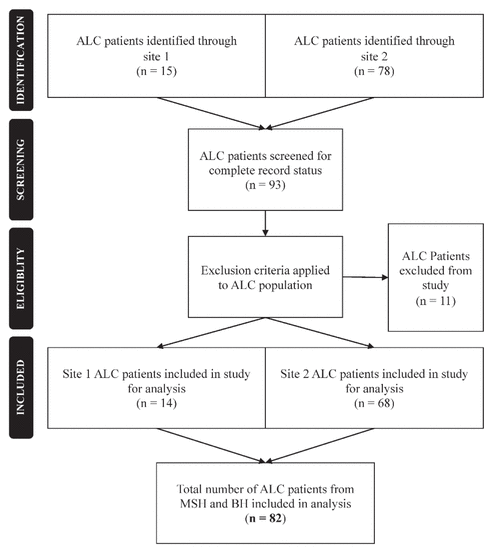
Figure 1 Flow chart of inclusion and exclusion criteria for study participants. ALC = alternate level of care, BP = Bridgepoint Active Healthcare, MSH = Mount Sinai Hospital.
Mehrdad Azimi, Lisa Burry, Christinne Duclos, Jordan Pelc, Jason X Nie, Ross UpshurABSTRACT
Background
The population of patients designated as alternate level of care (ALC) consists predominantly of frail older adults who are medically stable and awaiting discharge from hospital. They have complex medication regimens, often including potentially inappropriate medications (PIMs). There has been increasing emphasis on managing the burden that ALC patients place on the health care system, but little is known about their health care needs.
Objective
To characterize the medication regimens, including use of PIMs, of ALC patients at the study institution.
Methods
A cross-sectional chart audit of ALC patients was conducted between May and July 2017. For all patients in the sample, each medication was categorized by therapeutic class, and PIMs were categorized according to the Beers criteria, the STOPP/START criteria, and an established list of high-alert medications.
Results
A total of 82 patients met the audit criteria, for whom the mean number of chronic conditions was 6.4 (standard deviation [SD] 3.3) and the mean number of prescribed medications was 12.8 (SD 6.9). Twenty-four (29%) of the patients were receiving at least 1 drug from 7 different drug classes. All but one of the patients had PIMs in their regimen; the frequency of PIMs was highest according to the Beers criteria (mean 3.9 [SD 2.6] medications per patient).
Conclusions
At the study institution, ALC patients had on average more than 6 chronic conditions managed with at least 12 medications, of which one-quarter were PIMs. These data will be used to inform next steps in making recommendations to simplify, reduce, or discontinue medications for which there is an unclear indication, lack of effectiveness, or evidence of potential harm.
KEYWORDS: alternate level of care, polypharmacy, potentially inappropriate medications, older adults
RÉSUMÉ
Contexte
La population de patients désignés comme « niveaux de soins alternatifs » (NSA) se compose majoritairement d’aînés faibles, médicalement stables et en attente de leur congé hospitalier. Ils suivent des traitements médicamenteux complexes qui comprennent souvent des médicaments potentiellement contre-indiqués (MPCI). L’accent a été progressivement mis sur la gestion du fardeau que les patients NSA font peser sur le système de soins de santé, mais on connait peu de choses sur leurs besoins en matière de soins de santé.
Objectif
Décrire les traitements médicamenteux, y compris l’utilisation des MPCI, des patients NSA dans l’institution où s’est déroulée l’étude.
Méthodes
Une vérification transversale des dossiers de patients NSA a été menée entre mai et juillet 2017. Chaque médicament pris par les patients de l’échantillon a été classé selon sa catégorie thérapeutique, et les MPCI ont été catégorisés selon les critères de Beers, les critères STOPP/START ainsi qu’une liste établie de médicaments dont le niveau d’alerte est élevé.
Résultats
Au total, 82 patients remplissaient les critères de l’audit, car le nombre moyen de maladies chroniques était de 6,4 (écart type [ET] 3,3) et le nombre moyen de médicaments prescrits se montait à 12,8 (ET 6,9). Vingt-quatre (29 %) patients recevaient au moins un médicament de sept classes médicamenteuses différentes. Tous les patients sauf un avaient des MPCI dans leur programme. La fréquence des MPCI était plus élevée selon les critères de Beers (moyenne de MPCI par patient de 3,9 [ET 2,6]).
Conclusions
Sur le lieu de l’étude, les patients NSA avaient en moyenne plus de six maladies chroniques gérées à l’aide d’au moins 12 médicaments, dont un quart était des MPCI. Ces données seront utilisées pour informer les cliniciens sur les étapes suivantes et formuler des recommandations afin de simplifier, de réduire ou d’arrêter les médicaments pour lesquels l’indication n’est pas claire, dont l’efficacité est insuffisante ou sur lesquels il existe des données probantes faisant état de dangers potentiels.
MOTS CLÉS: autres niveaux de soins, polypharmacie, médicaments potentiellement contre-indiqués, ainés
Research indicates that older adults are taking increasingly more medications.1 Although there is disagreement about the best definition, polypharmacy is often defined as 5 or more medications for the same patient.2 Polypharmacy is known to increase the risk of adverse drug events, drug–drug and drug–disease interactions, nonadherence, inappropriate prescribing, falls, hospitalization, and death.3 In addition, polypharmacy may lead to medication wastage and a burden to society in terms of health care spending.4
Over the past 2 decades, methods have been developed to standardize the assessment of medication appropriateness for older adults, with the goal of reducing inappropriate prescribing and thus improving patients’ outcomes.5 These established methods include the Beers Criteria for Potentially Inappropriate Medication (PIM) Use in Older Adults,6 the STOPP/START criteria,7 and high-alert medications as identified by the Institute for Safe Medication Practices (US).8 Nonetheless, inappropriate medication use is common, especially in the setting of multimorbidity and polypharmacy.9,10
One population that may be particularly at risk for polypharmacy and exposure to PIMs consists of hospitalized patients who are medically appropriate for discharge from hospital, but who remain in hospital awaiting discharge disposition. Prolonged stay in hospital for disposition reasons, rather than clinical need, is known as alternate level of care (ALC).11 These patients are at risk of polypharmacy because of their advanced age, multiple chronic conditions, and the prolonged hospital stay. The ALC burden on Canadian health care is large and growing, representing 5% of all hospitalizations and contributing 14% of hospital days in acute care settings across Canada (data from 2009).11 ALC patients are mainly older adults (≥ 65 years of age) with frailty and cognitive or behavioural problems.12–14 Although the ALC population is growing, to date there have been few studies examining the medication regimens of these patients, and only one has investigated the potential appropriateness of medications.15 The objective of the current study was to present a descriptive analysis of medication regimens used by ALC patients in a large tertiary care centre and to characterize the potential inappropriateness of medications prescribed for this patient population.
Between May and July 2017, we conducted a cross-sectional audit of patients who were designated ALC at 2 of our institutional sites, Mount Sinai Hospital and Bridge-point Active Healthcare, in Toronto, Ontario. This quality improvement study was intended to gain a baseline understanding of medication use in this population and to help design future intervention work. Ethics approval was not required for the purpose of this quality improvement study.
Patients designated as ALC in the hospital’s computer system between May and July 2017 were considered for inclusion in this study. Patients with incomplete data and those missing one or more health-related reports from the electronic database (e.g., preadmission medications, ongoing chronic conditions) were excluded from the study. Patients who were discharged before assessment or for whom medication data were no longer available in the electronic database were also excluded.
Data were extracted from electronic records. For each individual patient, complete records were collected within a single day, providing a synopsis of their health status parameters. The research team designed an electronic case report based on previous work by the team. For each patient, the following data were extracted from the chart: demographic characteristics, reason for admission, expected postdischarge destination, length of stay in acute care hospital (calculated from admission date to audit date), length of ALC stay (calculated from date of ALC designation to audit date), history of falls, most current Morse fall risk,16 and preadmission health status (based on admission notes, including number of chronic conditions). For each patient, we calculated the complexity score (sum of number of chronic conditions and number of medications).17 We also extracted details of the medications in use at the time of admission to hospital from the best possible medication history and details of all medications given during the hospital stay (e.g., drug name, dose and frequency, and pill burden) from the medication administration record on the day of the audit. Pill burden was defined as the cumulative number of all solid oral dosage formulations prescribed per patient, which is a measure of the burden placed on a patient to take a specified number of solid oral medications (e.g., tablets, capsules). Data were entered into an Excel spreadsheet (Microsoft Corporation, Redmond, Washington), with standardized data entry using categorization by searchable drop-down menus.
The medications were coded in terms of specific drug classes generated by the team (based on general use rather than an official classification). The following specific drug classes were used: psychotropic, cardiovascular, hematologic, endocrine, analgesic/anti-inflammatory, anti-infective, genital/urinary, respiratory, musculoskeletal, topical, supplements/natural health products, gastrointestinal (including bowel routines), and “other”. An exhaustive list of these medication classes, as well as the specific drugs belonging to each class, is provided in Appendix 1 (available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/191/showToc). Medication appropriateness was assessed according to the following criteria for inappropriate medications: Beers criteria,6 STOPP/START criteria,7 and high-alert medications.8 The medications identified by these criteria may not be appropriate for certain patient populations (e.g., older adults with multimorbid conditions). Any medication used by a patient in the study sample that appeared on any of these lists was flagged as a PIM and was included in the analysis. This process was not intended to assess the clinical relevance of the prescribed medications, but rather to collect information on medications that might be deemed inappropriate according to the inappropriate medication references.6–8 In addition, flagged PIMs were not based on the wrong classes of medications being prescribed for specific conditions or a lack of prescribing. The proportion of each patient’s regimen represented by PIMs was calculated using each of the Beers criteria, the STOPP/START criteria, and the list of high-alert medications separately, and also using a combination of all 3 lists. Descriptive statistics, such as mean and standard deviation (SD) or median and interquartile range (IQR), were calculated as appropriate.
Of 93 patients with an ALC designation in the computer system, 82 patients (14 at Mount Sinai Hospital, 68 at Bridgepoint) met the inclusion criteria (Figure 1). The remaining 11 patients were excluded because health status information was incomplete upon audit. Forty-three (52%) of the patients were women, and the overall mean age was 75.6 (SD 15.1) years (Table 1). The mean number of chronic conditions before admission to hospital was 6.4 (SD 3.3) per patient, for which a mean of 12.8 (SD 6.9) distinct medications had been prescribed. A history of falls in the past 3 months was common (78% [64/81]), and 73% (59/81) of the patients were still considered to be at high risk of falling.
|
|
||
|
Figure 1 Flow chart of inclusion and exclusion criteria for study participants. ALC = alternate level of care, BP = Bridgepoint Active Healthcare, MSH = Mount Sinai Hospital. |
||
Table 1 Demographic Characteristics of Participants
Data about prescriptions for these ALC patients are presented in Table 2. The mean number of medications per patient at the time of the audit was 17.6 (SD 5.2), and 64.7% of the prescriptions (943/1458) were scheduled orders (i.e., not for as-needed administration). The overall mean daily pill burden, which represented solid oral dosage formulations for all standing and PRN prescriptions, was 18.5 (SD 9.5) per patient, whereas the mean daily pill burden for standing orders alone was 10.1 (SD 6.3) per patient.
Table 2 Characteristics of Prescriptions
The most frequently prescribed medication class was gastrointestinal drugs (mean 4.8 [SD 1.8] drugs per patient). Laxatives used for routine bowel preparation accounted for most of this class (mean 3.83 laxatives prescribed per patient; see Appendix 2, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/191/showToc). Supplements, cardiovascular drugs, and psychotropic agents were the next most frequently prescribed classes, with means of 2.8 (SD 1.6), 2.7 (SD 1.7), and 2.5 (SD 1.5) medications per patient, respectively (Table 3). Most psychotropics used by these patients were antipsychotics (36 patients) or antidepressants (31 patients). Figure 2 shows the proportion of patients by number of medication classes in their individual regimens. For 24 (29%) of the 82 patients, at least 1 drug from 7 different classes of medications was prescribed for concurrent use, and some patients had medications from 8 or 9 different classes (14/82 [17%] in each case). None of the patients were receiving drugs from fewer than 3 medication classes.
|
|
||
|
Figure 2 Frequency of concurrent medication classes used for alternate level of care patients. |
||
Depending on the particular list used to analyze PIMs, between 68 (83%) and 80 (98%) of the patients studied were receiving at least 1 PIM. Only 1 (1%) of the 82 patients in this study did not have a single medication on any of the 3 lists used here to identify PIMs (Figure 3). The number of PIMs ranged from 0 to 12 according to the Beers criteria (mean 3.9, SD 2.6), from 0 to 7 according to the STOPP/START criteria (mean 2.4, SD 1.9), and from 0 to 6 according to the ISMP list of high-alert medications (mean 1.4, SD 1.2) (Figure 4). Most prescribed PIMs belonged to the psychotropics, including antipsychotics, of which quetiapine accounted for 33 (2.26%) of the 1458 medications prescribed for the 82 patients. Appendix 3 (available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/191/showToc) lists the most commonly prescribed medications in our ALC cohort. A breakdown by medication class for the psychotropic and gastrointestinal medication classes is also provided in Appendix 2.
|
|
||
|
Figure 3 Distribution of patients with potentially inappropriate medications (n = 82 patients). |
||
|
|
||
|
Figure 4 Proportion of patients with potentially inappropriate medications (PIMs), according to various criteria (n = 82 patients). Beers = Beers Criteria,6 HAM = high-alert medications,8 S/S = STOPP/START criteria.7 |
||
This study was an audit of medication use by ALC patients admitted to the study institution between May and July 2017, with the aim of describing medication use and PIM exposure. Polypharmacy was common and exposure to PIMs was high in this cohort of ALC patients; these factors are known to put patients at high risk of adverse drug events, falls, medical complications, potential future repeat hospitalization, and death.18,19 The typical characteristics of ALC patients, such as age, frailty status, and relatively high cognitive impairment, suggests that many of these patients will need assistance with their medications. High pill burden, with or without PRN orders, also contributes to the need for ALC patients to have assistance in order to take their medications accurately. As members of our team have previously suggested, one solution might be consolidation of the medication regimen and reduction of regimen complexity.20
In the context of older adults with multimorbidity, application of clinical guidelines often results in polypharmacy, fostering complications and adverse reactions.21 We cross-referenced the medication regimens of these ALC patients with 3 lists that are frequently used to assess for PIMs in older adults. In the medication regimens of these ALC patients, PIMs were most frequent according to the Beers criteria, possibly because of the comprehensiveness of those criteria. At least 1 PIM from the Beers list was included in the medication regimen of 98% of the cohort, and only 1 patient had no PIMs in the medication regimen; these findings indicate a potential opportunity to improve prescribing in the ALC cohort at our institution. Although this work did not consider the clinical relevance of PIMs, our overview of the ALC medication regimen suggests that a more thorough review is needed to ensure appropriate prescribing.
Of particular interest, we found that psychotropic drugs were the third most commonly prescribed drug class, with about three-quarters of the ALC patients in our sample receiving at least one psychotropic drug. Antipsychotic use increases the risk of stroke, cognitive decline, and death among older persons with dementia.6 In addition, antipsychotic use by older adults may increase the risk of ventricular arrhythmia and cerebrovascular events, leading to a higher risk of death.22
Most of the patients in this study were at high risk of falling, and the association between psychotropic drugs and increased risk of falls among older adults has been well described.23–25 Furthermore, it has been shown that deprescribing medications associated with an increased risk of falls significantly lowers the incidence of falls,24 which suggests an opportunity for intervention in our institution.
We examined patients’ drug therapy regimens in detail and categorized exposure to PIMs using all of the commonly used scoring systems available. Our work was limited by the lack of a control group for comparison, by the retrospective study design, and by having a nonrandomized convenience sample, whereby individuals were predetermined for analysis. In addition, the indications for each medication were rarely documented, which limited our ability to discern clinical appropriateness.
ALC patients face many chronic conditions, which contribute to the complexity of their health status and for which large numbers of medications are prescribed. In this setting, prescribing often results in polypharmacy. The concerning presence of PIMs in the medication regimens of ALC patients warrants further attention. Psychotropics were the most commonly prescribed PIMs in the ALC cohort described here, and next steps for the study institution include developing and implementing initiatives to minimize their inappropriate use in this population, such as the introduction of deprescribing algorithms. More generally, the ALC population is only expected to grow, as life expectancy increases over the next couple of decades, along with new treatments for an array of chronic conditions; further work is needed to optimize prescribing safety for this population. Given that ALC patients have a high medication burden, which may include PIMs, there is an opportunity for pharmacists to become involved in deprescribing and optimizing medication use.
1 Drug use among seniors in Canada, 2016. Ottawa (ON): Canadian Institute for Health Information; 2018 [cited 2019 Jul 18]. Available from: https://www.cihi.ca/sites/default/files/document/drug-use-among-seniors-2016-en-web.pdf
2 Hilmer SN, Gnjidic D. The effects of polypharmacy in older adults. Clin Pharmacol Ther. 2009;85(1):86–8.
3 Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–72.

4 Woodward MC. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33(4): 323–8.
5 Gnjidic D, Tinetti M, Allore HG. Assessing medication burden and polypharmacy: finding the perfect measure. Expert Rev Clin Pharmacol. 2017;10(4):345–7.

6 Samuel MJ. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46.
7 O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Aging. 2015;44(2):213–8.
8 ISMP’s list of high-alert medications in community/ambulatory settings. Horsham (PA): Institute for Safe Medication Practices; 2011 [cited 2019 Jul 18]. Available from: https://www.ismp.org/recommendations/high-alert-medications-community-ambulatory-list
9 Steinman MA, Landefeld CS, Rosenthal GE, Berthenthal D, Sen S, Kaboli PJ. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54(10):1516–23.
10 Fick DM, Waller JL, Maclean JR, Heuvel RV, Tadlock JG, Gottlieb M, et al. Potentially inappropriate medication use in a medicare managed care population: association with higher costs and utilization. J Manag Care Pharm. 2001;7(5):407–13.
11 Alternate level of care in Canada. Ottawa (ON): Canadian Institute for Health Information; 2009 [cited 2019 Jul 18]. Available from: https://secure.cihi.ca/free_products/ALC_AIB_FINAL.pdf
12 Costa AP, Hirdes JP. Clinical characteristics and service needs of alternate-level-of-care patients waiting for long-term care in Ontario hospitals. Healthc Policy. 2010;6(1):32–46.
13 Costa AP, Poss JW, Peirce T, Hirdes JP. Acute care inpatients with long-term delayed-discharge: evidence from a Canadian health region. BMC Health Serv Res. 2012;12(1):172.

14 Manville M, Klein MC, Bainbridge L. Improved outcomes for elderly patients who received care on a transitional care unit. Can Fam Physician. 2014;60(5):263–71.
15 Slaney H, MacAulay S, Irvine-Meek J, Murray J. Application of the Beers criteria to alternate level of care patients in hospital inpatient units. Can J Hosp Pharm. 2015;68(3):218–25.

16 Morse JM, Morse RM, Tylko SJ. Development of a scale to identify the fall-prone patient. Can J Aging. 1989;8(4):366–75.
17 Upshur RE, Wang L, Nie JX, Tracy SC. The complexity score: towards a clinically-relevant, clinician-friendly measure of patient multi-morbidity. Int J Person Cent Med. 2012;2(4):799–804.
18 Leelakanok N, Holcombe AL, Lund BC, Gu X, Schweizer ML. Association between polypharmacy and death: a systematic review and meta-analysis. J Am Pharm Assoc (2003). 2017;57(6):729–38.e10.
19 Wastesson JW, Morin L, Tan ECK, Johnell K, Wastesson JW. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17(12):1185–96.
20 Fuller J, Upshur R. Medication regimen complexity and the care of the chronically ill patient. Int J Person Cent Med. 2011;1(4):719–25.
21 Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients. N Engl J Med. 2014; 294(6):716–24.
22 Wang PS, Schneeweiss S, Setoguchi S, Patrick A, Avorn J, Mogun H, et al. Ventricular arrhythmias and cerebrovascular events in the elderly using conventional and atypical antipsychotic medications. J Clin Psychopharmacol. 2007;27(6):707–10.
23 Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999;47(1):30–9.
24 van der Velde N, Stricker BHC, Pols HAP, van der Cammen TJM. Risk of falls after withdrawal of fall-risk-increasing drugs: a prospective cohort study. Br J Clin Pharmacol. 2007;63(2):232–7.
25 Browne C, Kingston C, Keane C. Falls prevention focused medication review by a pharmacist in an acute hospital: implications for future practice. Int J Clin Pharm. 2014;36(5):969–75.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors are grateful for the support of Janice Takata-Shewchuk and Kori Bilben, Department of Pharmacy, Bridgepoint Active Healthcare.
Canadian Journal of Hospital Pharmacy, VOLUME 72, NUMBER 4, July-August 2019