Safety of Rectal Administration of Acetaminophen in Neonates
Lori Chen, Monica Zhang, Jason Yung, Jennifer Chen, Carol McNair, Kyong-Soon Lee
ABSTRACT
Background
On the basis of pharmacokinetic modelling, high-dose acetaminophen by rectal administration has been recommended for neonates needing antipyretic or analgesic therapy, but the safety and efficacy of this approach have not been established in vivo.
Objectives
The primary objective was to assess the safety of rectal acetaminophen administration for neonates, as indicated by changes in the results of hepatic and renal function tests. The secondary objective was to assess the efficacy of rectal acetaminophen administration in terms of the Premature Infant Pain Profile-Revised (PIPP-R) score.
Methods
This single-centre retrospective chart analysis was conducted in the neonatal intensive care unit at a quaternary care children’s hospital. Neonates who received all prescribed doses of acetaminophen by continu - ous rectal administration for 24 h or more, from January 1, 2011, to December 31, 2012, were included. For the primary objective, hepatotoxicity was assessed in terms of changes in liver enzyme levels, and nephrotoxicity was assessed in terms of changes from baseline serum creatinine values.
Results
Twenty-five patients, who received a total of 27 courses of acetaminophen by rectal administration, met the inclusion criteria. Median gestational age at initiation of acetaminophen was 37.0 weeks (interquartile range 35.0–39.8 weeks). Values of alanine aminotransferase remained within normal limits during acetaminophen therapy for all but 3 patients, for whom the changes were attributable to confounding factors. Renal function remained unchanged. The secondary outcome of efficacy (based on PIPP-R score) could not be evaluated because of concurrent use of opioids for most patients.
Conclusions
Continuous rectal administration of acetaminophen over a short period (< 48 h) appeared to be well tolerated. The conclusions that can be drawn from these results are limited because of small sample size, the prescribing of doses lower than those recommended by the hospital’s formulary, and limited blood sampling. Further studies are required.
KEYWORDS: pharmacodynamics, neonatal intensive care unit, hepatotoxicity, nephrotoxicity, pain control
RÉSUMÉ
Contexte
Selon une modélisation pharmacocinétique, des doses élevées d’acétaminophène administré par voie rectale ont été recommandées comme traitement antipyrétique ou analgésique chez le nouveau-né, mais l’innocuité et l’efficacité de cette modalité d’administration n’ont pas été établies in vivo.
Objectifs
L’objectif principal était d’évaluer l’innocuité de l’acétaminophène administré par voie rectale chez le nouveau-né en observant les changements dans les résultats des bilans hépatique et rénal. L’objectif secondaire était d’évaluer l’efficacité de l’acétaminophène administré par voie rectale à l’aide du score obtenu dans le Premature Infant Pain Profile-Revised (PIPP-R).
Méthodes
La présente étude rétrospective menée dans un seul centre comportait une analyse des dossiers médicaux de patients admis à l’unité de soins intensifs néonatals d’un établissement de soins quaternaires pour enfants. Les nouveau-nés ayant reçu toutes les doses prescrites d’acétaminophène par administration rectale ininterrompue pendant 24 heures ou plus, entre le 1er janvier 2011 et le 31 décembre 2012, étaient admissibles à l’étude. Pour l’objectif principal, l’hépatotoxicité a été évaluée en fonction des variations observées dans les taux d’enzymes hépatiques et la néphrotoxicité a été évaluée en fonction des changements observés dans la créatininémie par rapport aux valeurs de départ.
Résultats
Vingt-cinq patients qui ont reçu un total de 27 traitements par acétaminophène administré par voie rectale répondaient aux critères d’inclusion. L’âge gestationnel médian lors de l’amorce du traitement par acétaminophène était de 37,0 semaines (écart interquartile de 35,0 semaines à 39,8 semaines). Les valeurs d’alanine-aminotransférase demeuraient à l’intérieur des limites normales pendant le traitement par acétaminophène pour tous les patients à l’exception de trois pour lesquels les changements étaient attribués à des facteurs de confusion. La fonction rénale demeurait inchangée. Le critère d’évaluation secondaire quant à l’efficacité (s’appuyant sur le score obtenu dans le PIPP-R) n’a pu être évalué en raison de la prise concomitante d’opioïdes chez la plupart des patients.
Conclusions
L’administration rectale ininterrompue d’acétaminophène pendant une courte période (moins de 48 heures) semblait être bien tolérée. Cependant, les conclusions qui peuvent être tirées de ces résultats sont limitées en raison de la petite taille de l’échantillon, de la prescription de doses plus faibles que celles recommandées dans la liste des médicaments de l’hôpital et de l’insuffisance des échantillons sanguins. De plus amples études sont nécessaires.
MOTS CLÉS: pharmacodynamique, unité de soins intensifs néonatals, hépatotoxicité, néphrotoxicité, contrôle de la douleur
INTRODUCTION
Acetaminophen is a commonly used antipyretic and analgesic agent for neonates. Although the drug is relatively safe, its well-documented toxicity can lead to hepatotoxicity and death. Relative to adults, neonates and infants have lower activity levels of the cytochrome P450 2E1 isozyme, which means they have lower ability to form the toxic metabolites of acetaminophen and greater ability to form glutathione.1 Nevertheless, these young patients are still able to form hepatotoxic metabolites to some degree, and the variable metabolism and clearance of acetaminophen in this population can still cause adverse effects.2,3 Case reports exist of hepatotoxicity occurring in neonates and infants who received high doses of acetaminophen, although the problem resolved after treatment with N-acetylcysteine.4,5
Rectal administration of acetaminophen is used when enteral administration is not possible or when IV administration is unavailable. Rectal administration of acetaminophen in neonates is poorly studied, and appropriate dosing is unclear because of high variability in absorption and clearance, along with poor absorption overall, in neonates.2,6,7
Two efficacy studies have suggested that rectal administration of acetaminophen at daily doses of 40–80 mg/kg is insufficient to achieve an analgesic effect,8,9 and some evidence suggests that higher-than-usual doses of acetaminophen (e.g., loading dose of 30–45 mg/kg and maintenance doses of 20–30 mg/kg) are required when the drug is administered rectally.2,10
In addition, several different formulations for rectal administration have been studied, including a diluted oral solution (to be administered rectally) and various types of suppositories, with widely differing pharmacokinetic parameters.10 Most previous studies of this drug in neonates have been pharmacokinetic in nature and have not focused on adverse effects.
Dosing references list a wide range of possible doses for neonates. The optimal dosage of acetaminophen for rectal administration is unknown because of the limited number of studies and the significant variation in dosing and other practices that these studies describe.2,10 At the authors’ institution, the defined formulary approach to rectal dosing for neonates,11 instituted in 2010, is based upon a pharmacokinetic paper by Anderson and others.10 According to that paper, the maximum daily dose for rectal solution for term neonates (120 mg/kg) is substantially higher than that for infants and older children (80 mg/kg); this suggested maximum is based upon a single-dose study involving 5 preterm neonates. Furthermore, the dosing suggested by Anderson and others10 has not been prospectively validated in neonates, and those authors stated that “these regimens may cause hepatotoxicity in some individuals if used for longer than 2–3 days.”
The primary objective of this study was to assess the safety of rectal administration of acetaminophen in neonates at dosages of up to 120 mg/kg daily. The secondary objective was to assess efficacy.
METHODS
Study Design, Setting, and Patient Population
A retrospective chart analysis was conducted in the neonatal intensive care unit (NICU) at The Hospital for Sick Children (Toronto, Ontario), a level 4 nursery in a quaternary care children’s hospital. No deliveries occur on site, and 40% of admissions involve a surgical condition. The study was approved by the SickKids Research Ethics Board.
All neonates who were admitted to the NICU from January 1, 2011, to December 31, 2012, and who had received aceta - minophen were screened for inclusion in the study. Neonates (postmenstrual age ≤ 44 weeks) who received all prescribed doses of acetaminophen by continuous rectal administration for at least 24 h in the NICU were included in the study. Patients were excluded if their medication administration records were not available in the electronic chart, if they had not received acetaminophen by rectal administration, or if the route of administration was uncertain. At the study site, usual practice is to avoid excessive use of acetaminophen for patients with pre-existing hepatic or renal dysfunction, although limited doses may be used, with close surveillance, in select cases; hence, pre-existing hepatic or renal dysfunction were not considered as exclusion criteria.
Acetaminophen Doses for Rectal Administration
Acetaminophen was ordered for neonates according to recommended doses by gestational age (GA), based on the pharmacokinetic paper by Anderson and others10:
-
GA < 30 weeks: not recommended for use
-
GA 30–33 weeks: 20 mg/kg per dose q12h (40 mg/kg per day)
-
GA 34–39 weeks: 25 mg/kg per dose q8h (75 mg/kg per day)
-
GA ≥ 40 weeks: 30 mg/kg per dose q6h (120 mg/kg per day)
Data Collection
Eligible patients were identified using drug utilization reports in the pharmacy medication management system (BDM Pharmacy, BDM IT Solutions, Saskatoon, Saskatchewan). Specifically, a list of patients in the NICU for whom acetaminophen had been prescribed was generated by the BDM Pharmacy system. Data from study participants were collected from the electronic medical records.
Data were collected for the following characteristics:
-
admission diagnosis
-
patient age (GA at birth, postmenstrual age at acetaminophen initiation)
-
weight (at birth, upon acetaminophen initiation)
-
indication for acetaminophen (pain, fever, or both)
-
surgical status (surgery performed within 7 days before acetaminophen administration)
-
total number of acetaminophen doses
-
doses, frequency, route, and duration of acetaminophen therapy
-
daily and weekly data for liver enzymes (alanine aminotransferase [ALT], alkaline phosphatase, γ-glutamyl transferase, bilirubin) and renal function markers (serum creatinine, urea) at baseline, during acetaminophen therapy, and up to 3 weeks after the last day of acetaminophen administration
-
pain, measured with the Premature Infant Pain Profile-Revised (PIPP-R) score,12 at 12 h before and during acetaminophen administration
-
name and duration of concurrent opioids, at 24 h before and during acetaminophen administration
-
name and duration of concurrent nephrotoxic drugs received during acetaminophen use and hepatotoxic drugs received during and up to 3 weeks after acetaminophen use, with dosages of these medications being reviewed for any outliers
Study Outcomes
The primary outcome was the safety of rectal administration of acetaminophen, in terms of hepatotoxicity and nephrotoxicity. Hepatotoxicity was defined as the result of any liver enzyme test (ALT, alkaline phosphatase, γ-glutamyl transferase, conjugated bilirubin, or unconjugated bilirubin) exceeding the upper limit of normal, as defined by Chang and others.13 Nephrotoxicity was defined as a relative increase in serum creatinine of 1.5 times or more from baseline.14
The secondary outcome was level of pain as measured by the PIPP-R score.
Statistical Analysis
Descriptive statistics were used to analyze the data for baseline characteristics and study outcomes. Available laboratory values at baseline and at 24 and 48 h after acetaminophen administration were analyzed. Descriptive statistics (minimum, maximum, median, interquartile range [25th to 75th percentiles], mean, standard deviation) are presented for all parameters with more than one value. Microsoft Excel (version 2007; Microsoft Corporation, Redmond, Washington) was used for the analyses.
RESULTS
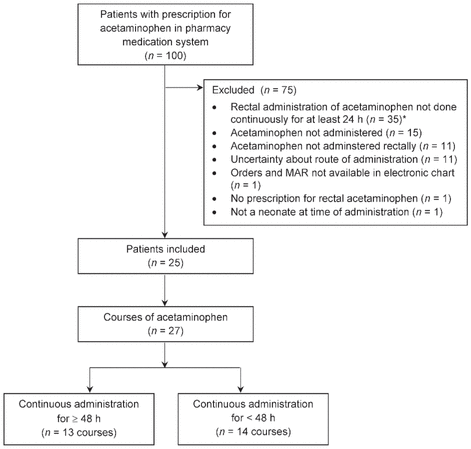
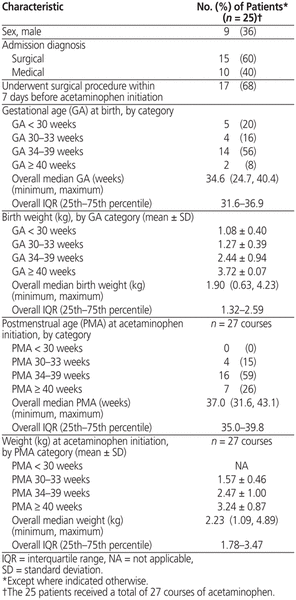
A total of 100 patients received acetaminophen during the study period; however, only a total of 27 courses of acetaminophen, administered to 25 patients, met the inclusion criteria and were evaluated in the analysis (Figure 1). Most of the patients were girls, most were between 34 and 39 weeks GA at acetaminophen initiation, and 60% of the patients had a surgical admission diagnosis (Table 1).
| |
|
|
|
Figure 1 Patient enrolment. *Patients with aceta - minophen therapy for less than 24 h were excluded because safety was the main end point, and patients receiving high doses for more than 24 h are thought to be at higher risk for toxic effects.
MAR = medication administration record.
|
Table 1 Baseline Characteristics of Neonates Receiving Rectal Administration of Acetaminophen
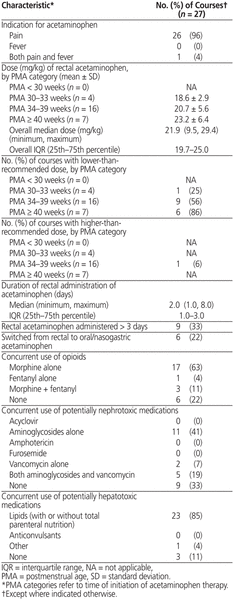
Among the 27 acetaminophen courses, 26 (96%) were for pain, and 21 (78%) involved concurrent opioids. Total parenteral nutrition (TPN) was the most common potentially hepatotoxic treatment, and aminoglycosides were the most common potentially nephrotoxic medication. Overall, 16 (59%) and 1 (4%) of the 27 courses used a lower-than-recommended or higher-than-recommended dose, respectively (Table 2). Six courses involved a higher frequency of lower doses or higher frequency of recommended doses (relative to the formulary).
Table 2 Acetaminophen Dosage Characteristics and Concurrent Toxic Medications
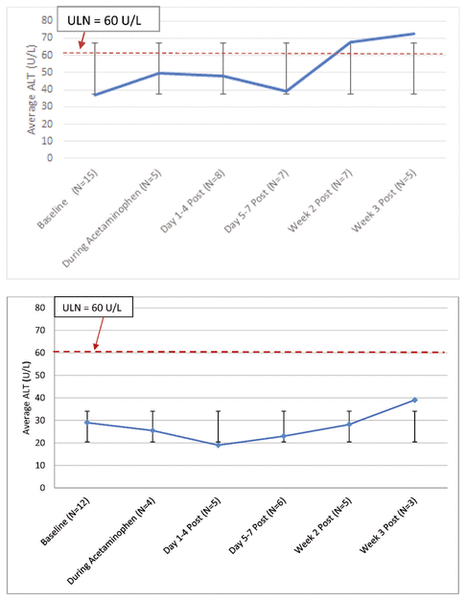
For the most part, mean ALT values across all courses of therapy remained below the upper limit of normal (Figure 2A). More specifically, individual ALT values were below the upper limit of normal during the evaluation period, with the exception of 3 patients, whose ALT values increased substantially from baseline while they were taking acetaminophen or within 2 weeks of discontinuing this drug. Each of these patients had confounding factors that have been described as causing elevated ALT. One of the patients had moderate to severe hypoxic ischemic encephalopathy with elevated results on liver function testing since birth.15 The second patient received isoniazid 5 days after acetaminophen initiation and was also receiving concurrent TPN, both of which are known to cause hepatotoxicity and elevated liver function results13,16; this patient’s liver values began increasing sometime between 4 and 8 days after initiation of isoniazid. The third patient’s increase in ALT was attributed to TPN cholestasis by the clinical team, on the basis of clinical assessment and other laboratory findings.16 The prescribed acetaminophen doses for these 3 patients were 20 mg/kg q6h, 22 mg/kg q8h, and 24 mg/kg q8h, respectively. When results for these 3 patients were omitted from the analysis, all mean ALT values remained below the upper limit of normal (Figure 2B). For all patients, serum creatinine remained the same or declined from baseline, thus showing no evidence of nephrotoxicity.
| |
|
|
|
Figure 2 Top: Mean alanine aminotransferase (ALT) levels for all neonates who received rectal administration of acetaminophen, from baseline to 3 weeks after the last acetaminophen dose. A total of 15 acetaminophen courses were included; however, data were not available at all time points for all patients. Bottom: Mean ALT levels for neonates without confounding factors (n = 12 courses). Error bars indicate standard deviations, and the dashed red line in each graph indicates 60 U/L (units per litre), which is the upper limit of normal (ULN).
|
The secondary outcome of efficacy (based on PIPP-R score) could not be evaluated because most patients were receiving opioids concurrently.
DISCUSSION
In this single-centre retrospective chart analysis, we attempted to evaluate the safety and efficacy of acetaminophen administered rectally to neonates in the NICU. In terms of safety, we found no evidence of hepatotoxicity or nephrotoxicity among neonates included in the study. However, the doses given were often discordant with the institution’s formulary guidelines, which were based on a study by Anderson and others.10 The doses administered were most often lower than recommended or were provided at higher frequency than recommended. This usage pattern may suggest that prescribers lack familiarity with dosing guidelines for rectal acetaminophen by GA; at least some of the dosage deviations may be due to use of dosing ranges appropriate for oral administration, with which most prescribers are familiar.
It proved impossible to evaluate the secondary objective of efficacy directly attributable to acetaminophen (according to PIPP-R scores), because the study centre commonly uses acetaminophen as an opioid-sparing agent; as such, most patients were also receiving some form of opioid therapy. The opioid doses were not consistently available or recorded, and the opioid-sparing effects of acetaminophen could therefore not be assessed. Conversely, patients receiving opioids were not excluded from the analysis, because doing so would have further reduced the already limited sample size.
The major limitations of this study were its small sample size and the short duration of rectal administration of acetaminophen. Use of acetaminophen beyond 2 or 3 days is more likely to increase the risk of toxicity,10 but only about half of this cohort (n = 13 courses) received rectal acetaminophen therapy for 2 days (48 h) or more. This limitation might be difficult to address even with larger sample sizes, given that the usual clinical indications for rectal administration of acetaminophen do not support prolonged or continuous use. The small number of patients included in this study may reflect the infrequent use of rectal acetaminophen for NICU patients in general. For instance, only 25% of patients receiving acetaminophen at the study institution were eligible for inclusion in the data analysis, even though the study setting was a high-volume surgical NICU and the recruitment period lasted for 2 years. This infrequent use may be due to lack of awareness of the availability and potential efficacy of this form of therapy. We were unable to find other studies reporting on the frequency of rectal administration of acetaminophen in NICUs. Another limitation was the relatively low number of results of hepatic and renal function tests available for review; ethical considerations would preclude more frequent monitoring even with a prospective study design.
We found substantial variation in dosing and other practices for acetaminophen administration in neonates. The extent of variation at other sites must be determined to evaluate whether our findings are generalizable to other sites.
CONCLUSION
Continuous rectal administration of acetaminophen in a surgical NICU setting was infrequent, and even when this form of therapy was used, it was often not prescribed according to formulary guidelines. We found no evidence of toxicity of rectally administered acetaminophen in these circumstances. The need for increased awareness of proper dosing was identified, and educational strategies are now being implemented in the NICU where this study was performed. Further studies are required to evaluate the safety and efficacy of rectal acetaminophen administration when utilized according to guideline recommendations.
References
1 Anderson BJ, Holford NHG, Woollard GA. Paracetamol kinetics in neonates [abstract]. Anesth Intensive Care. 1997;25(6):721–2.
2 van Lingen RA, Deinum HT, Quak CM, Okken A, Tibboel D. Multiple-dose pharmacokinetics of rectally administered acetaminophen in term infants. Clin Pharmacol Ther. 1999;66(5):509–15.

3 Warner A. Drug use in the neonate: interrelationships of pharmacokinetics, toxicity, and biochemical maturity. Clin Chem. 1986;32(5):721–7.
4 Muñiz AE, Rose SR 2nd, Liner SR, Foster RL. Unsuspected acetaminophen toxicity in a 58-day-old infant. Pediatr Emerg Care. 2004;20(12):824–8.

5 Walls L, Baker CF, Sarkar S. Acetaminophen-induced hepatic failure with encephalopathy in a newborn. J Perinatol. 2007;27(2):133–5.

6 Lin YC, Sussman HH, Benitz WE. Plasma concentrations after rectal administration of acetaminophen in preterm neonates. Paediatr Anaesth. 1997;7(6):457–9.

7 Hansen TG, O’Brien K, Morton NS, Rasmussen SN. Plasma paracetamol concentrations and pharmacokinetics following rectal administration in neonates and young infants. Acta Anaesthesiol Scand. 1999;43(8):855–9.

8 Tinner EM, Hoesli I, Jost K, Schöbi N, Ulrich Megged Y, Burkhardt T, et al. Rectal paracetamol in newborn infants after assisted vaginal delivery may increase pain response. J Pediatr. 2013;162(1):62–6.

9 van Lingen RA, Quak CM, Deinum HT, van de Logt F, van Eyck J, Okken A, et al. Effects of rectally administered paracetamol on infants delivered by vacuum extraction. Eur J Obstet Gynecol Reprod Biol. 2001;94(1):73–8.

10 Anderson BJ, van Lingen RA, Hansen TG, Lin YC, Holford NH. Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology. 2002;96(6):1336–45.

11 Acetaminophen. In: SickKids formulary (The Hospital for Sick Children electronic formulary). Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2016 Nov 28. Accessed through Lexicomp Online via institutional subscription.
12 Stevens BJ, Gibbins S, Yamada J, Dionne K, Lee G, Johnston C, et al. The Premature Infant Pain Profile-Revised (PIPP-R): initial validation and feasibility. Clin J Pain. 2014;30(3):238–43.

13 Chang SH, Nahid P, Eitzman SR. Hepatotoxicity in children receiving isoniazid therapy for latent tuberculosis infection. J Pediatr Infect Dis Soc. 2014;3(3):221–7.

14 Libório AB, Branco KMP, Torres de Melo Bezerra C. Acute kidney injury in neonates: from urine output to new biomarkers. Biomed Res Int. 2014;2014: Article 601568.


15 Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312(24):2629–39.


16 Gabe SM, Culkin A. Abnormal liver function tests in the parenteral nutrition fed patient. Frontline Gastroenterol. 2010;1(2):98–104.


Lori Chen, RPh, BScPhm, ACPR, is with the Department of Pharmacy, The Hospital for Sick Children, Toronto, Ontario
Monica Zhang, PharmD, is with the Department of Pharmacy, Joseph Brant Hospital, Burlington, Ontario
Jason Yung, BMSc, PharmD, is with the Department of Pharmacy, The Ottawa Hospital, Ottawa, Ontario
Jennifer Chen, BScPhm, PharmD, is with the Department of Pharmacy, The Hospital for Sick Children, Toronto, Ontario
Carol McNair, RN(EC), MN, PhD(c), NNP-BC, NP-Peds, is with the Division of Neonatology, The Hospital for Sick Children, Toronto, Ontario
Kyong-Soon Lee, MD, MSc, is with the Division of Neonatology, The Hospital for Sick Children, Toronto, Ontario
Acknowledgement
The authors would like to thank Simon Tran for his help with manuscript preparation.
Address correspondence to: Lori Chen, Department of Pharmacy, The Hospital for Sick Children, 555 University Avenue, Toronto ON M5G 1X8, e-mail:Lori.chen@sickkids.ca
(Return to Top)
Competing interests: None declared. (
Return to Text
)
Funding: None received. (
Return to Text
)
Canadian Journal of Hospital Pharmacy, VOLUME 71, NUMBER 6, November-December 2018