
Jia (Shermaine) Ngo, Man Hon (Mark) HoABSTRACT
Background
Rasburicase, a recombinant urate oxidase, is restricted in the Fraser Health Authority (FHA) to the “treatment of acute or at high risk of tumour lysis syndrome [TLS], when other therapeutic options are not suitable”. The manufacturer’s recommended dosage is 0.2 mg/kg daily for up to 7 days. Given the high cost of this drug, several studies have investigated other strategies and found that a single dose, repeated as needed, is effective in reducing serum uric acid. However, there are currently no guidelines in FHA for the use of rasburicase, which may result in different prescribing practices within the health authority.
Objectives
To describe the prescribing of rasburicase in FHA, including indications and doses, and to report the uric acid–lowering effects of rasburicase and any clinical outcomes, such as dialysis or death.
Methods
This retrospective descriptive chart review included adult patients receiving care in FHA for whom rasburicase was prescribed between June 1, 2010, and November 30, 2016. Descriptive statistics were used to summarize patient characteristics and results.
Results
The prescribing practices for rasburicase in this health authority were largely inconsistent, but the most common dose administered was 3 mg (8/12 [67%] among those receiving rasburicase for prophylaxis and 9/32 [28%] among those receiving rasburicase for treatment; combined total 17/44 or 39%). Regardless of dose, rasburicase reduced serum uric acid levels to less than 476 μmol/L and decreased the risk of TLS.
Conclusions
Having a uniform approach—involving a single dose that can be repeated as needed—for prevention and treatment of elevated serum uric acid levels could result in sufficient reduction of uric acid levels with fewer doses and lower cost. The results of this study support the need for a resource in FHA to guide and standardize the use of rasburicase.
KEYWORDS: rasburicase, tumour lysis syndrome, uric acid, hyperuricemia
RÉSUMÉ
Contexte
La Fraser Health Authorty limite l’usage de la rasburicase, une urate oxydase recombinée, au « traitement du syndrome de lyse tumorale (SLT) aigu ou comportant un risque élevé, lorsque les autres options thérapeutiques ne conviennent pas ». Le fabricant recommande une dose quotidienne de 0,2 mg/kg pendant une durée allant jusqu’à sept jours. Étant donné le coût élevé de ce médicament, plusieurs études ont exploré d’autres stratégies et ont permis de conclure qu’une dose unique, répétée au besoin, était efficace pour réduire le taux sérique d’acide urique. Cependant, il n’existe actuellement aucune ligne directrice provenant du Fraser Health relative à l’utilisation de la rasburicase, ce qui pourrait entraîner des pratiques différentes en matière de prescription au sein de l’institution.
Objectifs
Décrire la prescription de rasburicase au Fraser Health, y compris les indications et les doses, et rapporter les effets réducteurs de l’acide urique de la rasburicase et tout autre résultat clinique, comme la dialyse ou la mort.
Méthodes
Cet examen rétrospectif et descriptif des dossiers comprenait des patients adultes soignés au Fraser Health, qui avaient reçu une prescription de rasburicase entre le 1er juin 2010 et le 30 novembre 2016. Le résumé des caractéristiques des patients et des résultats de l’étude a été obtenu à l’aide de statistiques descriptives.
Résultats
Les pratiques en matière de prescription de la rasburicase au Fraser Health étaient largement incohérentes, mais la dose la plus communément administrée aux personnes recevant de la rasburicase en prophylaxie était de 3 mg (8/12 [67 %] alors que 9/32 [28 %] des personnes recevaient la même dose comme traitement, donc un total combiné de 17/44 ou 39 %). Quelle que soit la dose, la rasburicase réduisait le taux sérique d’acide urique à moins de 476 μmol/L et diminuait le risque de SLT.
Conclusions
L’adoption d’une approche uniforme—impliquant une dose unique pouvant être répétée au besoin—pour la prévention et le traitement du taux sérique élevé d’acide urique pourrait entraîner une réduction suffisante du taux d’acide urique avec une durée de traitement plus courte et des coûts moins importants. Les résultats de cette étude soutiennent le besoin d’une ressource au Fraser Health pour guider et standardiser l’utilisation de la rasburicase.
MOTS CLÉS: rasburicase, syndrome de lyse tumorale, acide urique, hyperuricémie
Tumour lysis syndrome (TLS) is an oncologic metabolic emergency, whereby lysis of malignant tumour cells and the rapid release of nucleic acid, potassium, and phosphate can lead to severe neuromuscular and cardiovascular complications and death.1 Hyperuricemia from the catabolism of nucleic acids is a key instigator for the laboratory abnormalities and clinical complications seen in this condition.2 The accumulation and precipitation of uric acid in the kidneys can cause acute uric acid nephropathy. The resulting renal dysfunction can create a vicious circle, whereby further increases in the build-up and crystallization of uric acid worsen electrolyte abnormalities, ultimately leading to further renal injury.
The likelihood of TLS depends on age, stage of malignancy, tumour burden, tumour grade and turnover rate, white blood cell (WBC) count, lactate dehydrogenase (LDH) level, pre-existing renal impairment, renal involvement by the tumour, intensity of cancer treatment, sensitivity of the tumour to cytotoxic therapy, patient’s sex, baseline uric acid levels, and use of drugs that increase uric acid.1–4 Malignancies with rapid cell proliferation or large tumour burden are considered to carry high risk for TLS, as it is theorized that high nucleoprotein turnover increases serum uric acid.5Box 1 lists conditions that are considered to be associated with high and intermediate risk for TLS.3,6 Although TLS is more commonly observed with rapidly dividing hematologic malignancies and after cytotoxic chemotherapy,1,4 it has been reported with nearly all malignancies, either spontaneously or after radiation therapy, cytolytic antibiotic therapy, intrathecal chemotherapy, dexamethasone, and newer chemotherapeutic agents.2,4,7–12
Hyperuricemia can develop rapidly, so it is important to identify and prevent TLS in high-risk patients and to treat appropriately if TLS does occur.2 The consequence of renal injury was highlighted in a prospective study by Canet and others,13 who showed that acute kidney injury in patients with high-grade hematological malignancies, including those caused by TLS, was associated with a higher mortality rate and lower 6-month complete remission rate.
Strategies for prophylaxis include aggressive hydration, urinary alkalinization, and, depending on the risk of TLS, administration of allopurinol or rasburicase.6 Allopurinol works by inhibiting xanthine oxidase, thereby decreasing the formation of uric acid.14 Its maximum effect for hyperuricemia associated with chemotherapy occurs 27 h after administration,4,14 and this drug could therefore be considered for patients at low and moderate risk.1,3,6 Rasburicase, a highly potent recombinant urate oxidase, metabolizes uric acid into a more soluble, inactive metabolite called allantoin.6,15 Unlike allopurinol, rasburicase affects the existing uric acid and has a faster onset, decreasing uric acid level within 4 h of administration.15,16 Thus, it is generally reserved for patients at high risk of TLS and those for whom allopurinol is contraindicated.1,4,6,16,17 A review by Dinnel and others18 showed that rasburicase reduced TLS, acute kidney injury, and need for renal replacement therapy, and was superior to allopurinol in the reduction of uric acid levels.
Box 1 Conditions with High and Intermediate Risk for Tumour Lysis Syndrome3,6
Treatment of TLS generally includes aggressive hydration with or without a loop diuretic, correction of electrolyte abnormalities, and administration of rasburicase and renal replacement therapy, if appropriate.1 Allopurinol, which only reduces the formation of uric acid, is not considered an effective treatment option because of its slow onset.1,14 The manufacturer’s recommended dosage for rasburicase is 0.2 mg/kg IV once daily for up to 7 days,15,19 with a maximum single dose of 0.2 mg/kg. This dose regimen has been found to rapidly decrease uric acid levels in 95% to 99% of patients.20 However, rasburicase is expensive, costing about $130 per 1.5-mg vial in the Fraser Health Authority (FHA). Therefore, for a 70-kg person, each dose costs about $1200. Because of this high cost, numerous investigators have trialled other strategies in attempts to lower costs while maintaining efficacy. Possible strategies include a single low, fixed dose ranging from 3 to 7.5 mg21–30 and a single weight-based dose ranging from 0.05 to 0.20 mg/kg,23,27,31–36 with subsequent doses given daily as needed. Although most of the cited studies were relatively small and retrospective, with uric acid levels used as a surrogate marker for TLS management, their results suggested that a single reduced dose of rasburicase, with subsequent doses given as needed, is sufficient to reduce and normalize uric acid levels. In their retrospective study, McBride and others25 reviewed patients who received rasburicase 3 mg, 6 mg, or 7.5 mg or a weight-based dose for prevention or treatment of TLS. They observed no statistically significant differences in uric acid normalization within 24 h (92.9% with 3-mg dose, 97.6% with 6-mg dose, 100.0% with 7.5-mg dose, 98.0% with weight-based dosing [mean 0.16 mg/kg]; p = 0.1238). In their meta-analysis, Feng and others37 found that a single dose ranging from 3 to 7.5 mg (fixed) or from 0.05 to 0.2 mg/kg (weight-based) effectively maintained the uric acid level below 267 μmol/L at 24 to 72 h. Although some patients needed more than one dose, implementing these dosing strategies generally resulted in management of TLS at a lower dose and with shorter treatment duration. In other studies, Coutsouvelis and others30 and Liu and others32 also showed that a single dose of rasburicase helped return renal function to within the normal range or creatinine level to baseline.
In terms of safety, rasburicase is considered to be well tolerated.20,37 Currently, the FHA formulary restricts rasburicase to the “treatment of acute or at high risk of tumour lysis syndrome, when other therapeutic options are not suitable”.38 However, it does not provide any definitions of high risk, nor does it include guidelines to direct the prescribing and monitoring of rasburicase. Unlike FHA, another health authority in British Columbia has a monograph for rasburicase to guide its use, which recommends the off-label dose of 3 mg.39
The overall goal of this study was to investigate and characterize how rasburicase is being prescribed in FHA and to describe the outcomes of patients receiving rasburicase. The lack of standardized and evidence-based guidelines in this health authority may be resulting in inconsistent prescribing practices and may also be unnecessarily increasing expenses. This study was undertaken to help identify any discrepancies between current prescribing practices and current formulary restrictions, guidelines, and evidence. The results might also help justify the need for a standardized approach to rasburicase prescribing in FHA. The primary objective was to describe the prescribing of rasburicase in FHA, including indication and dose. The secondary objectives were to report the uric acid–lowering effects of this drug, the clinical outcomes (such as need for dialysis or death), and adverse events.
This study was a retrospective descriptive chart review based on electronically scanned records from FHA. The data collected included demographic characteristics (age, sex, weight), indication for rasburicase, risk factors (underlying malignancy, baseline WBC count, baseline LDH level), characteristics of the patient’s condition (signs and symptoms of clinical TLS, laboratory markers of TLS), outcomes of treatment for TLS (serum uric acid level, TLS markers, dialysis, death), outcomes of prophylaxis for TLS (serum uric acid level, TLS markers, presence of TLS), and adverse drug reactions. The TLS markers were serum uric acid, potassium, and phosphate. Data points for continuous variables, specifically age, weight, serum creatinine, and dose of rasburicase, were plotted graphically in Excel software (Microsoft Corporation, Redmond, Washington) for visual examination; those that did not follow a normal distribution curve are reported as medians and interquartile ranges (IQRs; presented as 25th percentile to 75th percentile). Categorical variables, such as type of malignancy and sex, are reported as proportions.
This study was approved by the Fraser Health Research Ethics Board. No identifying information appears in this article.
Eligible patients were those 19 years of age or older for whom rasburicase was prescribed in FHA from June 1, 2010, to November 30, 2016. Patients were excluded if they had contraindications to rasburicase, such as hypersensitivity to the drug or glucose-6-phosphate deficiency.
The definitions used in this study were based on a widely used classification system developed by Cairo and Bishop.1 Laboratory TLS was defined as 2 or more of the following within 3 days before or 7 days after administration of cytotoxic therapy: uric acid ≥ 476 μmol/L; potassium ≥ 6.0 mmol/L; phosphorus ≥ 1.45 μmol/L; calcium ≤ 1.75 mmol/L; 25% increase from baseline in uric acid, potassium, or phosphorus; or 25% decrease from baseline in calcium. Clinical TLS was defined as laboratory TLS plus at least one of the following complications: increase in serum creatinine ≥ 1.5 times the upper limit of normal, cardiac arrhythmia, seizures, or sudden death. Cases that did not meet either of these definitions were classified as spontaneous TLS or suspected TLS. In this study, TLS was classified as spontaneous if it met the Cairo-Bishop criteria for laboratory or clinical TLS but did not occur within 3 days before or 7 days after cytotoxic therapy. TLS was classified as suspected if the Cairo-Bishop criteria for laboratory or clinical TLS were not met, but TLS was the documented indication for rasburicase. Rasburicase therapy was defined as prophylactic if the Cairo-Bishop criteria for laboratory and clinical TLS were not met and the patient was expected to receive chemotherapy; otherwise, rasburicase was deemed to have been ordered for treatment of TLS. For TLS prophylaxis, rasburicase is generally given 24 h before chemotherapy,15 whereas for TLS treatment, rasburicase is given at any time that TLS has been identified.
There were 46 orders for rasburicase from June 1, 2010, to November 30, 2016. Of these, 32 (70%) were for treatment of TLS, 12 (26%) were for prophylaxis of TLS, and 2 (4%) were for treatment of hyperuricemia, in the absence of confirmed malignancy. Patient characteristics and baseline TLS markers for prophylaxis and treatment of TLS are presented in Table 1. No patients were documented as having glucose-6-phosphate dehydrogenase deficiency.
Table 1 Patient Characteristics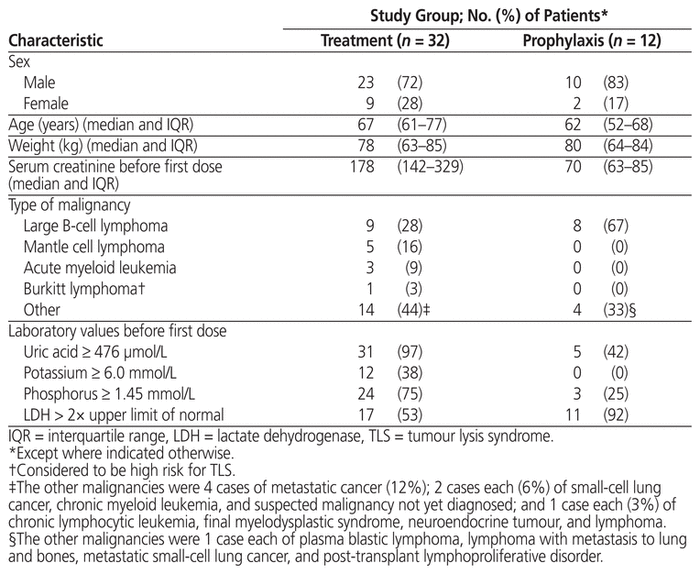
Overall, 30 (65%) of the orders were initially prescribed as single, one-time doses, whereas the other 16 (35%) were prescribed for daily administration. However, the duration of therapy for 9 of the daily orders was eventually reduced because of insufficient stock or upon liaison with pharmacy. For 6 (13%) of the orders, the dose was also reduced from the initial prescription for similar reasons. The results presented below refer to the first doses administered and exclude the 2 orders for treatment in absence of malignancy.
Because of the wide variation in doses administered, the first doses and results are presented relative to the 3-mg dose (the lowest effective dose studied), with other doses being presented in terms of ranges of weight-based doses (i.e., < 0.10 mg/kg, 0.10–0.14 mg/kg, 0.15–0.20 mg/kg, > 0.20 mg/kg) (Tables 2 and 3). Tables 4 and 5 show the administered first doses as prescribed (i.e., fixed dosing: 3, 6, or 7.5 mg and weight-based dosing).
Table 2 Prophylaxis of TLS: Dose of Rasburicase, TLS Markers, Risk Factors, and Clinical Outcomes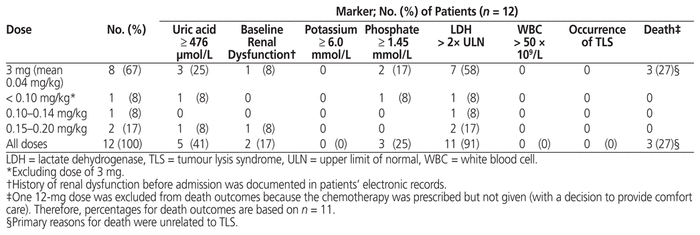
Table 3 Dose of Rasburicase for Treatment of Tumour Lysis Syndrome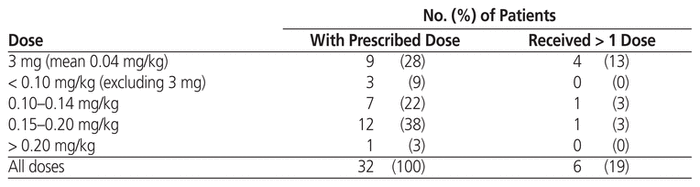
Table 4 Dose of Rasburicase as Prescribed for Prophylaxis of Tumour Lysis Syndrome
Table 5 Dose of Rasburicase as Prescribed for Treatment of Tumour Lysis Syndrome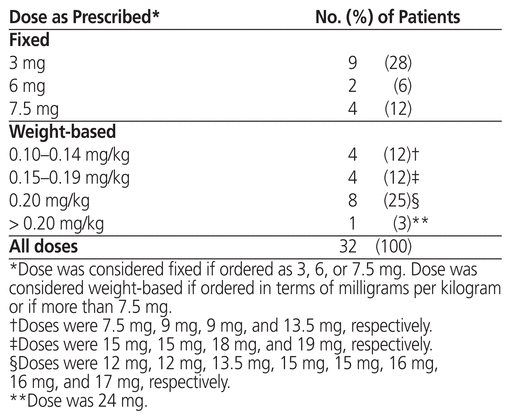
Patients who received prophylaxis were mostly male (10/12 or 83%), with median age 62 years (IQR 52–68 years) and median weight 80 kg (IQR 64–84 kg) (Table 1). The median serum creatinine before administration of rasburicase was 70 μmol/L (IQR 63–85 μmol/L). The most common underlying malignancy was large B-cell lymphoma (8/12 or 67%), which at a high tumour burden has been associated with increased risk of TLS.3 The doses for these patients ranged from 3 to 16 mg (median 3 mg, IQR 3–7.9 mg), and the most common dose was 3 mg (8/12 or 67%) (Tables 2 and 4). Before the first dose, 9 (75%) of the 12 patients did not have elevation of serum potassium and phosphate levels sufficient to meet the Cairo-Bishop criteria for TLS. Eleven (92%) of the patients had elevated LDH, indicating high tumour burden, and 5 (42%) of the patients had serum uric acid level above 476 μmol/L.
The use of allopurinol, an alternative for prophylaxis, was also documented. Of these 12 patients, 5 (42%) received allopurinol before the rasburicase was started. With the exception of one patient who had problems swallowing pills and another who received allopurinol while waiting for a supply of rasburicase, the reasons for either switching to rasburicase or using rasburicase in addition to allopurinol were not clearly documented. Two of these 5 patients had normalization of their elevated baseline serum uric acid level (to less than 476 μmol/L) with allopurinol, before administration of rasburicase. An additional 3 patients (25%) started allopurinol at the same time as rasburicase. For 5 of the 8 patients who received both rasburicase and allopurinol for prophylaxis, allopurinol was continued after initiation of rasburicase.
Of the 12 patients with rasburicase ordered for prophylaxis, one did not receive the dose that was ordered; as such, outcome data were available for 11 patients (Table 2). Only 3 patients, all of whom had an initial 3-mg dose, received more than 1 dose. For 1 of these 3 patients, the order was initially written for daily administration rather than a single dose; however, despite the patient’s serum uric acid levels remaining below 476 μmol/L after the first dose, a second dose was given for rising potassium and phosphate levels, which were still below the criteria for laboratory TLS. For another patient, additional doses were given because the patient had received chemotherapy on multiple days during the hospital stay. For another patient, an additional dose was given before repeat testing of serum uric acid level, and the rationale for the additional dose was not documented. Therefore, it is likely that the administration of additional doses for the 3-mg dose is not a reflection of lack of efficacy in reducing serum uric acid levels. The additional doses may have been prescribed on the basis of the patients’ overall clinical status or perceived risk of TLS, or due to not recognizing that the primary mechanism of action of rasburicase is to reduce serum uric acid levels. Regardless of the dose, no patients met the criteria for TLS. Three patients died for reasons unrelated to TLS.
As in the group receiving rasburicase for prophylaxis, patients who received rasburicase for treatment of TLS were mostly male (23/32 or 72%), with median age 67 years (IQR 61–77 years) and median weight 78 kg (IQR 63–85 kg) (Table 1). The median serum creatinine before administration of rasburicase was 178 μmol/L (IQR 142–329 μmol/L), and 22 (69%) of the 32 patients had elevated serum creatinine level. The most common malignancy was also large B-cell lymphoma (9/32 or 28%), followed by mantle cell lymphoma (5/32 or 16%) and metastatic cancer (4/32 or 12%). The indications for treatment included clinical TLS (12 patients [38%]), laboratory TLS (1 patient [3%]), spontaneous clinical TLS (10 patients [31%]), and spontaneous laboratory TLS (2 patients [6%]). Seven (22%) of the orders did not fall within any of these indication categories, but TLS was suspected. The treatment doses ranged from 3 to 24 mg (median 9 mg, IQR 5.2–15 mg) (Table 5), and the most common doses were 0.15–0.20 mg/kg (12/32 or 38%) and 3 mg (9/32 or 28%) (Table 3). Six (19%) of the patients received more than 1 dose, including 4 patients in the 3-mg dosing category; however, only 2 of these patients received additional doses because the serum uric acid level remained above 476 μmol/L. One patient in the 0.10–0.14 mg/kg dosing category received an additional dose, but for this patient, no sample was drawn between doses for uric acid testing. Another patient, in the 0.15–0.20 mg/kg dosing category, received an additional dose because uric acid levels remained elevated.
Of the 32 patients with rasburicase ordered for treatment, outcome data were available for 28 (Table 6); outcomes are not reported for patients who did not receive the ordered dose or those for whom monitoring could not be performed in FHA. At baseline, 1 of these 28 patients did not have elevation of serum uric acid level. Of the remaining 27 patients, 26 (96%) had normalization of serum uric acid level (≤ 476 μmol/L), which occurred within 24 h for 18 patients (67%). Despite normalization of serum uric acid levels in most patients, 9 (32%) of the 28 patients for whom outcomes are available required dialysis and 14 (50%) died during the hospital stay. Of the 14 deaths, 7 occurred when TLS was documented as ongoing or the acute kidney injury caused by TLS had not improved or had worsened. The initial dose administered to these 7 patients was 6 mg, 12 mg, 15 mg, 15 mg, 17 mg, 18 mg, and 24 mg, respectively. Although TLS may have contributed to the other deaths, there were other confounding factors as well, such as severity of the malignancy, which make it challenging to attribute death primarily to TLS.
Table 6 Normalization of Serum Uric Acid Level with Rasburicase for Treatment of TLS and Clinical Outcomes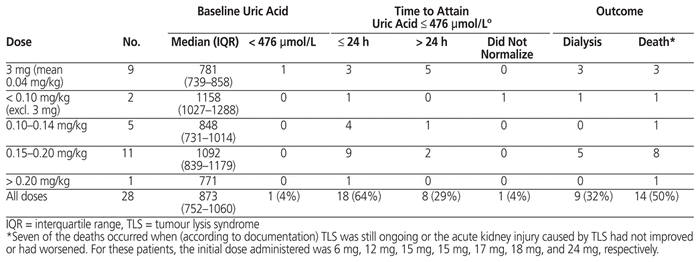
There were no adverse drug reactions reported with rasburicase.
In this study, which examined the prescribing of and outcomes with rasburicase for prophylaxis and treatment of TLS, the doses prescribed were generally inconsistent. Additionally, for reasons such as insufficient stock, the doses administered were not completely reflective of prescribing practices in FHA. No clear trends were observed, in terms of baseline serum uric acid levels or creatinine levels, that would explain the use of different doses. However, patients treated with the higher doses (0.15 to 0.20 mg/kg) might have had a poorer prognosis, given that a greater proportion of patients in that group died. In addition, prescribers might have been more inclined to use higher doses for treatment, given that the median dose was higher for treatment than for prophylaxis. The differences in doses could also be partly explained by the fact that a wide range of rasburicase doses have been studied in the setting of TLS. For most patients, monitoring of electrolytes, serum uric acid levels, and renal function was performed at least every 24 h after administration of rasburicase. However, for 3 orders, testing of serum uric acid level was not repeated before the subsequent dose(s), even though such testing would typically be used to help justify an additional dose. These inconsistencies in prescribing and monitoring support the need for a resource to guide the proper monitoring and rational use of rasburicase.
The Cairo-Bishop definitions are commonly used in the literature, but they exclude spontaneous TLS occurring in the absence of cytotoxic therapy. Another issue with these criteria is that their definition of acute kidney injury (serum creatinine level more than 1.5 times the upper limit of normal) could include patients with baseline chronic kidney disease.2 Of the 32 patients with a diagnosis of TLS, 97% had elevated serum uric acid levels, 75% had elevated serum phosphate levels, 68% had elevated serum creatinine levels, and 38% had elevated serum potassium levels. Therefore, in practice, these definitions and criteria guiding the use of rasburicase may not be appropriate for every patient.
For prophylaxis, rasburicase has been recommended for high-risk patients requiring immediate chemotherapy.1,3 In this review, 75% patients had high risk of TLS and 92% had chemotherapy planned within 72 h. Therefore, this group included patients who could have been received allopurinol instead of rasburicase for prophylaxis. Although there are insufficient data to make strong recommendations on how rasburicase should be prescribed, this review highlights the importance of having a reference (e.g., a monograph) to guide the prescribing and monitoring of rasburicase. Given that prompt recognition and management of TLS is imperative to prevent acute kidney injury (a strong predictor of death among patients with TLS2), it is critical that any restrictions or guidelines created for rasburicase do not inadvertently prevent its use in patients who could benefit from it. Aside from uric acid nephropathy, there are other possible mechanisms of acute kidney injury in TLS, such as acute phosphate nephropathy and precipitation of calcium phosphate within the renal parenchyma.40,41 Therefore, although rasburicase was observed to normalize serum uric acid levels, its efficacy in reducing the risk of and reversing acute kidney injury, as well as preventing death, is less clear. Even though the majority of patients had normalization of serum uric acid, some patients required dialysis and others died. Larger studies would be required to fully evaluate the clinical efficacy and safety of the various doses of rasburicase. Despite this need for additional study, this review does suggest that implementing a strategy whereby a single dose is prescribed and subsequent doses are given daily, as needed, could conserve stock and minimize unnecessary expenditure, without compromising the efficacy of rasburicase in normalizing serum uric acid levels.
This study had several limitations. Because of the retrospective design, it was challenging to assess the appropriateness and safety of each order, and potential confounding factors made it impossible to compare different doses. Some factors, such as kidney involvement due to malignancy or renal dysfunction due to other acute conditions (e.g., infection), might not have been consistently documented yet could have affected the clinical outcomes. In addition, the small sample size did not allow sufficient power to compare the different doses and detect a significance difference in outcomes, had such a difference been present. Furthermore, the doses evaluated were the first doses administered, not the total doses, which made it challenging to compare efficacy and safety. This study was also dependent on the documentation accessible from FHA; however, not all charts were completely scanned. The rationale for each particular dose and for the decision to use rasburicase instead of allopurinol for prophylaxis was often poorly documented. For 21 patients, allopurinol was prescribed for treatment, and for another 7 patients, allopurinol was prescribed for prophylaxis, at the clinician’s discretion. However, given the slower onset of allopurinol, the addition of this drug is unlikely to have significantly affected the efficacy of rasburicase. Moreover, it was unclear whether serum samples for determination of uric acid levels after the rasburicase dose were properly collected (in prechilled tubes containing heparin) and analyzed within 4 h. At room temperature, rasburicase causes ex vivo enzymatic degradation of uric acid, resulting in falsely low levels.
Overall, this study showed that prescribing practices and monitoring of rasburicase in FHA were not standardized. These results indicate the need for a resource in this health authority to help guide the prescribing and monitoring of rasburicase. Creating an institution-specific monograph that recommends a uniform approach—involving a single dose, to be repeated as needed—for treatment of elevated serum uric acid levels could result in appropriate management with shorter treatment duration and lower cost.
1 Jones GL, Will A, Jackson GH, Webb NJ, Rule S. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169(5):661–71.

2 Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21(1):18–26.

3 Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149(4):578–86.

4 Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767–78.

5 Larson RA, Pui CH. Tumor lysis syndrome: definition, pathogenesis, clinical manifestations, etiology and risk factors. In: UpToDate [database on Internet]. Wolters Kluwer; [updated 2018 Feb 7; cited 2019 Jan 10]. Available from: http://www.uptodate.com. Password required to access content.
6 Leukemia/BMT manual [E-edition]. Vancouver (BC): Leukemia/Bone Marrow Transplant Program of BC; [updated 2016 Aug; cited 2016 Jun 29]. Available from: http://www.leukemiabmtprogram.com/healthcare_professionals/cancer_management_guidelines/BMT_Manual.html. Password required to access content.
7 Saleh RR, Rodrigues J, Lee TC. A tumour lysis syndrome in a chemotherapy naïve patient with metastatic pancreatic adenocarcinoma. BMJ Case Rep. 2015;2015:bcr2014207748.
8 Vallianou N, Geladari E, Chroni P, Trigkidis K, Rontogianni D, Kokkinakis E. Spontaneous tumor lysis syndrome in a patient with angioimmunoblastic lymphoma. Arch Hellenic Med/Arheia Ellenikes Iatrikes. 2016;33(3):399–401.
9 Chow M, Yuwono A, Tan R. Tumour lysis syndrome: a rare acute presentation of locally advanced testicular cancer—case report and review of literature. Asian J Urol. 2016;3(1):49–52.


10 Weerasinghe C, Zaarour M, Arnaout S, Garcia G, Dhar M. Spontaneous tumor lysis syndrome in small-cell lung cancer: a rare complication. World J Oncol. 2015;6(5):464–71.


11 Melendez-Rosado JM, Zetir M, Stancampiano FF. 50-year-old man with new-onset acute kidney injury.Mayo Clin Proc. 2015;90(10):1420–3.

12 Vaidya GN, Acevedo R. Tumor lysis syndrome in metastatic breast cancer after a single dose of paclitaxel. Am J Emerg Med. 2015;33(2):308e1–308e2.
13 Canet E, Zafrani L, Lambert J, Thieblemont C, Galicier L, Schnell D, et al. Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: impact on remission and survival. PloS One. 2013;8(2):e55870.


14 Allopurinol: drug information [Lexicomp monograph]. In: UpToDate [database on Internet]. Wolters Kluwer; [cited 2016 Jul 11]. Available from: http://www.uptodate.com. Password required to access content.
15 Rasburicase: drug information [Lexicomp monograph]. In: UpToDate [database on Internet]. Wolters Kluwer; [cited 2016 Jul 11]. Available from: http://www.uptodate.com. Password required to access content.
16 Cortes J, Moore JO, Maziarz RT, Wetzler M, Craig M, Matous J, et al. Control of plasma uric acid in adults at risk for tumor lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28(27):4207–13.


17 Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson FL, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97(10):2998–3003.

18 Dinnel J, Moore BL, Skiver BM, Bose P. Rasburicase in the management of tumor lysis: an evidence-based review of its place in therapy. Core Evid. 2015;10:23–38.

19 Fasturtec® (rasburicase) powder for injection. 1.5 mg/mL/vial. Uricolytic agent [product monograph]. Laval (QC): sanofi-aventis Canada Inc; [revised 2016 Mar 7; cited 2016 Jul 11]. Available from: http://products.sanofi.ca/en/fasturtec.pdf
20 de Bont JM, Pieters R. Management of hyperuricemia with rasburicase review. Nucleosides Nucleotides Nucleic Acids. 2004;23(8–9):1431–40.

21 Trifilio SM, Pi J, Zook J, Golf M, Coyle K, Greenberg D, et al. Effectiveness of a single 3-mg rasburicase dose for the management of hyperuricemia in patients with hematological malignancies. Bone Marrow Transplant. 2011; 46(6):800–5.
22 Herrington JD, Dinh BC. Fixed, low-dose rasburicase for the treatment or prevention of hyperuricemia in adult oncology patients. J Oncol Pharm Pract. 2014:21(2):111–7.

23 Hummel M, Reiter S, Adam K, Hehlmann R, Buchheidt D. Effective treatment and prophylaxis of hyperuricemia and impaired renal function in tumor lysis syndrome with low doses of rasburicase. Eur J Haematol. 2008;80(4):331–6.
24 Malaguarnere M, Vacante M, Russoa C, Dipasquale G, Gargante MP, Motta M. A single dose of rasburicase in elderly patients with hyperuricaemia reduces serum uric acid levels and improves renal function. Expert Opin Pharmacother. 2009;10(5):737–42.
25 McBride A, Lathon SC, Boehmer L, Augustin KM, Butler SK, Westervelt P. Comparative evaluation of single fixed dosing and weight–based dosing of rasburicase for tumor lysis syndrome. Pharmacotherapy. 2013;33(3):295–303.

26 McDonnell AM, Lenz KL, Frei–Lahr DA, Hayslip J, Hall PD. Single–dose rasburicase 6 mg in the management of tumor lysis syndrome in adults. Pharmacotherapy. 2006;26(6):806–12.

27 Reeves DJ, Bestul DJ. Evaluation of a single fixed dose of rasburicase 7.5 mg for the treatment of hyperuricemia in adults with cancer. Pharmacotherapy. 2008;28(6):685–90.

28 Hutcherson DA, Gammon DC, Bhatt MS, Faneuf M. Reduced-dose rasburicase in the treatment of adults with hyperuricemia associated with malignancy. Pharmacotherapy. 2006;26(2):242–7.

29 Chiang J, Chan A, Lian T, Tay K, Quek R, Tao M, Lim ST. Management of tumor lysis syndrome with a single fixed dose of rasburicase in Asian lymphoma patients: a case series and literature review. Asia Pac J Clin Oncol. 2011;7(4):351–6.

30 Coutsouvelis J, Wiseman M, Hui L, Poole S, Dooley M, Patil S, et al. Effectiveness of a single fixed dose of rasburicase 3 mg in the management of tumour lysis syndrome. Br J Clin Pharmacol. 2013;75(2):565–8.
31 Knoebel RW et al. Evaluation of a low, weight-based dose of rasburicase in adult patients for the treatment or prophylaxis of tumor lysis syndrome. J Oncol Pharm Pract. 2010;17(3);147–54.

32 Liu CY, Sims-McCallum RP, Schiffer CA. A single dose of rasburicase is sufficient for the treatment of hyperuricemia in patients receiving chemotherapy. Leukemia Res. 2005;29(4):463–5.
33 Coiffier B, Mounier N, Bologna S, Fermé C, Tilly H, Sonet A, et al. Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-Hodgkin’s lymphoma: results of the GRAAL1 (Group d’étude des lymphomes de l’adulte Trial on Rasburicase Activity in Adult Lymphoma) study. J Clin Oncol. 2003;21(23):4402–6.

34 Campara M, Shord SS, Haaf CM. Single-dose rasburicase for tumour lysis syndrome in adults: weight-based approach. J Clin Pharm Ther. 2009; 34(2):207–13.

35 Vadhan-Raj S, Fayad LE, Fanale MA, Pro B, Rodriguez A, Hagemeister FB, et al. A randomized trial of a single-dose rasburicase versus five-daily doses in patients at risk for tumor lysis syndrome. Ann Oncol. 2012;23(6):1640–5.
36 Steel S, Coutsouvelis J, Mckendrick J. Single dose rasburicase in tumor lysis: one hospital’s experience. Asia Pac J Clin Oncol. 2008;4(1):18–20.
37 Feng X, Dong K, Pham D, Pence S, Inciardi J, Bhutada NS. Efficacy and cost of single-dose rasburicase in prevention and treatment of adult tumour lysis syndrome: a meta-analysis. J Clin Pharm Ther. 2013;38(4):301–8.

38 Restricted drugs. Fraser Health, Pharmacy Services; 2016 Jun 24.
39 Acute HSDA: rasburicase. In: Parenteral drug therapy manual. Vancouver (BC): Vancouver Coastal Health; [cited 2016 Jun 29]. Available from: https://vchconnect.vch-phc.ca/policies_manuals/pdtm_va/monographs/page_124842.htm. Access restricted to VCH staff.
40 El-Husseini A, Sabucedo A, Lamarche J, Courville C, Peguero A. Acute kidney injury associated with tumor lysis syndrome: a paradigm shift. Am J Emerg Med. 2012;30(2):390.e3–6.
41 Wilson FP, Berns JS. Onco-nephrology: tumor lysis syndrome. Clin J Am Soc Nephrol. 2012;7(10):1730–9.

Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 72, NUMBER 4, July-August 2019