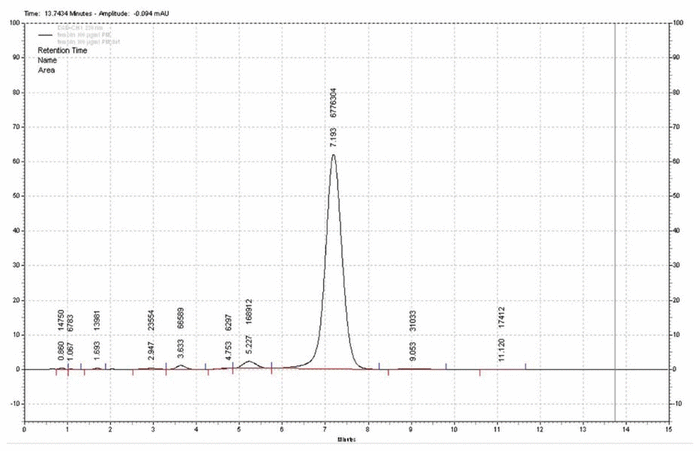
Figure 1 Chromatogram of vancomycin 100 μg/mL without degradation.
Élise d’Huart, Jean Vigneron, Alexandre Charmillon, Igor Clarot, Béatrice DemoréABSTRACT
Background
In severe infections, high-concentration vancomycin may be administered by continuous infusion. The dosage of vancomycin may reach 60 mg/kg per day.
Objectives
To study the feasibility of preparing high-concentration vancomycin solutions (40 to 83.3 mg/mL), to study the effect of an electric syringe pump on the physical stability of high-concentration vancomycin, and to study the stability of vancomycin 62.5 and 83.3 mg/mL in 0.9% sodium chloride (0.9% NaCl) or 5% dextrose in water (D5W) with storage up to 48 h at room temperature.
Methods
The following sets of syringes were prepared: (1) 4 syringes of vancomycin in 0.9% NaCl for each of 5 concentrations between 40 and 83.3 mg/mL (total 20 syringes); (2) 6 syringes at 83.3 mg/mL in 0.9% NaCl and 6 syringes at 83.3 mg/mL in D5W; and (3) 30 syringes at 83.3 mg/mL in D5W. Visual inspection was performed for all 3 syringe sets, and subvisual inspection for sets 1 and 2 (for periods of 24 h for set 1 and 48 h for sets 2 and 3). One syringe of vancomycin 83.3 mg/mL with each solvent was inserted into an electric syringe pump, and samples from the infusion line and collected after transit through the pump were inspected visually. Chemical stability was evaluated by high-performance liquid chromatography, and physical stability, pH, and osmolality were investigated.
Results
For all sets of syringes, no physical modification was observed over time, nor were any changes observed after transit through the electric syringe pump. In 0.9% NaCl, vancomycin 62.5 and 83.3 mg/mL retained more than 90% of the initial concentration after 48 and 24 h, respectively; however, for the 83.3 mg/mL solution, precipitate was visible after 48 h. In D5W, vancomycin at 62.5 and 83.3 mg/mL retained more than 90% of the initial concentration after 48 h.
Conclusion
It was feasible to prepare high-concentration solutions of vancomycin. The electric syringe pump did not cause any precipitation. Vancomycin in D5W at 62.5 and 83.3 mg/mL was stable over 48 h at room temperature. Precipitation occurred in 0.9% NaCl. D5W is therefore recommended as the solvent for this drug.
KEYWORDS: vancomycin, intensive care unit, high-performance liquid chromatography, stability
RÉSUMÉ
Contexte
En cas d’infection grave, de la vancomycine à forte concentration peut être administrée par perfusion continue à une dose pouvant atteindre 60 mg/kg par jour.
Objectifs
Mener une étude de faisabilité portant sur la préparation de solutions de vancomycine à forte concentration (de 40 à 83,3 mg/mL); étudier l’effet d’un pousse-seringue électrique sur la stabilité physique de la vancomycine à forte concentration; et étudier la stabilité de la vancomycine (62,5 et 83,3 mg/mL) dans une solution de chlorure de sodium à 0,9 % (NaCl à 0,9 %) ou dans une solution aqueuse de dextrose à 5 % (D5W) après 48 h à la température ambiante.
Méthodes
Trois ensembles de seringues ont été préparés : (1) quatre seringues de vancomycine dans une solution de NaCl à 0,9 %, à chacune des cinq concentrations comprises entre 40 et 83,3 mg/mL (20 seringues au total); (2) six seringues à 83,3 mg/mL dans une solution de NaCl à 0,9 % et six seringues à 83,3 mg/mL dans une solution de D5W; et (3) 30 seringues à 83,3 mg/mL dans une solution de D5W. Une inspection visuelle des trois ensembles de seringues et une inspection « sous-visuelle » des ensembles 1 et 2 ont eu lieu (période de 24 h pour l’ensemble 1 et de 48 h pour les ensembles 2 et 3). Une seringue contenant de la vancomycine à 83,3 mg/mL mélangée à chaque solvant a été insérée dans un pousse-seringue électrique, et les échantillons prélevés dans le tube de perfusion et ceux recueillis après leur passage dans la pompe ont été inspectés visuellement. La stabilité chimique a été évaluée par chromatographie liquide à haute performance et la stabilité physique, le pH ainsi que l’osmolalité ont eux aussi été étudiés.
Résultats
Les trois ensembles de seringues n’ont présenté aucune modification physique avec le temps. Aucun changement n’a non plus été observé après le passage dans le pousse-seringue électrique. Dans la solution de NaCl à 0,9 %, la vancomycine à 62,5 et à 83,3 mg/mL a conservé plus de 90 % de sa concentration initiale respectivement après 48 et 24 h. Cependant, le précipité de la solution à 83,3 mg/mL était visible après 48 h. Dans la solution de D5W, la vancomycine à 62,5 et à 83,3 mg/mL a conservé plus de 90 % de sa concentration initiale après 48 h.
Conclusion
La préparation de solutions de vancomycine à forte concentration est faisable. Le pousse-seringue électrique n’a pas causé de précipitation. La vancomycine dans la solution de D5W à 62,5 et à 83,3 mg/mL est restée stable pendant plus de 48 h à la température ambiante. Les précipitations se sont produites dans les solutions de NaCl à 0,9 %. On recommande donc la solution de D5W comme solvant pour ce médicament.
MOTS CLÉS: vancomycine, unité de soins intensifs, chromatographie liquide à haute performance, stabilité
Vancomycin is an antibiotic of the glycopeptide family produced by a soil bacterium, Amycolatopsis orientalis. Vancomycin kills bacteria in a time-dependent manner, which means that the amount of time the bacteria are exposed to a sufficiently high concentration is key to treatment success.1 This antibiotic is used to treat a variety of bacterial infections, such as infective endocarditis due to Staphylococcus (in penicillin-allergic patients) or methicillin-resistant Staphylococcus aureus (MRSA).2 In infective endocarditis caused by MRSA, the recommended dosage of vancomycin is 30–60 mg/kg daily; for osteoarticular MRSA infections, the recommended dosage is 60 mg/kg daily.3
In emergency clinical settings, such as the intensive care unit (ICU), the optimal serum concentration of vancomycin must be achieved rapidly and maintained over time. To avoid fluid overload, ICUs often use a high concentration of drug in a minimal volume of solution. Wysocki and others4 demonstrated that continuous infusion allowed rapid achievement of the target concentration. In clinical practice, a loading dose can be administered, followed by continuous infusion.1
Manufacturers have reported that vancomycin 5 mg/mL in 0.9% sodium chloride (0.9% NaCl) is stable for 48 h at 25°C,5 but caution that the final concentration should not exceed 10 mg/mL.6 For patients with body weight about 65 kg, the total daily dose of vancomycin, at the recommended dosage of 60 mg/kg daily, is 4 g. This amount of drug would have to be diluted with 400 mL of solvent to yield a final concentration not exceeding 10 mg/mL. However, for patients with fluid restrictions, this volume is excessive.
Previous studies have evaluated the stability of vancomycin at concentrations exceeding 10 mg/mL. Godet and others7 determined that vancomycin 41.7 mg/mL in 0.9% NaCl or 5% dextrose in water (D5W) stored in polypropylene syringes at a temperature between 18°C and 25°C was stable for 48 h. Masse and others8 determined that vancomycin 41.7 mg/mL in water for injection or 0.9% NaCl stored in polypropylene syringes at room temperature was stable for 24 h.
Longuet and others9 explained that vancomycin at a concentration of 80 mg/mL is often used in electric syringe pumps, but its safety has not been scientifically confirmed. In the authors’ hospital, high doses of vancomycin can be administered over 24 h in the ICU, using an electric syringe pump. The total volume is limited to 50 mL (and nurses usually use a final volume of 48 mL), which means that the concentration must be high, for example, 62.5 (3 g of drug in 48 mL of solvent) or 83.3 mg/mL (4 g of drug in 48 mL of solvent).
As reported in a poster presentation, Masse and others10 studied the physicochemical stability of vancomycin 80 mg/mL in 0.9% NaCl in polypropylene syringes, at 22°C. They used high-performance liquid chromatography (HPLC), visual inspection, particle count, and microbiological stability studies. The authors observed precipitation, with the number of particles (≥ 10 μm) in the unfiltered vancomycin solutions increasing as storage time increased. These authors used an electric syringe pump for a period of 24 h at 22°C, but they did not study the stability of vancomycin 80 mg/mL in D5W.
In the authors’ hospital, vancomycin is typically administered by intermittent infusion over 1 h, but high doses of the drug are also administered by continuous infusion in the ICU. The aim of this study was to validate the possibility of preparing highly concentrated solutions for administration by continuous IV infusion. Given the potential risk of precipitation highlighted by Masse and others,10 our first objective was to determine the feasibility of preparing vancomycin solutions at concentrations between 40 and 83.3 mg/mL in 0.9% NaCl or D5W in polypropylene syringes and to study the effect of an electric syringe pump on the physical stability of vancomycin 83.3 mg/mL in 0.9% NaCl and in D5W. The second objective was to study the stability of vancomycin 62.5 mg/mL and 83.3 mg/mL in 0.9% NaCl or D5W in polypropylene syringes, on the basis of analysis immediately after preparation and after storage for 6, 24, and 48 h at room temperature.
Before the stability study, we evaluated the risk of precipitation for vancomycin solutions in 0.9% NaCl or D5W at various concentrations between 40 and 83.3 mg/mL in polypropylene syringes at room temperature.
The first set of test solutions consisted of 4 syringes of vancomycin in 0.9% NaCl at each of 5 concentrations—40, 50, 58.8, 71, and 83.3 mg/mL—prepared and stored at room temperature. The second set consisted of 12 syringes of vancomycin 83.3 mg/mL—6 syringes in 0.9% NaCl and 6 syringes in D5W—prepared from 1-g vials of vancomycin. For the preparation of 3 syringes with each solvent, we used a polycarbonate spike adaptor (ChemoClave Universal Vented Vial Spike, ICU Medical Inc, San Clemente, California) to reconstitute the vancomycin; for the other 3 syringes, we used a needle and air intake. The third set consisted of 30 syringes of vancomycin 83.3 mg/mL in D5W, to study physical compatibility.
All syringes in each set were visually inspected by 2 technicians against a white background with the unaided eye. In addition, the subvisual aspect of the first 2 sets of syringes was investigated by evaluation of turbidity with a UVmc2 spectrophotometer (SAFAS Monaco), with absorbance determined at 350, 410, and 550 nm.11 For the first set of syringes, visual and subvisual inspections were performed after preparation and after storage for 8, 15, and 24 h. For the second set of syringes, visual and subvisual inspections were performed after preparation and after storage for 24 and 48 h. For the third set of syringes, visual inspection only was performed after storage for 6, 16, 24, and 48 h at room temperature.
To determine the influence on the solution (before administration) of an electric syringe pump with infusion line, incorporating a 0.22-μm filter (Agilia Injectomat, Fresenius Kabi), a syringe containing vancomycin 83.3 mg/mL in 0.9% NaCl (final volume 48 mL) was inserted into the pump. We added an extension for infusion (Doran International; 160 cm, VR = 1.334 mL, diameter = 1.0 × 3.0 mm; batch 261801P), with a 0.2-μm filter (Codan France; batch K73791-1), onto the syringe. The same process was repeated for a syringe containing vancomycin 83.3 mg/mL in D5W. The flow rate was 25 mL/h. Vancomycin samples collected from the infusion line underwent visual evaluation.
To assess the stability of vancomycin, solutions were prepared at 2 concentrations (62.5 and 83.3 mg/mL) in 0.9% NaCl or D5W; a total of 3 syringes were prepared for each combination of concentration and solvent (i.e., total of 12 syringes). For each syringe with concentration 62.5 mg/mL, 3 vials of vancomycin 1 g (Sandoz; batch EC0107) were each reconstituted with 16 mL of 0.9% NaCl (Easyflex 0.9% NaCl 500 mL, MacoPharma; batch 18B01B) or D5W (Easyflex D5W 500 mL, MacoPharma; batch 17I12D) and then combined for a total of 48 mL for each solvent. For each syringe with concentration 83.3 mg/mL, 4 vials of vancomycin 1 g were each reconstituted with 12 mL of 0.9% NaCl or D5W and then combined for a total of 48 mL for each solvent. Each 48-mL volume of drug was transferred to a polypropylene syringe (BD Plastipak, 50-mL Luer-lock syringes; batch 1803236) for storage at room temperature (20°C to 25°C), without protection from light.
The vancomycin solutions were analyzed by a stability-indicating reverse-phase HPLC method with photodiode array detection.8
The HPLC system consisted of an Elite LaChrom VWR/Hitachi plus autosampler, a VWR photodiode array detector L-2455, and a VWR L-2130 HPLC pump. Data were acquired and integrated with EZChrom Elite software (VWR). The column used contained LiChrospher 100 RP-18 gel carrier in a LiChroCART 125-4 cartridge, 12.5 cm long with 5-μm particle size (Merck). The mobile phase consisted of 0.1 mol/L buffer (8% acetonitrile [VWR Chemicals; batches D7G058267G and D5N045046A] and 92% monopotassium phosphate [Merck; batch AM09735277618]), adjusted to pH 3.5 with orthophosphoric acid 85% (VWR Chemicals; batch 15D200503). Water for chromatography was obtained from a reverse osmosis system (Millipore Iberica).
The flow rate was set at 1.5 mL/min, with an injection volume of 10 μL. The detection wavelength was set at 220 nm. The temperature of the injector was set at 15°C and that of the column oven at 30°C. The calibration curve was constructed from plots of peak area versus concentration. The linearity of the method was evaluated for 5 concentrations (50, 75, 100, 125, and 150 μg/mL).
For preparation of the standard curve, a solution of vancomycin 1 mg/mL was prepared from 100.0 mg of precisely weighed drug (vancomycin 125 mg, Sandoz; batch DA0012) diluted in 100.0 mL of the mobile phase. Further dilution of this 1 mg/mL solution with the mobile phase was used to generate solutions suitable for creating standard curves. The intra-day reproducibility was evaluated as recommended by the Q2 (R1) guideline of the International Conference on Harmonisation,12 using 3 determinations for each of 3 concentrations: 50, 100, and 150 μg/mL. To evaluate inter-day precision, the 3 concentrations (50, 100, and 150 μg/mL) were assayed 3 times on each of 3 different days. To demonstrate the specificity of the method and the absence of interaction between vancomycin and the solvent, solutions of 0.9% NaCl and D5W were analyzed by HPLC.
The stability-indicating capability of the assay was evaluated by analyzing vancomycin solutions that had been subjected to forced degradation. For acidic degradation, 1 mL of a 400 μg/mL vancomycin solution (vancomycin 125 mg, Sandoz; batch DA0012) was diluted with 1.0 mL of hydrochloric acid (HCl) 1.0 mol/L (VWR Chemicals; batch 17110005), stored at room temperature (20°C to 25°C) for 16 h, neutralized with 1.0 mL of sodium hydroxide (NaOH) 1.0 mol/L (VWR Chemicals; batch 17110003), and then diluted with 1.0 mL of the mobile phase to obtain a theoretical concentration of 100 μg/mL. For alkaline degradation, 1 mL of a 400 μg/mL vancomycin solution was diluted with 1.0 mL of NaOH 1.0 mol/L, stored at room temperature (20°C to 25°C) for 60 min, neutralized with 1.0 mL of HCl 1.0 mol/L, and then diluted with 1.0 mL of the mobile phase to obtain a theoretical concentration of 100 μg/mL. For oxidative degradation, 1 mL of a 400 μg/mL vancomycin solution was diluted with 1.0 mL hydrogen peroxide (H2O2) 3.0% or 30.0% (both concentrations prepared from 30% H2O2; Merck, batch K48743810713), stored at room temperature (20°C to 25°C) for 1 h, and then diluted with 2.0 mL of the mobile phase to obtain a theoretical concentration of 100 μg/mL. For heat degradation, a solution of 100 μg/mL vancomycin was exposed to a temperature of 80°C for 6 h.
The photodiode array detector could evaluate the ultraviolet (UV) spectrum of the chromatographic column effluent every 0.4 s, which allowed evaluation of the UV purity of each eluting peak. Variations in the UV spectrum over the elution profile of the peak of interest would indicate that the peak was contaminated, that the analytical method did not separate vancomycin from its degradation products, and that the method was therefore unsuitable.13
The stability of the diluted sample in the autosampler was also evaluated. Solutions of vancomycin diluted in ultrapure water were stored in the autosampler at 15°C. Vancomycin concentration was evaluated at various times up to 24 h.
At each analysis time, 5 mL of solution was removed from each syringe (62.5 mg/mL or 83.3 mg/mL). Each sample was then diluted with the mobile phase to obtain a theoretical concentration of 100 μg/mL (the middle of the standard curve). For each syringe, samples were prepared in triplicate for analysis by reverse-phase HPLC immediately after initial preparation and after 6, 24, and 48 h of storage. Total run time for HPLC was set at 15 min.
Chemical stability was defined as not less than 90.0% of the initial vancomycin concentration in relation to the evolution of potential degradation products.12,14
The pH of each solution (i.e., every syringe representing each combination of concentration and solvent) was measured with a Bioblock Scientific pH meter after initial preparation and after 6, 24, and 48 h of storage. The pH values were considered acceptable if they did not vary by more than 1.0 pH unit from the initial measurement.14
Osmolality was measured for each syringe at each analysis time using an osmometer (Roebling). Before each measurement, the osmometer was calibrated with a quality control solution (300 mOsm/kg) provided by the manufacturer.
Physical stability was defined as the absence of particulate formation, haze, colour change, or gas evolution.11 The samples from the stability study were visually inspected, with the unaided eye, against a white or black background by 2 technicians (working independently) after initial preparation and after storage for 6 h, 24 h, 48 h, and 5 days. The subvisual aspect was assessed, after manual agitation, with a UVmc2 spectrophotometer (SAFAS Monaco), with absorbance evaluated at 350, 410, and 550 nm.11
No change in appearance of the solutions was visible at any analysis time for any combination of concentration and solvent. Similarly, there were no differences in the visual appearance of syringes prepared with a needle and air intake or with a spike adaptor. The maximum variation in turbidity between the time of syringe preparation and assay time (for solutions in the first 2 sets of syringes) was less than 0.04 absorbance units (AU) at 350 nm, less than 0.02 AU at 410 nm, and less than 0.02 AU at 550 nm.
After use of the electric syringe pump, no precipitate was observed for vancomycin 83.3 mg/mL in 0.9% NaCl or D5W.
The calibration curve was linear, and the coefficient of determination was 0.9995. The equation for the calibration curve was y = 68080.757x – 339239.933. The intra-day precision, expressed as relative standard deviation, was between 0.22% and 1.37%. The intermediate precision, expressed as relative standard deviation, was 2.48% at 50 μg/mL, 1.70% at 100 μg/mL, and 2.33% at 150 μg/mL.
For the evaluation of stability in the autosampler, solutions were stable, with a degradation rate less than 1%.
The stability-indicating capability of the assay was tested under various conditions of forced degradation. More specifically, the UV spectral purity of the vancomycin peak in chromatograms of the degraded samples was compared with the spectrum of the undegraded sample of vancomycin obtained at time 0. A sample chromatogram of vancomycin without forced degradation is presented in Figure 1, with retention time of 7.19 min, and a chromatogram of vancomycin after alkaline degradation in Figure 2. The mass balance and retention of degradation products relative to the pure vancomycin peak are presented in Table 1. After forced degradation, the extent of degradation was 25% under acidic conditions, 23% under alkaline conditions, and 14% with heating. No degradation occurred under oxidative conditions.
|
|
||
|
Figure 1 Chromatogram of vancomycin 100 μg/mL without degradation. |
||
|
|
||
|
Figure 2 Chromatogram of vancomycin 100 μg/mL after alkaline degradation (sodium hydroxide 1.0 mol/L, 1 h). |
||
Table 1 Mass Balance of Vancomycin Solutions after Various Methods of Forced Degradation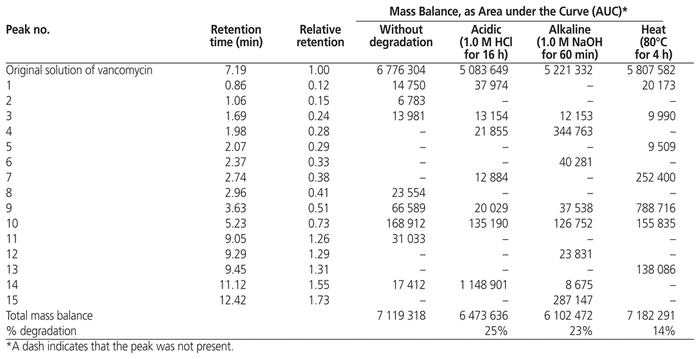
The various conditions tested allowed good separation and detection of degradation products. The extent of degradation was about 20%, the limit recommended in a guideline developed by the Société Française de Pharmacie Clinique (French Society of Clinical Pharmacy) and the Groupe d’Évaluation et de Recherche sur la Protection en Atmosphère Contrôlée (Evaluation and Research Group on Protection in Controlled Atmosphere).14
The percentage of vancomycin remaining in solutions with original concentration 62.5 or 83.3 mg/mL in 0.9% NaCl or D5W after storage at room temperature (20°C to 25°C) for various periods is shown in Table 2.
Table 2 Stability of Vancomycin in 0.9% Sodium Chloride (NaCl) and 5% Dextrose in Water (D5W)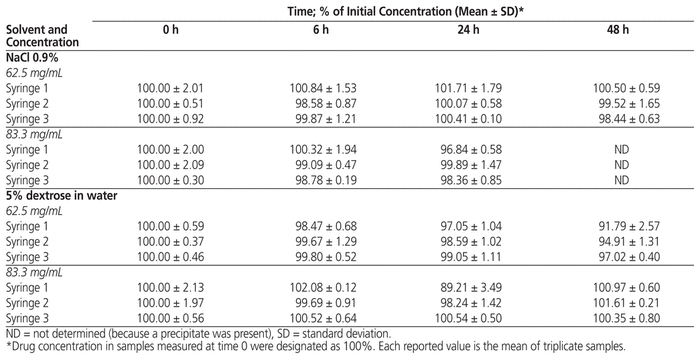
During the stability study, none of the degradation products that appeared with forced degradation were observed. Peaks 3, 9, and 10, with relative retention 0.24, 0.51, and 0.73, respectively, were consistently observed immediately after preparation and after storage for 24 and 48 h.
No significant change in pH was observed during the stability studies. For vancomycin 62.5 and 83.3 mg/mL in NaCl 0.9%, mean pH was 3.57 (standard deviation [SD] 0.02) and 3.54 (SD 0.02), respectively, over the course of the stability study. For vancomycin 62.5 mg/mL and 83.3 mg/mL in D5W, mean pH was 3.26 (SD 0.16) and 3.32 (SD 0.14), respectively, over the course of the stability study.
No significant change in osmolality was observed during the stability studies. Osmolality was slightly lower in solutions using 0.9% NaCl as the solvent than in solutions using D5W. With 0.9% NaCl, osmolality was 337–351 mOsm/kg at 62.5 mg/mL and 362–378 mOsm/kg at 83.3 mg/mL. With D5W, osmolality was 363–383 mOsm/kg at 62.5 mg/mL and 379–409 mOsm/kg at 83.3 mg/mL.
After storage for 48 h, precipitate was observed in syringes of vancomycin 83.3 mg/mL in 0.9% NaCl (Figure 3). These syringes were stored for an additional 3 days; upon visual inspection after a total of 5 days of storage, an extensive white precipitate was visible in all 3 syringes (Figure 4).
|
|
||
|
Figure 3 Appearance of vancomycin 83.3 mg/mL in 0.9% sodium chloride, after storage in syringe for 48 h. |
||
|
|
||
|
Figure 4 Appearance of vancomycin 83.3 mg/mL in 0.9% sodium chloride, after storage in syringes for 5 days. |
||
Solutions of vancomycin 83.3 mg/mL in D5W were slightly yellow in colour, but remained clear, with no precipitate, after 5 days of storage.
To confirm the absence of risk of precipitation at 83.3 mg/mL in D5W, a batch of 30 syringes at this concentration in this solvent was prepared and inspected visually by 2 technicians after 6, 16, 24, and 48 h of storage at room temperature. No precipitation or visual modification was observed at any observation time.
In terms of subvisual inspection, mean absorbance values for the 3 wavelengths (350, 410, and 550 nm) after the various storage periods are presented in Table 3.
Table 3 Absorbance Values for Vancomycin Solutions during Stability Study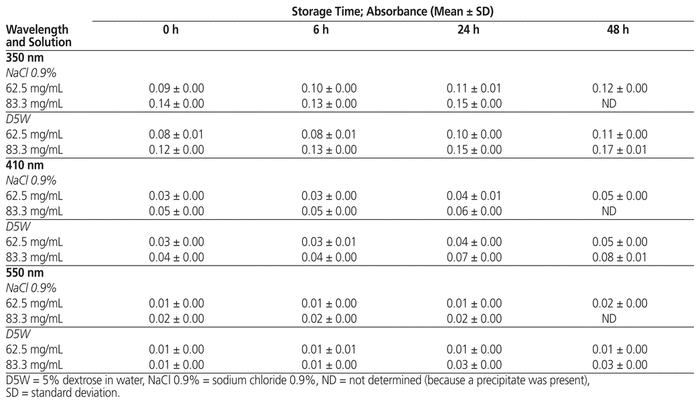
Vancomycin is most commonly administered by continuous infusion with an electric syringe pump.15 For some indications, the dose of vancomycin can be elevated, meaning that a higher concentration is required, given the volume limit of 48 mL in syringes used with this type of pump. Masse and others10 observed a precipitate in vancomycin solutions at 80 mg/mL in 0.9% NaCl stored in polypropylene syringes at 22°C (they did not study the stability of vancomycin in D5W).
In the first phase of this work, before performing the stability study, we tested the physical stability of 3 sets of vancomycin solutions (in 0.9% NaCl or D5W) in polypropylene syringes stored for 24 or 48 h at room temperature, but noted no visual or subvisual changes. Furthermore, the electric syringe pump did not cause any precipitate to form.
Diluted in D5W and stored at room temperature (20°C to 25°C), vancomycin solutions at 62.5 mg/mL and 83.3 mg/mL retained more than 90.0% of the initial concentration after 48 h.
At a concentration of 62.5 mg/mL and after storage for 48 h, syringe 1 retained 91.79% of the initial concentration with a standard deviation (SD) of 2.57%. This SD value is related to the low value obtained for 1 of the 3 samples prepared for this syringe. At a concentration at 83.3 mg/mL and after storage for 24 h, syringe 1 retained slightly less than 90% of the initial concentration, but after storage for 48 h, the value was more than 90%. We assume that these results are related to a technical problem, probably due to the 2 dilutions required to obtain the target (theoretical) concentrations.
During 48 h of storage at room temperature, no degradation products, no change of pH, and no visible modifications were observed for vancomycin solutions at 62.5 or 83.3 mg/mL in D5W.
Diluted in 0.9% NaCl and stored at room temperature (20°C to 25°C), vancomycin solutions at 62.5 mg/mL and 83.3 mg/mL retained more than 90.0% of the initial concentration after 48 h and 24 h, respectively. Barbault and others16 studied the stability of vancomycin eye drops 50 mg/mL in 0.9% NaCl in glass vials. They concluded that the eye drops were stable for 15 days at 25°C, with the appearance of new peaks on the chromatogram after 7 days of storage.
The 3 syringes of vancomycin 83.3 mg/mL in 0.9% NaCl precipitated after 48 h of storage, in contrast to the 62.5 mg/mL solutions, which did not show any physical modification during the stability study. These results are discrepant with the results in the first stage of this study, described above. When we evaluated the potential risk of precipitation for vancomycin between 40 and 83.3 mg/mL, we observed no visible precipitate. The risk of precipitation for vancomycin 80 mg/mL was demonstrated by Masse and others,10 who observed the formation of precipitates after 12 h of storage. They used an electric syringe pump over 24 h at 22°C. In our study, mechanical pumping did not cause precipitation.
However, it must be kept in mind that the formation of precipitates can be influenced by excipients present in the product; for example, the vancomycin used in this study (from Sandoz) contained mannitol, sodium hydroxide, and hydrochloric acid. Therefore, the results cannot be extrapolated to products containing other excipients.
In the first part of this study, which investigated the feasibility of high-concentration solutions of vancomycin, solutions with concentrations between 40 and 83.3 mg/mL in 0.9% NaCl and D5W showed no visual or subvisual modification over 48 h of storage at room temperature. In addition, mechanical pumping by means of an electric syringe pump did not cause the precipitation of vancomycin at these high concentrations.
Vancomycin in D5W at concentrations of 62.5 mg/mL and 83.3 mg/mL was physically and chemically stable over a period of 48 h at room temperature. These stability data provide additional knowledge to assist intensive care services in daily practice. Vancomycin in 0.9% NaCl at a concentration of 62.5 mg/mL was physically and chemically stable over a period of 48 h at room temperature; at a concentration of 83.3 mg/mL, the solution was physically and chemically stable over a period of 24 h. Therefore, for high concentrations of vancomycin, D5W is recommended as the solvent.
1 Murphy M, Jamieson E, Alrifai Z. Vancomycin continuous infusions in adult critical care: guideline. Nottingham (UK): NHS, Nottingham University Hospitals; 2018 Nov [cited 2019 Mar 27]. Available from: http://www.facm.ucl.ac.be/vancomycin/Administration-of-vancomycin-by-continuous-infusion.pdf
2 Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al.; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–128.
3 Antibioguide V8-2016. Référentiel Lorrain d’antibiologie en établissements de soins. Antibiolor (Réseau Lorrain d’antibiologie); 2016.
4 Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B, et al. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother. 2001;45(9):2460–7.

5 Vancomycin 1000 mg. Powder for concentrate for solution for infusion. Summary of product characteristics [product monograph]. Consilient Health Ltd; updated 2018 May.
6 Vancomycin 1000 mg. Powder for concentrate for solution for infusion. Summary of product characteristics [product monograph]. Mylan; updated 2018 Dec.
7 Godet M, Simar J, Closset M, Hecq JD, Braibant M, Soumoy L, et al. Stability of concentrated solution of vancomycin hydrochloride in syringes for intensive care units. Pharm Technol Hosp Pharm. 2018;3(1):23–30.
8 Masse M, Genay S, Carta N, Delannoy-Rousselière C, Moreau F, Faure K, et al. Étude de la stabilité de la vancomycine à 40 mg/mL au cours d’une perfusion de 24 heures [poster presentation]. Hopipharm: 65th French Hospital Pharmacy Congress; Nancy, France; 2017 May 10–12.
9 Longuet P, Lecapitaine AL, Cassard B, Batista R, Gauzit R, Lesprit P, et al.; Groupe des référents en infectiologie d’Île-de-France (GRIF). Preparing and administering injectable antibiotics: how to avoid playing God / Préparation et administration des antibiotiques par voie injectable : comment éviter de jouer à l’apprenti sorcier. Med Mal Infect. 2016;46(5):242–68.
10 Masse M, Genay S, Carta N, Moreau F, Delannoy-Rousselière C, Barthélémy C, et al. Stabilité de la vancomycine à des doses méningées dans les seringues au cours d’une perfusion de 24 heures [poster presentation]. Journées nationales d’infectiologie; Saint Malo, France; 2017 Jun 21–23.
11 Bardin C, Astier A, Vulto A, Sewell G, Vigneron J, Trittler R, et al. Guidelines for the practical stability studies of anticancer drugs: a European consensus conference. Ann Pharm Fr. 2011;69(4):221–31.
12 ICH harmonised tripartite guideline. Validation of analytical procedures: text and methodology Q2 (R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 2005 [cited 2018 Feb 23]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1_Guideline.pdf
13 Metha AC. Practice research: strategies for stability studies on hospital pharmaceutical preparations. Int J Pharm Pract. 1993;2(1):49–52.
14 Sautou V, Brossard D, Chedru-Legros V, Crauste-Manciet S, Fleury-Souverain S, Lagarce F, et al. Methodological guidelines for stability studies of hospital pharmaceutical preparations. Part 1: liquid preparations. 1st ed. SFPC and GERPAC; 2013. Available from: https://www.gerpac.eu/IMG/pdf/guide_stabilite_anglais.pdf
15 Ampe E, Delaere B, Hecq JD, Tulkens PM, Glupczynski Y. Implementation of a protocol for administration of vancomycin by continuous infusion: pharmacokinetic, pharmacodynamics and toxicological aspects. Int J Antimicrob Agents. 2013;41(5):439–46.
16 Barbault S, Aymard G, Feldman D, Pointereau-Bellanger A, Thuillier A. Étude de stabilité d’un collyre à la vancomycine à 50 mg/mL. J Pharm Clin. 1999;18(2):183–9.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors thank Jacques Kuhnlé for editorial assistance in advance of submission and Franck Blaise, Nathalie Sobalak, and Hubert Zenier for technical assistance during the study.
Canadian Journal of Hospital Pharmacy, VOLUME 72, NUMBER 5, September-October 2019