part I, 2018;152(24). Available from: www.gazette.gc.ca/rp-pr/p1/2018/2018-06-16/html/reg5-eng.html [cited 2018 Jul 9].
32 McMorran M, McEnaney J. Adverse reaction and incident reporting—2012. Can Advers React Newsl. 2013;23(3):2–5. Available from: www.hcsc.gc.ca/dhp-mps/alt_formats/pdf/medeff/bulletin/carn-bcei_v23n3-eng.pdf
33 Mirbaha F, Shalviri G, Yadizadeh B, Gholami K, Majdzadeh R. Perceived barriers to reporting adverse drug events in hospitals: a qualitative study using theoretical domains framework approach. Implement Sci. 2015;10:110.


34 Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009; 32(1):19–31.

35 Stewart D, MacLure K, Paudyal V, Hughes C, Courtenay M, McLay J. Non-medical prescribers and pharmacovigilance: participation, competence and future needs. Int J Clin Pharm. 2013;35(2):268–74.

36 Green CF, Mottram DR, Rowe PH, Pirmohamed M. Attitudes and knowledge of hospital pharmacists to adverse drug reaction reporting. Br J Clin Pharmacol. 2001;51(1):81–6.


37 Sweis D, Wong IC. A survey on factors that could affect adverse drug reaction reporting according to hospital pharmacists in Great Britain. Drug Saf. 2000;23(2):165–72.

38 Gavaza P, Brown CM, Lawson KA, Rascati KL, Wilson JP, Steinhardt M. Influence of attitudes on pharmacists’ intention to report serious adverse drug events to the Food and Drug Administration. Br J Clin Pharmacol. 2011;72(1):143–52.


39 Part A and Part B register. Toronto (ON): Ontario College of Pharmacists; [cited 2017 Aug 17]. Available from: www.ocpinfo.com/registration/register-pharmacist/two-part-register/
40 Johnstone DM, Kirking DM, Vinson BE. Comparison of adverse drug reactions detected by pharmacy and medical records departments. Am J Health Syst Pharm. 1995;52(3):297–301.
41 Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. JAMA. 1991;266(20):2847–51.

42 Chen AM, Kiersma ME, Shepler BM, Murawski MM. Pilot testing of checklists to discern adverse drug reactions and adverse drug events. J Am Pharm Assoc. 2013;53(1):61–9.
43 Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–9.

44 Gallo M, Clavenna A, Bonati M, Siani P, Irpino A, Rossi F, et al. Active surveillance of adverse drug reactions in children in five Italian paediatric wards. Open J Ped. 2012;2(2):111–7.
45 Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362(9391):1225–30.

Competing interests: Development of the study protocol was initiated by Renaud Roy in partial fulfilment of the academic requirements for the entry-level PharmD degree at the Université de Montréal. No other competing interests were declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors wish to express their thanks to Julie Lanouette, who diligently administered the telephone surveys, and to Cdr Sylvain Grenier, Maj Cecilia Reyes, Debra Willcox, and Dr Yousef Al-Enzi, who provided constructive comments regarding the survey questions and potential interpretation of preliminary results. The input of all pharmacists who responded to the survey is also gratefully acknowledged.
Canadian Journal of Hospital Pharmacy, VOLUME 71, NUMBER 4, July-August 2018