Ouida Antle, Ashley Kenny, Julie Meyer, Luciana G MacedoABSTRACT
Background
Postoperative nausea and vomiting (PONV) is one of the most commonly reported adverse experiences after surgery. PONV is a major risk factor for delayed patient mobilization and consequently increased length of hospital stay.
Objectives
The primary objective was to compare the effectiveness of scheduled versus as-needed administration of antiemetic for the prevention and treatment of PONV in the first 48 h after elective hip or knee arthroplasty. The secondary objective was to determine whether PONV affected mobilization on either postoperative day 0 or postoperative day 1 in each study group.
Methods
This retrospective cohort study used chart reviews for collection of patient data. PONV and mobilization were compared for patients who received antiemetics on a scheduled or as-needed basis following elective hip or knee arthroplasty performed between January and September 2016.
Results
Of the 132 patients included in the study, 65 received antiemetics on an as-needed basis and 67 had scheduled antiemetic therapy. Thirty-one (46%) of the patients in the “scheduled” group received antiemetics as intended; the others missed one or more of the scheduled doses. There was no statistical difference in PONV between treatment groups with either intention-to-treat or as-treated analysis. Furthermore, there was no statistically significant difference in mobilization, on either POD 0 or POD 1, between patients who received scheduled antiemetic and those who received antiemetic on an as-needed basis.
Conclusions
Scheduled use of antiemetics did not significantly affect PONV, nor did it positively influence mobilization in the postoperative period for patients undergoing elective arthroplasty. Further high-quality prospective studies are needed to confirm these results.
KEYWORDS: postoperative nausea and vomiting, ondansetron, antiemetic, arthroplasty, scheduled versus as-needed therapy
RÉSUMÉ
Contexte
Les nausées et vomissements postopératoires sont parmi les réactions indésirables les plus fréquentes après une intervention chirurgicale. Elles représentent un facteur de risque important de retard de mobilisation et par conséquent de prolongation du séjour à l’hôpital.
Objectifs
L’objectif principal visait la comparaison de l’efficacité d’une administration régulière d’antiémétiques à une administration au besoin pour la prévention et le traitement des nausées et vomissements postopératoires au cours des 48 heures suivant une arthroplastie nonurgente de la hanche ou du genou. L’objectif secondaire était de déterminer si les nausées et vomissements postopératoires avaient des répercussions sur la mobilisation des patients durant la journée postopératoire 0 ou 1 dans chaque groupe à l’étude.
Méthodes
Les données de la présente étude de cohorte rétrospective proviennent des analyses de dossiers des patients. La comparaison portait sur les nausées et vomissements postopératoires et la mobilisation de patients ayant reçu des antiémétiques prescrits régulièrement ou au besoin après avoir subi une arthroplastie non-urgente de la hanche ou du genou, réalisée entre janvier et septembre 2016.
Résultats
Parmi les 132 patients admis à l’étude, 65 ont reçu des antiémétiques au besoin et 67 en ont pris régulièrement. Trente et un (46 %) patients du groupe auquel on avait prescrit une prise régulière ont reçu des antiémétiques comme prévu, les autres ont sauté une ou plusieurs doses prévues. Aucune différence statistique n’a été relevée quant aux nausées et vomissements postopératoires entre les groupes, que ce soit à l’aide d’une analyse selon l’intention de traiter ou selon le traitement reçu. De plus, il n’y avait aucune différence statistiquement significative du point de vue de la mobilisation, pour les jours postopératoires 0 et 1 entre les patients ayant pris régulièrement des antiémétiques et ceux en ayant pris au besoin.
Conclusions
L’administration régulière d’antiémétiques n’a pas eu d’effet significatif sur les nausées et vomissements postopératoires tout comme elle n’a pas influencé positivement la mobilisation au cours de la période postopératoire des patients ayant subi une arthroplastie non-urgente. De plus amples études prospectives de grande qualité sont nécessaires pour confirmer ces résultats.
MOTS CLÉS: nausées et vomissements postopératoires, ondansétron, antiémétique, arthroplastie, comparaison entre traitement régulier et traitement au besoin
Postoperative nausea and vomiting (PONV) is one of the most commonly reported adverse experiences after surgery. It occurs in about 30% of the general surgical population and in up to 80% of high-risk surgical patients.1,2 PONV is a major risk factor for delayed patient mobilization and consequently increased length of hospital stay and prolonged overall recovery in hospital; it may therefore be indirectly associated with increased health care costs.
The 2014 consensus guidelines for the management of PONV,3 compiled under the auspices of the Society for Ambulatory Anesthesia, proposed that establishing the baseline risk of PONV is of value when determining ways to reduce the risk for this adverse effect, identifying effective regimens for prophylaxis, and recommending strategies for treatment when PONV occurs.3 The use of risk stratification for PONV is supported by the literature.1,2,4
The consensus guidelines suggest use of the simplified Apfel score1 to predict a patient’s risk of PONV. The Apfel score ranges from 0 to 4, with 1 point assigned to each of the following 4 independent risk factors: female sex, nonsmoker status, history of PONV or motion sickness, and use of postoperative opioids. The risk of PONV increases with increasing number of risk factors and has been reported to be about 10% with no risk factors, about 20% with 1 risk factor, about 40% with 2 risk factors, about 60% with 3 risk factors, and about 80% with 4 risk factors.3
The Apfel score was prospectively validated in patients undergoing surgery with general anesthesia and was found to have good predictive accuracy.1 However, it has not been specifically validated in patients undergoing regional anesthesia, nor has it been specifically examined in patients undergoing orthopedic surgery.
The consensus guidelines3 recommend considering PONV prophylaxis using 1 or 2 interventions in adults at moderate risk for PONV and 2 or more interventions in adults at high risk for PONV. The recommended interventions include nonpharmacological, anesthesia-specific, and pharmacological options. The guidelines do not support giving prophylactic antiemetics to all patients undergoing surgical procedures. In addition, there is little guidance in these guidelines concerning the treatment of PONV occurring in the period immediately after an elective surgical procedure but before the patient has been discharged from the hospital.
Many different strategies have been trialled to facilitate patients’ early mobilization and discharge from hospital. Over the years at our own institution, we have observed utilization of new surgical approaches (e.g., direct anterior versus lateral approach for hip replacements), changes to pain management regimens (e.g., IV versus oral administration of opioids), and use of spinal anesthesia instead of general anesthesia. Recently, the orthopedic surgeons have started using scheduled antiemetic therapy for patients undergoing hip and knee replacement surgery, regardless of each individual’s risk factors. The purpose of this intervention is to decrease the incidence of PONV. Although antiemetic medications are generally well tolerated, adverse events may still occur, and scheduled use of these medications may be associated with significant issues, such as increased side effects or concerns about drug–drug interactions. We found no previous studies suggesting that implementation of this protocol in clinical practice can significantly reduce PONV or the length of hospital stay in this patient population. The typical length of stay for elective hip and knee arthroplasty at the study institution, based on the Alberta Bone and Joint Health Institute knee and hip replacement patient care plans (commonly referred to as the “pathways”), is a total of 4 days in hospital (i.e., POD 0 to POD 3).5,6 On the study unit, we aim to discharge patients as soon as possible after their surgery. A desire to shorten the length of stay in hospital is a primary driver for changing how antiemetic medications are ordered and administered in this patient group.
The current study was undertaken, in a spirit of inquiry, following the local practice change to use of scheduled antiemetics. The primary objective was to compare the effectiveness of scheduled versus as-needed antiemetic therapy in the first 48 h after surgery (i.e., on POD 0 and POD 1) for the prevention and treatment of PONV in patients undergoing elective hip or knee arthroplasty. Although a variety of antiemetics may be appropriate for treating PONV in this population, ondansetron is the drug most widely used for this indication and is the first-line antiemetic used for PONV at the study institution; it was therefore the focus of our study. Clinical observations at our institution indicate that patients typically require an antiemetic only within the first 48 h after surgery, and it is rare for an antiemetic to be administered beyond the first 48 h. Therefore, for the purposes of this study, antiemetic use was evaluated on POD 0 and POD 1.
The secondary objective was to determine, within each study group, whether PONV was associated with mobilization on POD 0 or POD 1. The exploratory objective was to evaluate whether Apfel scores (independent of the intervention) predicted PONV in this population of patients undergoing elective arthroplasty.
A retrospective chart review was used to collect data for this pilot study. Notes and documentation by physicians, nurses, and allied health professionals were reviewed within the electronic medical record and in the paper chart for each included patient. Data were obtained for consecutive adult patients admitted to an acute orthopedic surgery inpatient unit at Foothills Medical Centre in Calgary, Alberta, from January 1 to September 30, 2016. Data collection for consecutive patient charts continued until we attained convenience samples (groups of nearly equal size) of patients receiving scheduled or as-needed antiemetic therapy. The start date for data collection was based on when surgeons began ordering ondansetron for “scheduled” postoperative use. Some surgeons were slower to adopt this practice change than others, which allowed for a comparator group (receiving antiemetics on an as-needed basis) within the same timeframe.
Ethics approval was obtained from the Health Research Ethics Board of Alberta (Community Health Committee) (approval #HREBA.CHC-16-0044). A waiver of consent was granted because of the retrospective nature of the study.
Patients were identified through the hospital’s electronic medical record software Allscripts Sunrise Clinical Manager (SCM) (Eclipsys), which contains detailed clinical information about all patients and their hospital stay, including demographic information, diagnostic imaging results, laboratory values, procedures and treatments received, progress notes, and discharge summaries. SCM records and supplementary paper charts were used to obtain all of the data for the study. The information about PONV and mobilization was taken from documentation by both nursing and physiotherapy staff. More specifically, PONV was documented as present or absent with a yes/no question in the nursing flowsheets. PONV was documented by physiotherapy staff (in the electronic progress notes) if it occurred during mobilization. Documentation of PONV and mobilization is part of standard nursing assessment and practice on the unit.
Patients 18 years of age or older who had been admitted for elective total knee or total hip arthroplasty and who remained on the study unit after their surgery were included in the study. Patients were excluded if they had undergone bilateral joint arthroplasty, unicompartmental (partial) knee arthroplasty, revision of previously performed surgery, resurfacing surgery, or trauma and fracture surgery of the hip or knee. Also excluded were patients taking any type of chemotherapeutic agent for cancer treatment (oral or IV) and patients who were pregnant or breastfeeding.
Demographic data were collected from review of each patient’s electronic health record to determine age, sex, type of surgery, date of surgery, and date of discharge. The following data concerning administration of antiemetics were also collected: dose, frequency, and number of doses administered.
This study did not itself involve any interventions, but instead evaluated the intervention (antiemetic therapy) ordered by the surgeons. Patients for whom a scheduled dose of ondansetron was prescribed were compared with those for whom any other antiemetic regimen was prescribed. The “scheduled” antiemetic regimen was ondansetron 4–8 mg IV every 6–8 h (q6–8h) for 48 h after surgery. For patients within this group, a prescriber could order other antiemetics to be used on an as-needed basis, in addition to the scheduled ondansetron, for example, metoclopramide 10 mg PO or IV q6–8h as needed or dimenhydrinate 25–50 mg PO or IV q6–8h as needed. The “as-needed” antiemetic regimen involved orders for a medication to be administered at the patient’s request to treat acute symptoms of PONV. The as-needed antiemetic regimen always included ondansetron 4–8 mg PO or IV q6–8h as needed (the first-choice antiemetic for this population); patients could also have additional orders for either or both of the following: metoclopramide 10 mg PO or IV q6–8h as needed or dimenhydrinate 25–50 mg PO or IV q6–8h as needed. If any patient experienced intractable nausea or vomiting, the prescriber was contacted and, upon appropriate clinical assessment, could order a one-time dose of dexamethasone or methylprednisolone sodium succinate IV. These options are not part of the regular arthroplasty pathway, and their use was assessed on a case-by-case basis. The choice of antiemetic or antiemetics ordered and whether the drugs were ordered on a scheduled or as-needed basis was at the surgeon’s discretion. Most antiemetic prescribing in this patient population is driven by an electronic order set listing ondansetron and metoclopramide; however, physicians may order different antiemetics (outside of the electronic order set) as deemed clinically appropriate.
The primary outcome was the occurrence of PONV on POD 0 and POD 1 (i.e., within 2 timeframes: 0–24 h after surgery and 24–48 h after surgery).
The secondary outcome was whether the patient was able or unable to mobilize on POD 0 or POD 1. As set out in the unit’s care pathway, a patient was considered to have met the criteria for mobilization, from a physiotherapy standpoint, on POD 0 if the patient could stand at the bedside and do bed exercises (ankle pumping, static quadriceps, and buttock exercises). The minimum requirement for mobilization on POD 1 was considered to have been met if the patient walked a minimum of 10 m twice during the day, progressing to an eventual distance goal of 15–20 m, as well as repeating the bed exercises performed on POD 0.
Adherence to the protocol was assessed for patients in the “scheduled” group by considering the number of doses administered within the first 24 h. In this group, adherence was defined as receiving at least 3 doses in the first 24 h.
Demographic characteristics are reported with means and standard deviations (for normally distributed variables) or with medians and interquartile ranges (for variables not normally distributed and for ordinal variables).
Logistic regression was conducted to evaluate the relation between intervention groups and the primary and secondary outcomes. Given previous literature3 reporting that higher Apfel scores are associated with occurrence of PONV, Apfel score was included in the model as a potential effect modifier (treatment × Apfel score interaction). The inclusion of the Apfel score in the model also controlled for age, sex, and smoking status. Both intention-to-treat (a priori) and as-treated (post hoc) analyses were conducted. The as-treated analysis was conducted because a large number of those for whom scheduled administration was prescribed did not receive all of their scheduled doses, and we wanted to explore potential trends in effects.
Chi-square tests were used to investigate whether Apfel score was associated with the primary and secondary outcomes.
One of the main reasons for using antiemetics is to decrease PONV and thus to facilitate earlier mobilization, potentially reducing the time to hospital discharge. We therefore used logistic regression to investigate the association between PONV and mobilization, controlling for the intervention.
All data analyses were conducted using Stat version 14.1 (StataCorp LP). The level of significance was set at 0.05 for all statistical analyses.
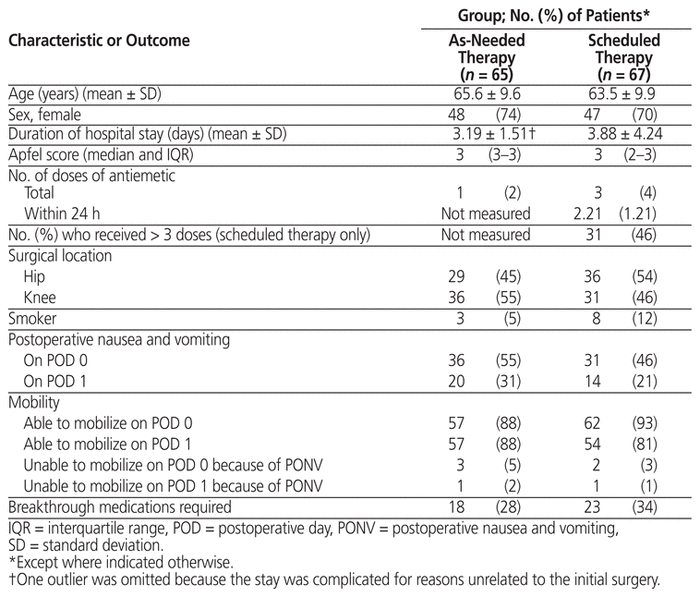
A total of 132 patient charts were reviewed, with 65 of the patients receiving “as-needed” antiemetic treatment, and 67 receiving “scheduled” antiemetic treatment. Demographic characteristics are presented in Table 1. Overall, there were more women than men in both groups (48 women in the “as-needed” group and 47 women in the “scheduled” group), and there were few smokers in either group (3 and 8, respectively). There were no statistically significant differences between groups in terms of baseline measures.
Table 1 Characteristics of Sample and Primary Outcomes
About half of the patients in both groups (36 [55%] in the “as-needed” group and 31 [46%] in the “scheduled” group) had PONV on POD 0; the proportions decreased to 20 (31%) and 14 (21%), respectively, by POD 1. Most patients were able to mobilize on POD 0 (57 [88%] in the “as-needed” group and 62 [93%] in the “scheduled” group). Of patients who were unable to mobilize on POD 0, the reason was PONV for 3 patients in the “as-needed” group and 2 patients in the “scheduled” group. There were no statistically significant differences between groups in terms of these measures.
Thirty-one (46%) of the patients in the “scheduled” group received antiemetics as intended (i.e., therapy was adherent with the physician’s orders); the others missed one or more of the scheduled doses.
The Apfel score was associated with PONV on POD 0 (χ2 = 30.52, df = 3; p < 0.001) but not with PONV on POD 1 (χ2 = 3.91, df = 3; p = 0.27), mobilization on POD 0 (χ2 = 3.39, df = 3; p = 0.34), or mobilization on POD 1 (χ2 = 0.29, df = 3; p = 0.96).
There were no differences in results between the intention-to-treat and the as-treated analyses; therefore, only results from the intention-to-treat analysis are presented below.
Apfel score did not act as an effect modifier for any of the primary or secondary outcomes, and thus interaction effects were not included in the final model. However, the Apfel score was a predictor of the association between intervention and PONV in the first 24 h after surgery. The intervention was not significantly associated with the primary outcome of PONV on either POD 0 or POD 1, nor was it associated with the secondary outcome of mobilization on either POD 0 or POD 1 (Tables 2 and 3).
Table 2 Logistic Regression for Association of Intervention with Primary and Secondary Outcomes (Intention-to-Treat Analysis)*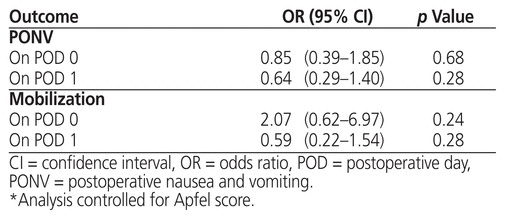
Table 3 Logistic Regression for Association between PONV and Mobilization*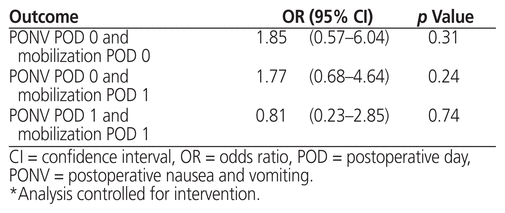
Eighteen patients in the “as-needed” group and 23 patients in the “scheduled” group had intractable PONV and required additional rescue medication (defined as “breakthrough medication”) for PONV (Table 1). A χ2 test showed no significant difference in the use of rescue medication for PONV between the treatment groups (χ2 = 0.5, df = 1; p = 0.48).
The notion that scheduled ondansetron treatment in the immediate postoperative period would decrease nausea and vomiting and therefore encourage earlier mobilization and reduce the length of stay in the hospital is elegant in theory. However, in this study, the scheduled administration of antiemetic, specifically ondansetron, did not result in a statistically significant difference in either PONV or mobilization. Notably, Apfel score did indeed predict PONV outcomes in patients undergoing elective arthroplasty, who at the study facility largely undergo regional anesthesia.
The routine use of scheduled administration of antiemetics for all patients undergoing surgery is not supported by the most recent consensus guidelines3 or by a number of independent researchers.4,7 This is based on a lack of evidence for the use of scheduled antiemetics, the possibility of rare but unwanted side effects, and economic reasons. The results of this study support the current clinical guidelines. However, some clinicians have argued for a more liberal use of postoperative antiemetics, to reduce the risk of PONV and to facilitate early hospital discharge.8 Further research is needed to confirm or refute this benefit.
To the best of our knowledge, this is the first study to explore the question of whether scheduled administration of ondansetron in the postoperative period results in less PONV among adult patients undergoing elective knee or hip replacement. Our results do not support the hypothesis that administering ondansetron postoperatively to all patients undergoing this type of surgical procedure will lessen the occurrence of PONV or improve early mobilization for patients undergoing hip or knee replacement. We acknowledge that we examined postoperative antiemetic use only while the patient was on the inpatient unit. We did not look at intraoperative use of antiemetics or administration in the Post Anesthesia Care Unit, as this information would have been difficult to retrieve. However, it is possible that patients received a dose of ondansetron at the end of the surgical procedure, since ondansetron is prescribed at the anesthesiologist’s discretion.
The limitations of this study include the small sample size (132 participants), which limited the statistical power, and the retrospective nature of the study design. The small sample limits considerably any conclusions that can be drawn from the results. Another limitation was poor adherence to the regimen for scheduled use of ondansetron after surgery. Poor adherence was likely due to the lack of education provided to members of the health care team before implementation of this new regimen for around-the-clock medication. Our data collection demonstrated a lack of consistency within the physician group as to how ondansetron was ordered, as well as a lack of consistency within the nursing group as to how ondansetron was administered. Furthermore, although we were able to control for known factors that contribute to a patient’s risk of PONV, such as the Apfel score, larger studies are needed to identify other currently unknown confounders. The strengths of this study included collection of retrospective data from consecutive patients undergoing care within one hospital unit, which had a standardized protocol for patient care and mobilization after surgery; this limited potential confounders in patients’ outcomes.
This study has provided initial data on the effectiveness of “scheduled” versus “as-needed” ondansetron and allows for further research to be conducted in this patient population related to PONV, mobilization, and length of stay. Specifically, one area of further research could be to examine more closely patients with known higher Apfel scores (3 or 4 out of 4) who are undergoing elective orthopedic arthroplasty to determine the effectiveness of “scheduled” versus “as-needed” antiemetics in this population. Although we did not find an interaction effect in our study, a prospective clinical trial with a larger sample might be useful to evaluate this intervention in a higher-risk population. This study also contributes to the scarce data about the use of specific antiemetics in patients undergoing orthopedic surgery, particularly elective arthroplasty.
In this study, scheduled use of antiemetics in the immediate postoperative period did not significantly affect the occurrence of PONV in patients undergoing elective arthroplasty. Further high-quality prospective clinical trials are needed to confirm these results.
1 Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700.
2 Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for prevention of postoperative nausea and vomiting. N Engl J Med. 2004;35(24):2441–51.
3 Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113.
4 Eberhart LHJ, Morin AM. Risk scores for predicting postoperative nausea and vomiting are clinically useful tools and should be used in every patient: Con – ‘life is really simple, but we insist on making it complicated’. Eur Soc Anaesthesiol. 2011;28(3):155–9.
5 Knee replacement patient care plan. Calgary (AB): Alberta Bone and Joint Health Institute; [cited 2019 Apr 2]. Available from: https://albertaboneandjoint.com/wp-content/uploads/2013/08/alberta_bone_and_joint_knee_replacement_care_plan.pdf
6 Hip replacement patient care plan. Calgary (AB): Alberta Bone and Joint Health Institute; [cited 2019 Apr 2]. Available from: https://albertaboneandjoint.com/wp-content/uploads/2013/08/alberta_bone_and_joint_hip_replacement_care_plan.pdf
7 Hill RP, Lubarsky DA, Phillips-Bute B, Fortney JT, Creed MR, Glass PSA, et al. Cost-effectiveness of prophylactic antiemetic therapy with ondansetron, droperidol, or placebo. Anesthesiology, 2000;92(4):958–67.
8 Kranke P, Eberhart LHJ. Possibilities and limitations in the pharmacological management of postoperative nausea and vomiting. Eur J Anaesthesiol. 2011;28(11):758–65.
Competing interests: None declared. ( Return to Text )
Funding: The Research Challenge of Alberta Health Services provided financial support for this work. ( Return to Text )
The authors thank Pharmacy Services of Alberta Health Services and the management team of the orthopedic and general surgery unit at the Foothills Medical Centre, Calgary, for supporting the staff members who worked on this study and for allowing data collection. They also thank Michael Antle, PhD, for presubmission editing and review of the manuscript.
Canadian Journal of Hospital Pharmacy, VOLUME 72, NUMBER 2, March-April 2019