Efficacy and Safety of Infliximab in Pediatric Crohn Disease: A Systematic Review and Meta-Analysis
Sophia Li, Christopher Reynaert, Annie Ling Su, Sonja Sawh
ABSTRACT
Background
Crohn disease is an inflammatory bowel disease with intermittent symptoms relating to damage to the gastrointestinal tract. Compared with adult-onset Crohn disease, the childhood-onset form is more likely to be severe. Infliximab has shown efficacy in adult patients.
Objective
To examine the efficacy and safety of infliximab in pediatric Crohn disease, by means of a systematic review.
Data Sources
Three databases (MEDLINE, Embase, and Cochrane Central Register of Controlled Trials) and regulatory documents were searched from inception to December 2017. Clinical trial registries, conference abstracts, and reference lists were searched to March 2018.
Study Selection and Data Extraction
Randomized controlled trials (RCTs) and prospective cohort studies that compared infliximab with active control were included in the analysis. Two reviewers independently performed screening, extracted data, and assessed risk of bias. The primary outcomes were induction and maintenance of endoscopic remission and severe adverse effects.
Data Synthesis
Three eligible RCTs comparing different dose regimens, 16 prospective cohort studies comparing infliximab with other therapies (adalimumab, exclusive enteral nutrition, or standard of care), and 3 prospective cohort studies comparing different infliximab regimens were identified. Meta-analysis of the RCTs showed no significant difference between infliximab every 8 weeks compared with longer intervals for maintenance of clinical remission (risk ratio [RR] 1.76, 95% confidence interval [CI] 0.98–3.19). Meta-analyses of the prospective cohort studies showed no significant differences between infliximab and adalimumab for maintenance of endoscopic remission (RR 1.07, 95% CI 0.60–1.92), between infliximab and exclusive enteral nutrition for induction of clinical remission (RR 1.09, 95% CI 0.82–1.45), or between infliximab and standard of care for maintenance of clinical remission at 6 and 12 months (RR 1.12, 95% CI 0.58–2.17, and RR 1.24, 95% CI 0.84–1.84, respectively).
Conclusions
Current evidence suggested comparable efficacy for infliximab and other therapies; however, the available literature was limited by risk of bias and small sample size. Further prospective studies are needed to confirm the efficacy and safety of this drug in pediatric Crohn disease.
KEYWORDS: inflammatory bowel disease, anti-tumour necrosis factor alpha, infliximab, Crohn disease
RÉSUMÉ
Contexte
La maladie de Crohn est une maladie inflammatoire de l’intestin, dont les symptômes intermittents sont liés à des lésions du tractus gastro-intestinal. Comparativement à la maladie de Crohn se déclarant à l’âge adulte, celle qui se déclare dans l’enfance risque d’être plus grave. L’infliximab s’est avéré efficace chez l’adulte.
Objectif
Étudier l’efficacité et l’innocuité de l’infliximab chez l’enfant atteint de la maladie de Crohn à l’aide d’une analyse systématique.
Sources des données
Trois bases de données (MEDLINE, Embase, ainsi que le Registre central Cochrane des essais comparatifs) ont été interrogées et des documents réglementaires ont fait l’objet d’une recherche depuis leur création jusqu’en décembre 2017. Une consultation des registres d’essais cliniques, des résumés de conférences et des listes de références a eu lieu jusqu’en mars 2018.
Sélection des études et extraction des données
L’analyse a porté sur des essais cliniques à répartition aléatoire (ECRA) et des études de cohorte prospectives comparant l’infliximab au traitement actif. Deux examinateurs indépendants ont procédé à la sélection et à l’extraction des données ainsi qu’à l’évaluation des risques de biais. L’induction et le maintien d’une rémission endoscopique ainsi que les effets indésirables graves étaient les principaux paramètres d’évaluation.
Synthèse des données
Trois ECRA admissibles comparant différents schémas posologiques, 16 études de cohorte prospectives comparant l’infliximab à d’autres traitements (l’adalimumab, une alimentation exclusivement entérale et les soins d’usage) et trois études de cohorte prospectives comparant différents schémas posologiques d’infliximab ont été sélectionnées. Une méta-analyse des ECRA ne montrait aucune différence significative entre un traitement à l’infliximab toutes les huit semaines comparativement à des intervalles plus longs pour le maintien d’une rémission clinique (risque relatif [RR] de 1,76, intervalle de confiance [IC] à 95 % de 0,98–3,19). Des méta-analyses des études de cohorte prospectives ne montraient aucune différence significative entre l’infliximab et l’adalimumab pour le maintien d’une rémission endoscopique (RR de 1,07, IC à 95 % de 0,60–1,92), aucune différence non plus entre l’infliximab et une alimentation exclusivement entérale pour l’induction d’une rémission clinique (RR de 1,09, IC à 95 % de 0,82–1,45) ni entre l’infliximab et les soins d’usage pour le maintien d’une rémission clinique à six et douze mois (respectivement : RR de 1,12, IC à 95 % de 0,58–2,17 et RR de 1,24, IC à 95 % de 0,84–1,84).
Conclusions
Les données probantes actuelles laissaient entendre que l’efficacité de l’infliximab était comparable à celle des autres traitements; cependant, les articles disponibles étaient insuffisants en raison du risque de biais et de la faible taille de l’échantillon. De plus amples études prospectives sont nécessaires pour confirmer l’efficacité et l’innocuité de ce médicament chez l’enfant atteint de la maladie de Crohn.
MOTS CLÉS: maladies inflammatoires de l’intestin, inhibiteur du facteur de nécrose tumorale-alpha, infliximab, maladie de Crohn
INTRODUCTION
Crohn disease is an immune-mediated condition characterized by inflammation along the entire length of the gastrointestinal tract.1,2 It is a chronic, progressive condition with a relapsing and remitting course.3,4 The incidence of Crohn disease is increasing internationally, and it is estimated that 20% to 25% of cases present during childhood.2,5 Childhood-onset Crohn disease is associated with higher disease activity and a more complicated disease course.2,6
The goals of treatment are to relieve symptoms, improve quality of life, and minimize drug-related adverse effects.7 An additional goal specific to pediatric Crohn disease is to optimize the patient’s growth, which may be impaired because of intestinal inflammation and inadequate nutrition.2,8 Recently, there has been interest in mucosal healing or endoscopic remission as a treatment target.1,2,4 In adults with inflammatory bowel disease, mucosal healing has been associated with sustained remission, decreased complications, and decreased corticosteroid use, relative to patients without mucosal healing.4
Infliximab is a chimeric monoclonal antibody that binds to and interferes with the activity of human tumour necrosis factor alpha (TNF-α). Current guidelines for pediatric Crohn disease, from both the European Crohn’s and Colitis Organisation and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition, recommend anti-TNF-α as a second-line therapy after failure of standard-of-care therapy, such as exclusive enteral nutrition and corticosteroids for induction of remission and immunomodulators for maintenance of remission.7 This recommendation is in line with Health Canada’s indications for infliximab in the pediatric population.9 A consensus statement from the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition has affirmed that anti-TNF-α agents have demonstrated benefit in induction and maintenance treatment of pediatric Crohn disease.10
This systematic review aimed to examine the efficacy and safety of infliximab, compared with conventional therapy, for inducing and maintaining endoscopic remission in pediatric patients with luminal or fistulizing Crohn disease.
METHODS
This systematic review was conducted in accordance with the PRISMA guidelines11 and the Cochrane Handbook for Systematic Reviews of Interventions.12 A protocol detailing the conduct of this systematic review and meta-analysis was registered a priori with PROSPERO (registration identifier CRD42016037820).
Eligibility Criteria
The initial search strategy was developed to identify randomized controlled trials (RCTs) examining participants less than 18 years of age who had moderate to severe luminal or fistulizing Crohn disease. Studies involving any dose and regimen of infliximab, compared with active controls or standard of care, were eligible. For the purpose of this review, the definitions of active control used by the authors of included studies were accepted. These active controls included but were not limited to corticosteroids, immunomodulators, aminosalicylates, exclusive enteral nutrition, and other biologics. In the event that no RCTs satisfying the eligibility criteria were found, the protocol allowed for inclusion of prospective comparative nonrandomized studies and studies comparing different regimens of infliximab. Language and publication status restrictions were not imposed.
Outcomes
The primary outcomes were induction of endoscopic remission by 14 weeks, maintenance of endoscopic remission at 6 months, and incidence of severe adverse effects. The definitions of endoscopic remission used by the authors of included studies were accepted. The secondary outcomes were induction and maintenance of clinical remission as measured by the Pediatric Crohn Disease Activity Index (PCDAI), maintenance of endoscopic or clinical remission at 1 year, change in PCDAI, change in height, serum infliximab antibodies, serum infliximab levels, corticosteroid use (in prednisone equivalents), corticosteroid-free remission, need for surgery, hospitalization, and all-cause adverse effects. The incidence of each adverse effect, such as infections, malignancy, and infusion-related reactions, was individually examined.
Search and Study Selection
The search strategies were developed by 3 of the authors (S.L., C.R., and S.S.), with the assistance of a clinical librarian. Three databases (MEDLINE, Embase, and the Cochrane Central Register of Clinical Trials) and relevant regulatory documents were searched from inception to December 2017. Clinical trial registries, conference abstracts, and reference lists of included studies and systematic reviews were searched through to March 2018. The search strategies are provided in Appendix 1 (available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/190/showToc).
Duplicate citations were identified and removed using Mendeley software.13 Multiple reports based on the same study were identified and linked. A screening tool was developed a priori and pilot-tested.
Data Collection and Risk-of-Bias Assessment
Before extraction of study characteristics and outcome data from eligible studies, an electronic data collection form, based on the Cochrane data extraction and assessment template,12 was developed. For trials with missing, unclear, or discrepant information, an attempt was made to contact study authors. If clarification was unsuccessful, reviewers used their judgment to determine the trial’s eligibility on the basis of available information. For RCTs, the risk of bias was assessed with the Cochrane risk-of-bias tool.14 For nonrandomized studies, the risk of bias was assessed with the Ottawa–Newcastle scale.15
Screening, data collection, and risk-of-bias assessment were performed by the authors, in duplicate, in an independent, unbiased, and standardized manner. Disagreements were resolved by discussion and consensus among 3 authors, if necessary.
Synthesis of Results
A random-effects model (that of DerSimonian and Laird16) was used for the meta-analysis, with calculation of χ2 and I2 as indicators of heterogeneity. Values of P less than 0.10 and values of I2 greater than 50% were used as thresholds defining significant heterogeneity.17 Data from prospective cohort studies were analyzed separately from RCT data. Studies examining different comparators were analyzed separately, with grouping by comparator drug class or infliximab dosing regimen.
RESULTS
Summary of Study Characteristics
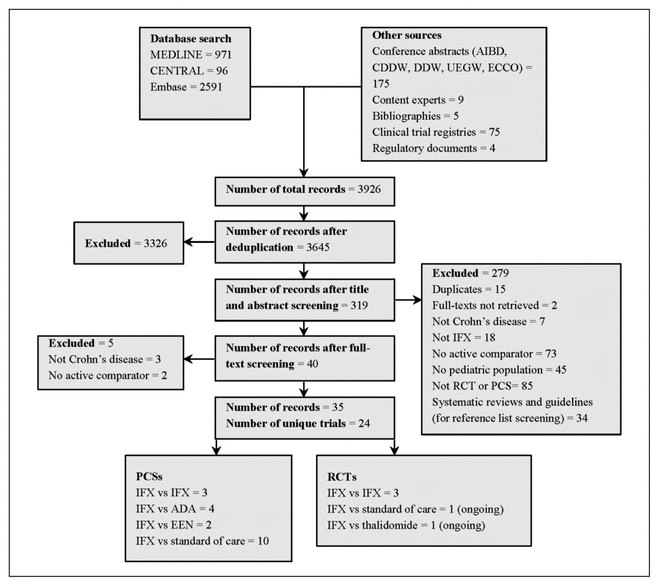
A total of 24 unique trials (some reported in multiple articles) were retrieved. Of the 24 trials included, 22 were complete (Figure 1, Table 1)18–47 and 2 were still in progress at the time of our analysis (Table 2). Three of the studies were RCTs, and the remainder were prospective cohort studies. Most of the studies were conducted in North America,18–21,44,46 Europe,22–31,43,45 or both,32–40 with only 2 studies from Asia.41,42,47
| |
|
|
|
Figure 1 Flow chart of literature selection. ADA = adalimumab, AIBD = Advances in Inflammatory Bowel Disease, CDDW = Canadian Digestive Diseases Week, DDW = Digestive Diseases Week, ECCO = European Crohn’s and Colitis Organisation, EEN = exclusive enteral nutrition, IFX = infliximab, PCS = prospective cohort study, RCT = randomized controlled trial, UEGW = United European Gastroenterology Week.
|
Table 1 Characteristics of Included Trials
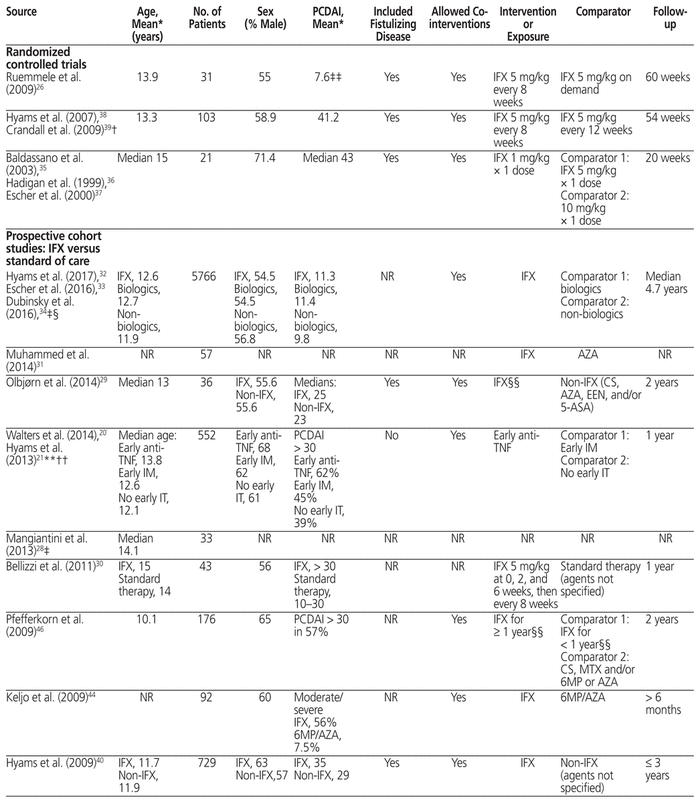
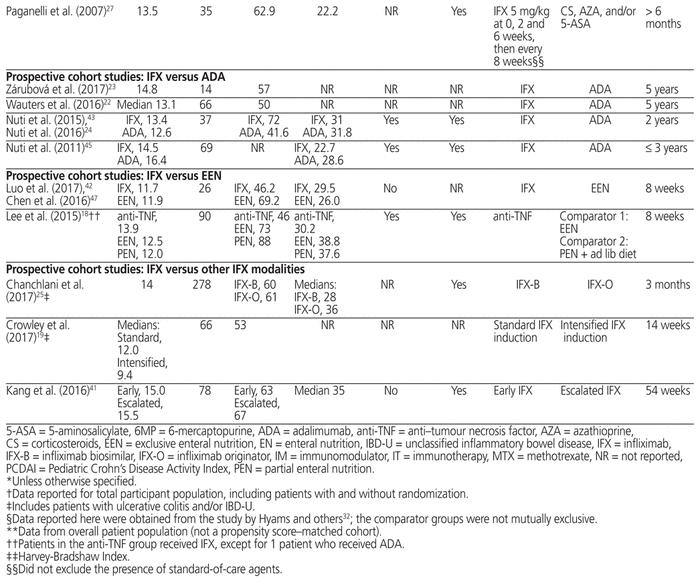
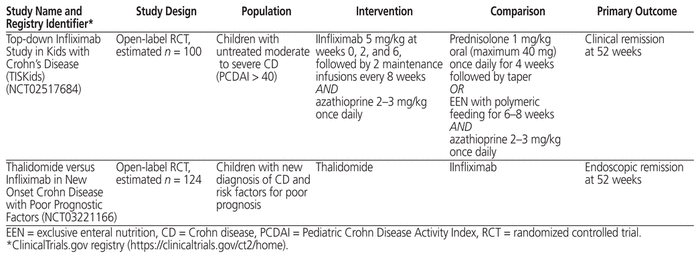
Table 2 Characteristics of Trials That Were in Progress at the Time of Analysis
In 7 of the 22 completed studies, the mean or median PCDAI was above 30, signifying moderate to severe disease.18,24,25,35–39,41,43,46 In 4 additional studies, only patients in the infliximab group had moderate to severe disease.20,21,30,40,44 In 6 studies, the mean or median PCDAI was between 10 and 30, which indicated mild disease on average.27,29,32–34,42,45,47 Five studies did not report disease severity.19,22,23,28,31 Eight studies included patients with fistulizing disease,18,24,26,29,35–39,40,43,45 although only 1 of these studies examined fistulizing disease in a separate analysis.39
The 3 RCTs compared different dose regimens of infliximab.26,35–39 One study examined the efficacy of a single induction dose of 1 mg/kg, 5 mg/kg, or 10 mg/kg,35–37 whereas the other 2 RCTs compared different maintenance regimens.26,38,39
Of the 19 prospective cohort studies, 2 studies compared infliximab with exclusive enteral nutrition,18,42,47 4 studies compared infliximab with adalimumab,22–24,43,45 3 studies compared different infliximab modalities (biosimilars versus originator,25 standard versus intensified induction,19 and early versus escalated therapy41), and 10 studies compared infliximab with other standard-of-care regimens.20,21,27–34,40,44,46 These regimens included other biologics,32–34 5-aminosalicylate,20,21,27,29,32–34 corticosteroids,27,29,32–34,46 azathioprine,20,21,27,29,31–34,44,46 6-mercaptopurine,20,21,32–34,44,46 methotrexate,20,21,32–34,46 antibiotics,32–34 and exclusive enteral nutrition.20,21,29 Three studies did not define or report standard therapy agents.28,30,40
Common outcomes examined were endoscopic remission,23,24,26,27,31,35,41–43 clinical remission,18,20–22,24,26,27,35,38–42,44 and clinical response.18,20,24,26,35,38–40,42 Other reported outcomes included partial endoscopic remission,21,24 changes in PCDAI27,45 or the Simple Endoscopic Score for Crohn Disease,42 height for age,20,27,44,46 body mass index for age,20 weight for age,20 quality of life,18 malignancy,32 serious infections,33 and adverse reactions.27,42 Studies varied in their outcome definitions (see Appendix 2, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/190/showToc): endoscopic remission or mucosal healing was defined using the Crohn Disease Index of Severity in 2 studies31,42 and using the Simple Endoscopic Score for Crohn Disease in 2 studies.24,41 One study used an endoscopic lesion severity score that involved a visual analogue scale,35 and another defined endoscopic remission as disappearance of ulcerations, multiple erosions, bleeding and friability.29 Clinical remission was defined as PCDAI ≤ 10 in 8 studies18,20,21,24,35,40–42 and Physician Global Assessment of inactive disease in 2 studies.22,40 Studies also varied in their definition of clinical response,24,28,35,38–40,42 although this was most commonly defined as reduction in PCDAI ≥ 15 or final PCDAI ≤ 10.18,42
Follow-up time ranged from 8 weeks18,42,47 to 5 years,22,23 with the median follow-up time being 54 weeks. For 8 studies, only abstracts were available for data extraction.19,22,23,25,28,31,44,45
Risk of Bias
One of the RCTs was rated as having a low risk of bias,35–37 whereas the other 2 RCTs were considered to have unclear risk of bias26,38,39 (see Appendix 3, parts A and B; available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/190/showToc).
Of the prospective cohort studies, 5 studies were rated as having a low risk of bias,20,21,24,32–34,40,41,43 11 studies as having unclear risk of bias,18,19,22,23,27,28,31,42,44–47 and 3 studies as having a high risk of bias25,29,30 (see Appendix 3, parts C and D).
Meta-analyses
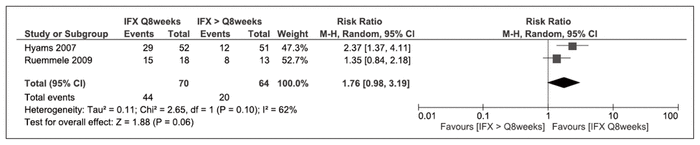
Maintenance of clinical remission at 1 year was reported by 2 RCTs.26,38 There was no significant difference between regimens of infliximab every 8 weeks and infliximab given less frequently (risk ratio [RR] 1.76, 95% confidence interval [CI] 0.98–3.19, I2 = 62%) (Figure 2).
| |
|
|
|
Figure 2 Forest plot examining maintenance of clinical remission in randomized controlled trials, as defined by Pediatric Crohn Disease Activity Index, at 1 year with infliximab (IFX) administered every 8 weeks compared with IFX administered less frequently.26,38 CI = confidence interval, M-H = Mantel-Haenszel analysis.
|
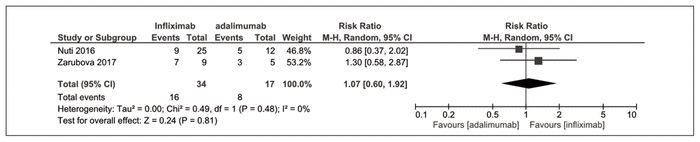
Maintenance of endoscopic remission at 6 to 12 months was reported by 2 nonrandomized trials.23,24 There was no significant difference between infliximab and adalimumab (RR 1.07, 95% CI 0.60–1.92, I2 = 0%) (Figure 3).
| |
|
|
|
Figure 3 Forest plot of prospective cohort studies examining maintenance of endoscopic remission at 6 to 12 months with infliximab compared with adalimumab.23,24 CI = confidence interval, M-H = Mantel-Haenszel analysis.
|
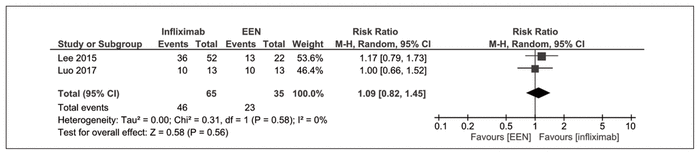
Induction of clinical remission at 8 weeks, as measured by PCDAI, was reported by 2 nonrandomized trials.18,42,47 There was no significant difference between infliximab and exclusive enteral nutrition (RR 1.09, 95% CI 0.82–1.45, I2 = 0%) (Figure 4).
| |
|
|
|
Figure 4 Forest plot of prospective cohort studies examining induction of clinical remission at 8 weeks with infliximab compared with exclusive enteral nutrition (EEN).18,42,47 CI = confidence interval, M-H = Mantel-Haenszel analysis.
|
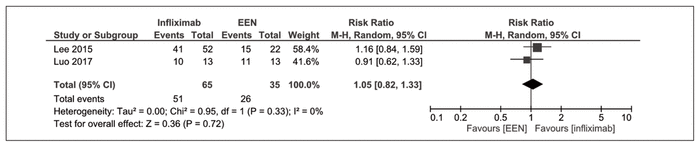
Clinical response at 8 weeks, as measured by PCDAI, was reported by 2 nonrandomized trials.18,42,47 There was no significant difference between infliximab and exclusive enteral nutrition (RR 1.05, 95% CI 0.82–1.33, I2 = 0%) (Figure 5).
| |
|
|
|
Figure 5 Forest plot of prospective cohort studies examining clinical response at 8 weeks with infliximab compared with exclusive enteral nutrition (EEN).18,42,47 CI = confidence interval, M-H = Mantel-Haenszel analysis.
|
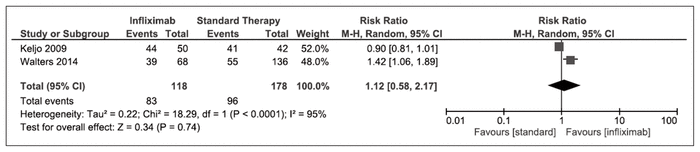
Maintenance of remission at 6 months, as measured by PCDAI, was reported by 2 nonrandomized studies.20,44 No significant difference was found between infliximab and standard of care (RR 1.12, 95% CI 0.58–2.17, I2 = 95%) (Figure 6).
| |
|
|
|
Figure 6 Forest plot of prospective cohort studies examining maintenance of clinical remission, as defined by Pediatric Crohn Disease Activity Index, at 6 months with infliximab compared with standard of care.20,44
CI = confidence interval, M-H = Mantel-Haenszel analysis.
|
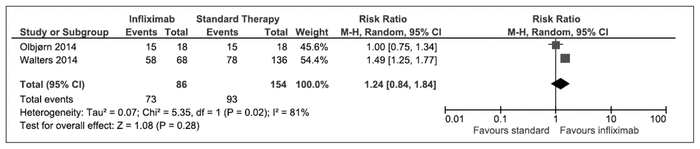
Maintenance of remission at 1 year, as measured by PCDAI, was reported by 2 nonrandomized studies.20,29 No significant difference was found between infliximab and standard of care (RR 1.24, 95% CI 0.84–1.84, I2 = 81%) (Figure 7).
| |
|
|
|
Figure 7 Forest plot of prospective cohort studies examining maintenance of clinical remission, as defined by Pediatric Crohn Disease Activity Index, at 1 year with infliximab compared with standard of care.20,29
CI = confidence interval, M-H = Mantel-Haenszel analysis.
|
Notable Studies Not Included in Meta-analysis
One RCT randomly assigned 21 pediatric patients with moderate to severe luminal or fistulizing Crohn disease to receive 1 dose of infliximab 1 mg/kg, 5 mg/kg, or 10 mg/kg.35–37 At week 4, the median decrease in endoscopic severity scores was 6.6%, 69.4%, and 52.2% in the 1 mg/kg, 5 mg/kg, and 10 mg/kg groups, respectively.35 Sixty-one percent of all patients had a clinical response, and 16.7% of all patients were in clinical remission by week 12.35 The study authors observed that the 5 and 10 mg/kg doses of infliximab were more effective than 1 mg/kg in achieving clinical remission.35
One nonrandomized study enrolled 78 pediatric patients with moderate to severe luminal Crohn disease of nonpenetrating, nonstricturing behaviour.41 Patients received either escalated combined immunosuppression, in which corticosteroid induction and azathioprine were trialled before escalation to infliximab, or early combined immunosuppression, in which infliximab and azathioprine were initiated within 1 month after diagnosis without corticosteroid induction.41 At week 14 from the first dose of infliximab, mucosal healing rates were higher in the early combined immunosuppression group, although no significant difference was observed between groups (32% versus 51%, p = 0.121).41 At week 54, mucosal healing rates were significantly higher in the early combined immunosuppression group (42% versus 74%, p = 0.007), although rates of clinical remission and laboratory remission did not differ significantly between groups.41 Z-scores for weight for age, height for age, and body mass index for age at weeks 15 and 54 did not significantly differ between groups.41 No significant difference in adverse effects was observed between the 2 groups (p = 0.804).41
A multicentre cohort study enrolled 5766 pediatric patients with inflammatory bowel disease, including 4047 with Crohn disease.32 When stratified by exposure to thiopurine agents, the infliximab cohort did not have an increased incidence (expressed in terms of events/100 patient-years) of malignancy compared with patients who received nonbiologic agents with thiopurines (0.53 [95% CI 0.14–1.35] versus 0.69 [95% CI 0.19–1.76]) and without thiopurines (0.31 [95% CI 0.01–1.75] versus 0.32 [95% CI 0.01–1.79]).32 There were 5 cases of hemophagocytic lymphohistiocytosis, all of which occurred during active thiopurine therapy; none involved exposure to infliximab, adalimumab, or methotrexate.32
A second report from the same cohort study included a subset of 5402 pediatric patients with inflammatory bowel disease.33 A greater cumulative incidence of serious infections (expressed in terms of events/100 patient-years) was reported in the infliximab cohort than the nonbiologics cohort (4.06 [95% CI 3.65–4.49] versus 2.25 [95% CI 1.92–2.61]), although the incidence of serious opportunistic infections was similar (0.35 [95% CI 0.24–0.5] versus 0.2 [95% CI 0.11–0.32]).33 In patients with Crohn disease, monotherapy with infliximab or corticosteroid and combination therapy including infliximab, immunomodulators, or corticosteroid were associated with increased risk of first serious infection.33
DISCUSSION
In the current analysis, the combined rates of clinical remission at 1 year were not significantly different between infliximab given every 8 weeks and infliximab given less frequently. This finding was interesting, given that the results of the individual trials were reported as statistically significant,26,38 and could be due to the difference in statistical methods between the current review and the study by Ruemmele and others.26 The heterogeneity in this outcome could be attributed to the different comparator regimens: whereas one study compared the standard regimen (5 mg/kg per dose every 8 weeks) to administration every 12 weeks,38 the other used infliximab on demand as the comparator.26
There were no significant differences in maintenance of clinical remission at 6 months and 1 year when infliximab was compared with standard of care. Of note, the 3 studies that were combined in this meta-analysis varied in their definitions of the comparator regimen: in one study, standard therapy consisted of immunomodulators,44 and in another, standard therapy was defined as corticosteroids, immunomodulators, aminosalicylates, and/or exclusive enteral nutrition.29 In the third study, “early anti-TNF-α therapy” (defined as initiation of anti-TNF-α within 3 months of diagnosis) was compared with “early immunomodulators” and “no early immunotherapy groups”.20 The differences in comparator group definitions likely contributed to the high heterogeneity for this outcome. In 2 of the 3 studies, patients exposed to infliximab had features of higher disease severity than patients exposed to standard therapy.29,44 In both studies, no adjustments were made to control for these differences in disease severity, which might have led to an outcome favouring the standard therapy group.29,44 In 1 of the 3 studies included in this meta-analysis, propensity score analysis was used to control for differences in disease severity.20 In this study, a significantly greater proportion of patients receiving early anti-TNF-α achieved clinical remission at 6 months and 1 year relative to patients receiving early immunotherapy or no early immunotherapy (p = 0.0003 and p = 0.036, respectively).20
The combined results of 2 prospective cohort studies demonstrated that infliximab was not significantly more effective than exclusive enteral nutrition in inducing clinical response or clinical remission at 8 weeks. This result was consistent with findings from the individual studies.18,42,47 Of note, the 2 studies were conducted in 2 different geographic locations (China and North America), and the patient population in the study by Luo and others42,47 had lower clinical disease severity than that of Lee and others.18
The combined results of 2 prospective cohort studies showed that infliximab was not significantly more effective than adalimumab in maintaining remission over a period of 6 to 12 months. This result was consistent with findings from the individual studies.23,24,43 Of note, Zárubová and others23 enrolled children with Crohn disease who had residual disease after ileocecal resection, whereas Nuti and others24,43 excluded patients who needed immediate surgery.
An older published systematic review of nonrandomized trials, including retrospective and noncomparative studies, summarized the risks of serious infection or lymphoma with anti-TNF-α agents in pediatric inflammatory bowel disease.48 The authors concluded that the rate of serious infections among pediatric patients treated with anti-TNF-α agents was similar to that of pediatric patients who received immunomodulator monotherapy, but lower than the expected rate for pediatric patients treated with corticosteroids.48 In contrast to these findings, the current systematic review found a recent prospective cohort study examining the risk of serious infections and malignancies in pediatric patients receiving infliximab over a median follow-up time of 4.7 years.32–34 This study concluded that there was an increased risk of serious infections in pediatric patients receiving infliximab relative to those receiving nonbiologic agents, although there was no increase in the risk of serious opportunistic infection.33
Dulai and others48 also found that the incidence of lymphoma was similar for pediatric patients receiving anti-TNF-α agents and the general pediatric population, but lower than for pediatric patients receiving thiopurine monotherapy. Hyams and others32,34 confirmed that there was no increase in the incidence of malignancy among pediatric patients receiving infliximab, relative to those receiving nonbiologic regimens, with stratification by thiopurine exposure.
The question of whether the more aggressive “top–down” approach should be favoured in certain patient populations is a current topic of debate in the field of pediatric Crohn disease.2,3 The top–down approach involves treatment with infliximab with or without concurrent immunomodulators early in the disease course, with the goal of attaining mucosal healing.3 The current review retrieved 2 prospective studies that compared the top–down approach with the conventional “step-up” approach: Walters and others20 demonstrated that in children newly diagnosed with severe Crohn disease, early monotherapy with anti-TNF-α agents (mainly infliximab) produced better clinical and growth outcomes at 1 year than early immunomodulators or no immunotherapy. Kang and others41 demonstrated that in children with moderate to severe luminal Crohn disease of nonpenetrating, nonstricturing behaviour, initiation of infliximab within 1 month after diagnosis yielded improved mucosal healing at 1 year compared with initiation of infliximab after failure of conventional therapy with corticosteroids. An ongoing RCT will compare the efficacy of top–down and step-up therapy in children with newly diagnosed moderate to severe Crohn disease.49
The current guidelines of the European Crohn’s and Colitis Organisation and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition recommend infliximab for induction and maintenance of remission in children with chronically active luminal Crohn disease despite prior optimized immunomodulator therapy, for induction of remission in children with steroid-refractory disease, or for primary induction and maintenance therapy for children with active perianal fistulizing disease.7 The current systematic review found no significant differences in efficacy between infliximab and standard of care regimens, exclusive enteral nutrition, or adalimumab in pediatric patients with luminal Crohn disease. However, the risk of bias in the existing literature, the small sample size, and the low number of studies available limited strong conclusions about this association. Our review has confirmed the current role of infliximab as second-line therapy in pediatric patients with luminal Crohn disease for whom conventional treatment has failed, until further evidence from RCTs and prospective cohort studies becomes available.
Future research should examine the comparative efficacy of infliximab (including biosimilars) using prospective study designs. Given the challenges of conducting RCTs in children,50 prospective comparative studies using registry data can be well designed to compare efficacy and safety between matched cohorts.51 Although mucosal healing is the gold standard for evaluation of disease remission, the invasiveness of endoscopic assessment limits its feasibility.8 The pediatric European Crohn’s and Colitis Organisation committee accepts clinical remission as a primary outcome for induction and maintenance of remission for drugs with demonstrated efficacy in mucosal healing in adult trials, as for infliximab.8 Primary outcomes should include induction and maintenance of endoscopic remission and corticosteroid-free clinical remission, and secondary outcomes should include growth, quality of life, and adverse effects over long-term follow-up.8
Limitations
Differences in study design and variability in definitions of standard therapy may have contributed to heterogeneity in some of the meta-analysis outcomes.
Unreported or incompletely reported outcomes and variability in outcome definitions limited the data available for meta-analysis. Although some trials reported endoscopic remission,23,24,41,42 variability in comparator groups meant that only 2 trials could be combined to examine this outcome.23,24
The RCT is considered the most scientifically rigorous study design for evaluating effectiveness of interventions.52 However, there was a lack of completed RCTs comparing infliximab with an active comparator for inclusion in this review. This could be explained by hesitancy to enroll pediatric patients in such trials, given their higher disease severity and ethical issues regarding consent.50 Several organizations—the European Crohn’s and Colitis Organisation; the European Society for Paediatric Gastroenterology, Hepatology and Nutrition; the Pediatric IBD Network for Research and Improvement; and the Canadian Children Inflammatory Bowel Disease Network—agree that placebo-controlled RCTs should not be conducted for a drug that has demonstrated superiority in adult studies.50 Prospective trial designs in which the comparator would be an active arm of established standard treatment should be considered.50
Most of the prospective comparative cohort studies included in this systematic review were deemed to have unclear or high risk of bias: 10 of the 19 studies did not adjust for confounding variables between study groups,19,23,25,29,30,31,40,42,44,45 which resulted in lack of comparability of cohorts at the start of the trial. Because 8 of the 19 studies were available only in format,19,22,23,25,28,31,44,45 outcome extraction and risk-of-bias assessment were limited. For many trials, length and adequacy of follow-up were unclear or were of concern.18,19,22,23,25,27,30–32,40,42,44–46 The majority of the included studies were limited by small sample size: of the 9 studies included in the quantitative analysis, only 2 examined more than 100 patients.20,38
Combination therapy with immunomodulators was not addressed by this systematic review and meta-analysis. There were limited comparative data available to consider the efficacy of biosimilar products and the role of infliximab levels in pediatric Crohn disease.
Supplementary Information
References
1 Conrad MA, Rosh JR. Pediatric inflammatory bowel disease. Pediatr Clin North Am. 2017;64(3):577–91.


2 Oliveira SB, Monteiro IM. Diagnosis and management of inflammatory bowel disease in children. BMJ. 2017;357:j2083.


3 Cozijnsen MA, Samsom JN, de Ridder L. Anti-tumour necrosis factor therapy for paediatric Crohn’s disease: improved benefits through treatment optimisation, deeper understanding of its risks, and reduced costs due to biosimilar availability. Pediatr Drugs. 2018;20(1):19–28.

4 Baldwin KR, Kaplan JL. Medical management of pediatric inflammatory bowel disease. Semin Pediatr Surg. 2017;26(6):360–6.


5 Kapoor A, Bhatia V, Sibal A. Pediatric inflammatory bowel disease. Indian Pediatr. 2016;53(11):993–1002.


6 Grossi V, Hyams JS. The safety of treatment options for pediatric Crohn’s disease. Expert Opin Drug Saf. 2016;15(10):1383–90.


7 Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8(10):1179–207.


8 Ruemmele FM, Hyams JS, Otley A, Griffiths A, Kolho KL, Dias JA, et al. Outcome measures for clinical trials in paediatric IBD: an evidence-based, expert-driven practical statement paper of the paediatric ECCO committee. Gut. 2015;64(3):438–46.

9 Infliximab product monograph. Toronto (ON): Janssen Inc; 2017 Aug 4.
10 De Zoeten EF, Pasternak BA, Mattei P, Kraer RE, Kader HA. Diagnosis and treatment of perianal Crohn disease: NASPGHAN clinical report and consensus statement. J Pediatr Gastroenterol Nutr. 2013;57(3):401–12.


11 Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.



12 Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Chichester (UK): The Cochrane Collaboration; 2011 [cited 2018 Aug 20]. Available from: http://www.handbook.cochrane.org
13 Mendeley [website]. Amsterdam (Netherlands): Elsevier Inc; 2017 [cited 2016 Dec 13]. Available from: http://mendeley.com
14 Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Chichester (UK): The Cochrane Collaboration; 2011 [cited 2018 Aug 20]. Available from: http://www.handbook.cochrane.org
15 The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute; 2014 [cited 2018 Aug 20]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
16 DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.


17 Hatala R, Keitz S, Wyer P, Guyatt G. Tips for learners of evidence-based medicine: 4. Assessing heterogeneity of primary studies in systematic reviews and whether to combine their results. CMAJ. 2005;172(5):661–5.



18 Lee D, Baldassano RN, Otley AR, Albenberg L, Griffiths AM, Compher C, et al. Comparative effectiveness of nutritional and biological therapy in North American children with active Crohn’s disease. Inflamm Bowel Dis. 2015;21(8):1786–93.


19 Crowley E, Carman N, Arpino V, Frost K, Ricciuto A, Sherlock M, et al. Early use of therapeutic drug monitoring to individualize infliximab therapy in paediatric IBD: a multicentre prospective cohort study [abstract]. Gastroenterology. 2017;152(5 Suppl 1):S218–S219.

20 Walters TD, Kim MO, Denson LA, Griffiths AM, Dubinsky M, Markowitz J, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn’s disease. Gastroenterology. 2014;146(2):383–91.

21 Hyams JS, Kim MO, Denson LA, Griffiths AM, Dubinsky M, Markowitz J, et al. Early anti-TNF therapy is superior to early immunomodulator therapy in newly diagnosed children with Crohn’s disease [abstract]. Gastroenterology. 2013;144(5 Suppl 1):S146.

22 Wauters L, Smets F, Paul J, Vermeire S, Veereman G. Characteristics of children with Crohn’s disease failing sustained remission despite anti-TNF exposure [abstract]. J Pediatr Gastroenterol Nutr. 2016;63 Suppl 2:S231–S232.
23 Zárubová K, Hradsky O, Copova I, Škába R, Rousková B, Poš L, et al. Endoscopic relapse-rate in Crohn’s disease patients treated postoperatively by anti-TNF for residual disease [abstract]. J Pediatr Gastroenterol Nutr. 2017;64 Suppl 1:556.
24 Nuti F, Civitelli F, Bloise S, Oliva S, Aloi M, Latorre G, et al. Prospective evaluation of the achievement of mucosal healing with anti-TNF-α therapy in a paediatric Crohn’s disease cohort. J Crohns Colitis. 2016; 10(1):5–12.

25 Chanchlani N, Mortier K, Willmott A, Rodrigues A, Wilson D, Puntis J, et al. Equivalent effectiveness and short term safety of infliximab biosimilars mean savings of 1,000,000 euro lost in first year; now is the time for universal adoption [abstract]. J Pediatr Gastroenterol Nutr. 2017;64 Suppl 1:459–60.
26 Ruemmele FM, Lachaux A, Cézard JP, Morali A, Maurage C, Giniès JL, et al. Efficacy of infliximab in pediatric Crohn’s disease: a randomized multicenter open-label trial comparing scheduled to on demand maintenance therapy. Inflamm Bowel Dis. 2009;15(3):388–94.

27 Paganelli M, Albanese C, Borrelli O, Civitelli F, Canitano N, Viola F, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2007; 13(4):416–23.


28 Mangiantini F, Stagi S, Russo ML, Pela I, de Martino M, Seminara S, et al. Does disease treatment in influence bone mineral density in children and adolescents with inflammatory bowel disease? [abstract]. Horm Res. 2013;80 Suppl 1:S222–S223.
29 Olbjørn C, Nakstad B, Småstuen MC, Thiis-Evensen E, Vatn MH, Perminow G. Early anti-TNF treatment in pediatric Crohn’s disease. Predictors of clinical outcome in a population-based cohort of newly diagnosed patients. Scand J Gastroenterol. 2014;49(12):1425–31.


30 Bellizzi A, Anzivino E, Ferrari F, Di Nardo G, Colosimo MT, Fioriti D, et al. Polyomavirus JC reactivation and noncoding control region sequence analysis in pediatric Crohn’s disease patients treated with infliximab. J Neurovirol. 2011;17(4):303–13.


31 Muhammed R, Bremner R, Theodoric W, Protheroe S, Murphy S. Mucosal healing in children with Crohn’s disease on long term maintenance treatment [abstract]. J Crohns Colitis. 2014;8 Suppl 1:S235.

32 Hyams JS, Dubinsky MC, Baldassano RN, Colletti RB, Cucchiara S, Escher J, et al. Infliximab is not associated with increased risk of malignancy or hemophagocytic lymphohistiocytosis in pediatric patients with inflammatory bowel disease. Gastroenterology. 2017;152(8): 1901–1914.e3.


33 Escher J, Faubion W, Baldassano RN, Colletti RB, Cucchiara S, Dubinsky MC, et al. Risk of serious infection in pediatric inflammatory bowel disease: results from the DEVELOP registry [abstract]. J Pediatr Gastroenterol Nutr. 2016;62 Suppl 1:57–8.
34 Dubinsky MC, Hyams JS, Baldassano RN, Colletti RB, Cucchiara S, Escher J, et al. Risk of malignancy in paediatric inflammatory bowel disease: results from the DEVELOP registry [poster abstract]. 11th Congress of the European Crohn’s and Colitis Organization; 2016 Mar 16–19; Amsterdam, The Netherlands.
35 Baldassano R, Braegger CP, Escher JC, DeWoody K, Hendricks DF, Keenan GF, et al. Infliximab (Remicade) therapy in the treatment of pediatric Crohn’s disease. Am J Gastroenterol. 2003;98(4):833–8.


36 Hadigan C, Baldassano R, Braegger C, Vasiliauskis E, Escher J, Sinaasappel M, et al. Pharmacokinetics of infliximab (anti-TNFα) in children with Crohn’s disease: a multicenter trial [abstract]. J Pediatr Gastroenterol Nutr. 1999;29(4):525.

37 Escher JC, van den Brink G, Kate FT, te Velde A, van Deventer S. Mucosal healing and down-regulation of inflammation with anti-tumor necrosis factor α (infliximab) in children with refractory Crohn’s disease [abstract]. J Pediatr Gastroenterol Nutr. 2000;31 Suppl 2:S19.
38 Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007; 132(3):863–73.


39 Crandall W, Hyams J, Kugathasan S, Griffiths A, Zrubek J, Olson A, et al. Infliximab therapy in children with concurrent perianal Crohn disease: observations from REACH. J Pediatr Gastroenterol Nutr. 2009;49(2): 183–90.


40 Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Kugathasan S, Evans J, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn’s disease. Inflamm Bowel Dis. 2009;15(6):816–22.

41 Kang B, Choi SY, Kim HS, Kim K, Lee YM, Choe YH. Mucosal healing in paediatric patients with moderate-to-severe luminal Crohn’s disease under combined immunosuppression: escalation versus early treatment. J Crohns Colitis. 2016;10(11):1279–86.


42 Luo Y, Yu J, Lou J, Fang Y, Chen J. Exclusive enteral nutrition versus infliximab in inducing therapy of pediatric Crohn’s disease. Gastroenterol Res Pract. 2017;2017:6595048.

43 Nuti F, Viola F, Aloi M, Civitelli F, Oliva S, Cucchiara S. Impact of mucosal healing on the clinical course in a cohort of paediatric patients affected by Crohn’s disease [abstract]. J Crohn Colitis. 2015;9 Suppl 1:S352–S353.

44 Keljo DJ, Markowitz J, Langton CR, Lerer T, Bousvaros A, Carvalho RS, et al. Growth in pediatric patients with IBD treated with infliximab or 6-mercaptopurine/azathioprine [abstract]. Gastroenterology. 2009;136(5 Suppl 1):A673.

45 Nuti F, Alessandri C, Conte F, Civitelli F, Aloi M, Del Giudice E, et al. Outcome of biological therapy in pediatric inflammatory bowel disease at a single tertiary center [abstract]. Gastroenterology. 2011;140(5 Suppl 1):S510.

46 Pfefferkorn M, Burke G, Griffiths A, Markowitz J, Rosh J, Mack D, et al. Growth abnormalities persist in newly diagnosed children with Crohn disease despite current treatment paradigms. J Pediatr Gastroenterol Nutr. 2009;48(2):168–74.


47 Chen J, Luo Y. Comparative effectiveness of infliximab and exclusive enteral nutrition therapy in pediatric Crohn’s disease [abstract]. J Pediatr Gastroenterol Nutr. 2016;63 Suppl 2:S365.
48 Dulai PS, Thompson KD, Blunt HB, Dubinsky MC, Siegel CA. Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol. 2014;12(9):1443–51.


49 Cozijnsen MA, van Pieterson M, Samsom JN, Escher JC, de Ridder L. Top-down infliximab study in kids with Crohn’s disease (TISKids): an international multicentre randomised controlled trial. BMJ Open Gastro. 2016;3(1):e000123.

50 Turner D, Koletzko S, Griffiths AM, Hyams J, Dubinsky M, de Ridder L, et al. Use of placebo in pediatric inflammatory bowel diseases: a position paper from ESPGHAN, ECCO, PIDBnet, and the Canadian Children IBD Network. J Pediatr Gastroenterol Nutr. 2016;62(1):183–7.

51 Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9.


52 Evans D. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare interventions. J Clin Nurs. 2003;12(1):77–84.


Sophia Li, PharmD, RPh, was, at the time this study was initiated, with the Pharmacy Department, London Health Sciences Centre, London, Ontario. She is now is a Clinical Pharmacist with the Pharmacy Department, Providence Healthcare, Vancouver, British Columbia
Christopher Reynaert, BScPhm, RPh, is a Pharmacist with the Pharmacy Department, London Health Sciences Centre, London, Ontario
Annie Ling Su is a candidate in the PharmD program of the Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, British Columbia
Sonja Sawh, BScPhm, RPh, ACPR, was, at the time this review was initiated, the Evidence-Based Medicine Pharmacist with the Pharmacy Department, London Health Sciences Centre, London, Ontario. She is now Clinical Director, Pharmacy Services, with Mohawk Medbuy Corporation, London, Ontario
Competing interests: None declared. (
Return to Text
)
Address correspondence to: Dr Sophia Li, Pharmacy Department, Providence Healthcare, 1081 Burrard Street, Vancouver BC V6Z 1Y6, e-mail:sli12@providencehealth.bc.ca
(Return to Top)
Funding: Funding was provided by the authors’ employer (London Health Sciences Centre) through the course of employment. (
Return to Text
)
Acknowledgements
The authors thank colleagues in the Pharmacy Department at London Health Sciences Centre, who provided feedback that assisted with the systematic review. They thank Dhandapani Ashok and Kevin Bax for their insights as content experts, and are grateful to Elizabeth Wong and Florence Chan for comments that greatly improved the manuscript. The authors also thank Brenda Sampson, who assisted with the screening stage of this review, and Sandra McKeown, who assisted with development of the search strategy.
Canadian Journal of Hospital Pharmacy, VOLUME 72, NUMBER 3, May-June 2019












