Stability of Methadone Hydrochloride for Injection in Saline Solution
M Mihaela Friciu, Hugo Alarie, Mylène Beauchemin, Jean-Marc Forest, Grégoire Leclair
ABSTRACT
Background
Until October 2014, methadone hydrochloride for injection (10 mg/mL) was available in Canada through Health Canada’s Special Access Programme. Diluted to 5 mg/mL in saline, it was used in pediatrics for acute and cancer-related pain. More recently, the department of pharmacy of the Centre hospitalier universitaire Sainte-Justine in Montréal, Quebec, proposed compounding a solution of methadone hydrochloride for injection (5 mg/mL) from bulk powder and saline solution for pediatric administration.
Objective
To assess the stability of the proposed compounded preparation.
Methods
Solutions of methadone hydrochloride in saline were prepared from bulk powder and stored in clear glass vials for up to 180 days at room temperature (25 °C) or with refrigeration (5 °C), with testing on days 0, 7, 14, 30, 60, 90, and 180. The appearance of the solutions and presence of particulate matter were assessed. A stability-indicating high-performance liquid chromatography (HPLC) method was developed to assay the concentration of methadone over time.
Results
No notable changes in appearance of the methadone solution were observed, particle counts did not exceed limits specified by the United States Pharmacopeia, and no microbial growth was observed. The HPLC analysis showed that the concentration of methadone remained above 90% on all study days.
Conclusions
Methadone hydrochloride for injection prepared from bulk powder in saline solution at a concentration of 5 mg/mL remained chemically stable for at least 180 days when stored in clear glass vials at 5 °C and at 25 °C.
KEYWORDS: methadone, powder, injection, stability
RÉSUMÉ
Contexte
Jusqu’en octobre 2014, le chlorhydrate de méthadone pour injection (10 mg/mL) était disponible au Canada à l’aide du Programme d’accès spécial de Santé Canada. Dilué à 5 mg/mL dans une solution saline, il était utilisé en pédiatrie contre les douleurs aigües et celles liées au cancer. Plus récemment, le département de pharmacie du Centre hospitalier universitaire Sainte-Justine de Montréal au Québec a proposé de préparer une solution de chlorhydrate de méthadone pour injection (5 mg/mL) à partir d’une poudre en vrac et d’une solution saline en vue de l’administrer en pédiatrie.
Objectif
Évaluer la stabilité de la préparation proposée.
Méthodes
Les préparations de chlorhydrate de méthadone diluées dans une solution saline se font à partir d’une poudre en vrac, elles sont stockées dans des fioles en verre transparent pendant180 jours à la température ambiante (25 °C) ou réfrigérées (5 °C) et sont soumises à des tests les jours 0, 7, 14, 30, 60, 90 et 180. L’apparence des solutions et la présence de substances particulaires ont fait l’objet d’une évaluation. Une méthode de chromatographie liquide à haute performance (CLHP) indiquant la stabilité a été développée pour tester la concentration de méthadone dans le temps.
Résultats
Aucun changement notable de l’apparence de la solution de méthadone n’a été observé, le nombre de particules ne dépassait pas les limites précisées par l’United States Pharmacopeia et aucune croissance microbienne n’a été observée. L’analyse CLHP a indiqué que la concentration de méthadone était demeurée au-delà de 90% durant toute la durée de l’étude.
Conclusions
Le chlorhydrate de méthadone pour injection préparé à partir de poudre en vrac dans une solution saline à une concentration de 5 mg/mL est resté chimiquement stable pendant au moins 180 jours lorsqu’il était stocké dans des fioles en verre transparent à 5 °C et à 25 °C.
MOTS CLÉS: méthadone, poudre, injection, stabilité
INTRODUCTION
Methadone is a long-acting opiate analgesic with μ-receptor agonist activity.1 It is approved for the treatment of severe chronic pain and opiate addiction in adult patients. It can be administered orally or intravenously in its salt form, methadone hydrochloride.2 The dosage is individualized according to many factors, including the severity of the pain and the patient’s tolerance of opioid analgesic.3
In the pediatric setting, methadone can be used in palliative care, for severe and chronic cancer-related nociceptive and neuropathic pain, for refractory noncancer pain, and for weaning from opioid therapy.4–6 In the hospital care setting, it is important to have access to an IV formulation of methadone as an alternative opioid therapy for pain that is refractory to conventional opioid treatment or to avoid treatment interruption for patients who are already receiving methadone and are unable to take medications by mouth. It has been proposed that methadone hydrochloride for injection (5 mg/mL) be compounded from bulk powder and saline solution for the purpose of pediatric administration. This study was undertaken to determine the stability of the proposed compounded preparation.
METHODS
A compounded formulation of methadone hydrochloride (5 mg/mL) was prepared aseptically from bulk powder dissolved in saline solution on October 27, 2016. More specifically, methadone hydrochloride USP powder (10 g, lot 12766-5313, expiry January 2017; Galenova Inc, Saint-Hyacinthe, Quebec) was dissolved in saline solution (2 L, 0.9%, lot w6i06ml, expiry March 2018; Baxter, Mississauga, Ontario) and then filtered through a sterile 0.22-μm membrane into 10-mL clear sterile glass vials (100 vials per batch, 2 batches in total). Twelve vials were used for the initial analyses, 50 vials were stored at 5 °C ± 2 °C, and the remaining 50 vials were stored at 25 °C ± 2 °C. At predetermined time points (0, 7, 14, 30, 60, and 90), 3 vials from each temperature condition were retrieved for analysis. At the end of the study (180 days), 9 vials from each temperature condition were retrieved for analysis. Unused vials and residual volumes of solution were destroyed at the end of the study or were used for microbial testing.
On each study day, each vial was inspected for visual appearance against a white and black background, and the concentration of methadone in the solution was assayed using a stability-indicating high-performance liquid chromatography (HPLC) method with ultraviolet (UV) detection. At the beginning and the end of the study, a particle count was obtained using a light obscuration method (LS-20 particle counter, Lighthouse Worldwide Solutions, Fremont, California).
The particle count was performed initially and at the end of the study (180 days) using combined solutions from 2 vials (total volume 20 mL), repeated 3 times (i.e., total of 6 vials). To count the particles in each 20-mL sample, a first fraction (4 mL) was used to flush the instrument, then 3 counts (using 5 mL each) were performed; the remaining solution was discarded. The specification was based on test 1.B of General Chapter <788> in the United States Pharmacopeia–National Formulary, for solutions intended for injection supplied in containers smaller than 100 mL: “The preparation complies with the test if the average number of particles present in the units tested does not exceed 6000 per container equal to or greater than 10 μm and does not exceed 600 per container equal to or greater than 25 μm.”7
For the HPLC analyses, each test sample (20 μL) was placed in an HPLC injection vial, diluted with a 20:80 mixture of methanol and water (1000 μL), and mixed with a vortex mixer (20 s). Solutions for HPLC analysis (nominal concentration 98 μg/mL) were analyzed immediately after preparation using an HPLC system (Prominence UFLC, Shimadzu, Laval, Quebec) equipped with an LC-20AD binary pump, a DGU-20A5 solvent degasser, a SPD-M20A multiple-wavelength photodiode array detector set at 220 nm, an SIL-20AC HT refrigerated autosampler set at 5 °C, a CTO-20AC column oven set at 40 °C, and a Kinetex XB-C18 column (4.6 × 100 mm, 5 μm, Phenomenex, Torrance, California). An isocratic method (1:1 methanol: phosphate buffer, 20 mmol/L, pH 3) with a flow rate of 0.9 mL/min was used. The methadone peak eluted at approximately 5.5 min; the peak area was used to perform the quantification. Six test samples were prepared for the initial analysis, whereas 3 test samples were prepared for each condition of the stability study. Each test sample (10 μL) was injected in duplicate (n = 6 × 2 at time zero and n = 3 × 2 for each time point during the stability study). The HPLC analysis of collected samples was performed at each study time point.
To perform the calibration, a stock solution of methadone base in methanol (1 mg/mL, lot FE06221502, expiry July 2020; Cerilliant Corporation, Round Rock, Texas) was diluted to concentrations of 80, 90, 100, 110, and 120 μg/mL using a methanol-water mixture (20:80) and analyzed by HPLC (r2 not less than 0.999; n = 3). The 100 μg/mL standard was also injected after every 24 sample injections to ensure system stability (n = 3). The highest intraday coefficient of variation observed was 0.4% (n = 3, all concentrations) and the highest interday coefficient of variation was 1.57% (3 consecutive days, n = 3 × 3, target concentration).
Aliquots (0.5 mL) of a methadone stock solution prepared at 5 mg/mL in saline solution were mixed with water (0.5 mL), aqueous solution of 3% hydrogen peroxide (0.5 mL), hydrochloric acid 1 mol/L (0.5 mL), and sodium hydroxide 1 mol/L (0.5 mL) and then submitted to forced degradation by heating at 60 °C for 4 h. After cooling, aliquots (40 μL) of these solutions were diluted with the 20:80 mixture of methanol and water (960 μL) and assayed by HPLC.
RESULTS
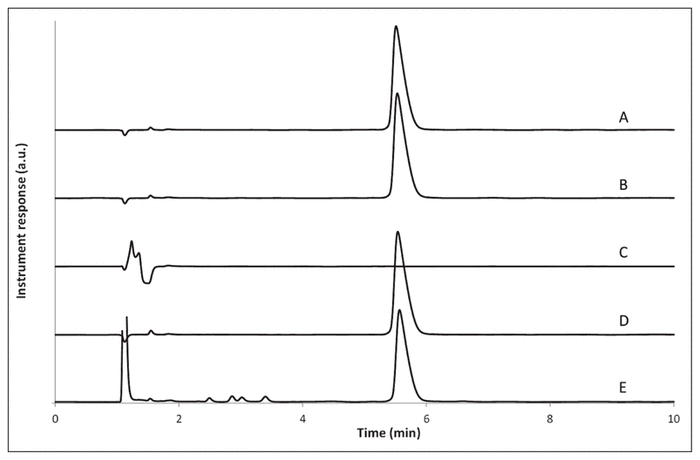
After forced degradation, recovery of methadone hydrochloride was variable: 102% after heating at 60 °C for 4 h in one volume of purified water, 0% after addition of sodium hydroxide 1 mol/L, 101% after addition of hydrochloric acid 1 mol/L, and 87% after addition of hydrogen peroxide 3% (Figure 1). These results suggest that methadone precipitates in alkaline conditions and is prone to oxidation. No peak overlap of methadone with impurities or degradation products was observed. Similarity of the UV spectra at all of the sampling points on the peak was compared using the HPLC system software (LabSolution version 5.54; Shimadzu, Laval, Quebec) to compute a similarity index and determine the presence of multiple components within the peak. With this system, the similarity index can vary between −1 (dissimilar) and 1 (identical).8 Here, the methadone peak purity index calculated between 190 and 250 nm was not less than 0.9999 in all cases.
| |
|
|
|
Figure 1 A: Representative chromatogram of methadone injectable solution in water (1:1) after storage at 5 °C for 4 h. B–E: Representative chromatograms of methadone injectable solution submitted to forced degradation at 60 °C for 4 h in (B) water, (C) sodium hydroxide 1 mol/L, (D) hydrochloric acid 1 mol/L, and (E) hydrogen peroxide 3%.
|
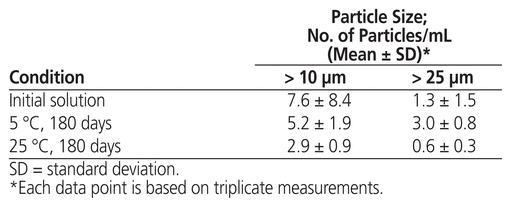
At the beginning of the stability study, the study preparation was clear, and the concentration of methadone hydrochloride was 5.02 (standard deviation 0.01) mg/mL. No notable changes in appearance were observed after storage at either temperature for up to 180 days. Over the study period, the concentration of methadone hydrochloride in solution stored at either temperature remained not less than 90.0% of the initial concentration at all time points (Table 1). The particle count complied with United States Pharmacopeia–National Formulary specifications at the start and end of the study under both storage conditions (Table 2). No microbial growth was noted in samples tested at the end of the study.
Table 1 Concentration of Methadone Hydrochloride Remaining after Storage
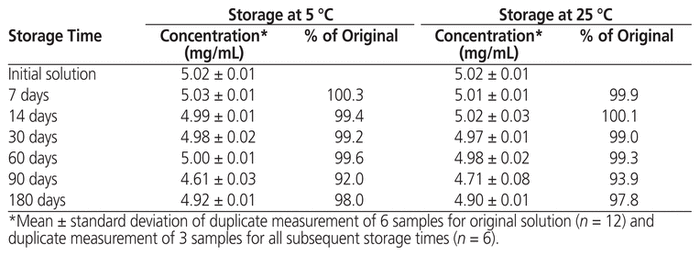
Table 2 Particulate Matter Observed in Samples
DISCUSSION
Methadone hydrochloride injection USP (Synastone 10 mg/mL, Auden Mckenzie Group, Ruislip, England) was available in Canada through Health Canada’s Special Access Programme until production of this drug was discontinued in October 2014. The stability of methadone hydrochloride in aqueous solutions is good.9,10 Methadone hydrochloride for injection diluted in saline solutions (at 1, 2, or 5 mg/mL) was reported to be stable for at least 4 weeks at room temperature.9 Solutions of methadone hydrochloride prepared in orange-flavoured Tang® drink mix for oral administration were stable for 91 days at room temperature or under refrigeration.10 With methadone hydrochloride injection USP no longer available, this study proposes a compounded methadone hydrochloride solution for injection (5 mg/mL) prepared from bulk powder and saline solution as an alternative for pediatric administration.
CONCLUSION
This study demonstrated that methadone hydrochloride for injection prepared from bulk powder in saline solution at a concentration of 5 mg/mL remained chemically stable and conformed to the specifications for particulate matter in solutions intended for injection for at least 180 days when stored in clear glass vials at 5 °C and at 25 °C.
References
1 Anderson IB, Kearney TE. Medicine cabinet: use of methadone. West J Med. 2000;172(1):43–6.


2 Reisine T, Pasternak G. Opioid analgesics and antagonists. In: Goodman LS, Limbird LE, Milinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 9th ed. McGraw-Hill; 1996:544–5.
3 Fainsinger R, Schoeller T, Bruera E. Methadone in the management of cancer pain: a review. Pain. 1993;52(2):137–47.

4 Anghelescu DL, Faughnan LG, Hankins GM, Ward DA, Oakes LL. Methadone use in children and young adults at a cancer center: a retrospective study. J Opioid Manag. 2011;7(5):353–61.


5 Giby K, Vaillancour R, Varughese N, Vadeboncoeur C, Pouliot A. Use of methadone for opioid weaning in children: prescribing practices and trends. Can J Hosp Pharm. 2014;67(2):149–56.

6 Habashy C, Spring E, Hall EA, Anghelescu DL. Methadone for pain management in children with cancer. Paediatr Drugs. 2018;20(5):409–16.

7 <788> Particulate matter in injections. In: United States Pharmacopeia–National Formulary. USP 36/NF 31. United States Pharmacopeial Convention; 2012:350–3.
8 LabSolutions data acquisition and processing theory guide. Instruction manual. Shimadzu Corporation; 2012 Mar.
9 Denson DD, Crews JC. Stability of methadone hydrochloride in 0.9% sodium chloride injection in single-dose plastic containers. Am J Hosp Pharm. 1991;48(3):515–7.
10 Donnelly R. Chemical stability of methadone concentrate and powder diluted in orange-flavored drink. Int J Pharm Compound. 2004;8(6):489–91.
M Mihaela Friciu, MSc, is a Research Associate with the Faculty of Pharmacy, Université de Montréal, Montréal, QuebecHugo Alarie, BSc, is a Master of Science candidate in the Faculty of Pharmacy, Université de Montréal, Montréal, QuebecMylène Beauchemin, PharmD, MSc, is a Pharmacist with the Department of Pharmacy, Centre hospitalier universitaire Sainte-Justine, Montréal, QuebecJean-Marc Forest, BPharm, DPH, MSc, is a Pharmacist with the Compounding Service (Fabrication) in the Department of Pharmacy, Centre hospitalier universitaire Sainte-Justine, Montréal, QuebecGrégoire Leclair, BPharm, PhD, is an Associate Professor with the Faculty of Pharmacy, Université de Montréal, Montréal, Quebec
Competing interests: None declared. (
Return to Text
)
Address correspondence to: Jean-Marc Forest, Département de pharmacie, Centre hospitalier universitaire Sainte-Justine, 3175, côte Sainte-Catherine, Montréal QC H3T 1C5, e-mail:jean-marc.forest.hsj@ssss.gouv.qc.ca
(Return to Top)
Funding: This study was sponsored by the Centre hospitalier universitaire Sainte-Justine in the form of an unrestricted grant. (
Return to Text
)
Canadian Journal of Hospital Pharmacy, VOLUME 73, NUMBER 2, March-April 2020