Arden R Barry, Candy LeeABSTRACT
Background
Sacubitril/valsartan is a first-in-class angiotensin receptor–neprilysin inhibitor indicated in the management of heart failure with reduced ejection fraction, based on the results of the PARADIGM-HF trial. Practice-based studies are needed to validate its effect in real-world settings. Clinical pharmacists are ideally situated to assess and titrate sacubitril/valsartan.
Objective
To evaluate the utilization, safety, and tolerability of sacubitril/valsartan in a multidisciplinary heart failure clinic, with assessment and titration by a clinical pharmacist or a nurse practitioner.
Methods
A retrospective cohort study was conducted at a heart failure clinic in Abbotsford, Canada. Included were adult patients with heart failure who were currently or formerly taking sacubitril/valsartan. Data collected for the period October 2015 to February 2019 included patient characteristics, New York Heart Association (NYHA) classification, concurrent medications, sacubitril/valsartan dose, adverse effects, and discontinuation rate.
Results
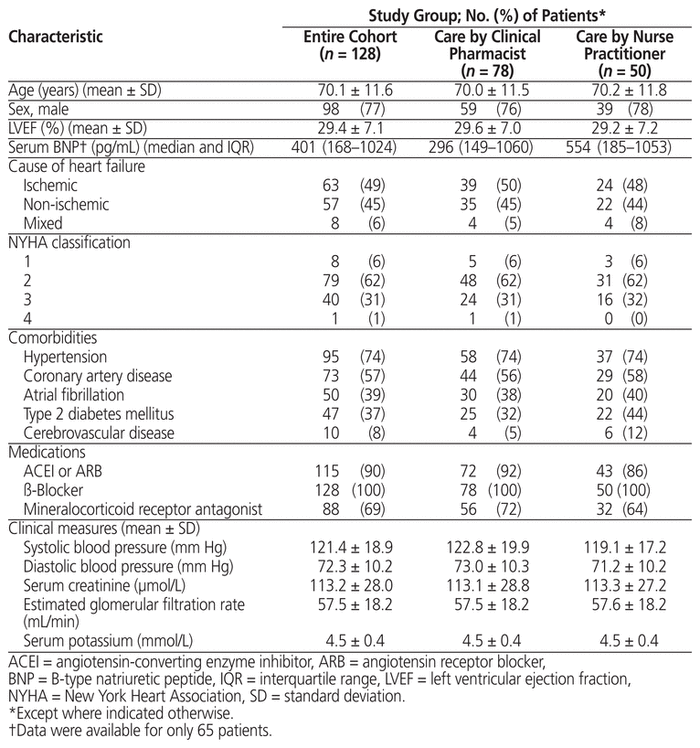
In total, 128 patients were included. Mean age was 70.1 years, 98 (77%) of the patients were men, and 79 (62%) had NYHA class 2 heart failure. The clinical pharmacist managed care for 78 (61%) of the patients, and the nurse practitioner managed care for 50 (39%). Forty-one (32%) of the patients met modified PARADIGM-HF inclusion criteria. Eighty-five (66%) of the patients achieved the target dose of sacubitril/valsartan, with similar proportions for the clinical pharmacist and nurse practitioner groups, over a mean of 2.2 clinic visits. Patients who achieved the sacubitril/valsartan target dose, relative to those who did not, were significantly younger and had higher mean systolic blood pressure at baseline. Twenty-nine percent of patients (35/119) had an improvement in NYHA classification from before initiation of sacubitril/valsartan to achievement of target or maximally tolerated dose. Eighty-five (66%) of the patients experienced an adverse effect, primarily hypotension, and 12 (9%) required a dose reduction. Only 9 (7%) patients discontinued therapy.
Conclusions
This study demonstrates the real-world safety and tolerability of sacubitril/valsartan in the treatment of heart failure, and reinforces that clinical pharmacists can effectively assess and titrate medications in a multidisciplinary heart failure clinic.
KEYWORDS: sacubitril/valsartan, angiotensin receptor-neprilysin inhibitor, heart failure, clinical pharmacists, clinical medicine
RÉSUMÉ
Contexte
Le sacubitril-valsartan est un inhibiteur novateur des récepteurs de l’angiotensine-néprilysine, indiqué dans la gestion de l’insuffisance cardiaque accompagnée d’une baisse de la fraction d’éjection, selon les résultats de l’essai PARADIGM-HF. Des études fondées sur la pratique sont nécessaires pour valider ses effets en contexte réel. Les pharmaciens cliniciens sont bien placés pour évaluer et titrer le sacubitril-valsartan.
Objectif
Évaluer l’utilisation, l’innocuité et le seuil de tolérance du sacubitril-valsartan en clinique multidisciplinaire d’insuffisance cardiaque, l’évaluation et le titrage étant effectués par un pharmacien clinicien ou une infirmière praticienne.
Méthodes
Une étude de cohorte rétrospective a été menée au sein d’une clinique d’insuffisance cardiaque à Abbotsford, au Canada. Les patients adultes inclus dans l’étude souffraient d’insuffisance cardiaque, ils prenaient ou avaient pris du sacubitril-valsartan. Les données recueillies entre octobre 2015 et février 2019 comprenaient les caractéristiques des patients, la classification de la New York Heart Association (NYHA), les médicaments pris de façon concomitante, la dose de sacubitril-valsartan, les effets secondaires et le taux d’abandon.
Résultats
Au total, 128 patients ont participé à l’étude. L’âge moyen des patients était de 70,1 ans, 98 d’entre eux (77 %) étaient des hommes et 79 (62 %) souffraient d’une insuffisance cardiaque de classe 2 selon la classification de la NYHA. Le pharmacien clinicien gérait les soins de 78 patients (61 %) et la pharmacienne praticienne gérait ceux de 50 patients (39 %). Quarante-et-un patients (32 %) répondaient aux critères d’inclusion modifiés de PARADIGM-HF. Quatre-vingt-cinq (66 %) patients atteignaient le dosage ciblé de sacubitril-valsartan dans des proportions similaires entre le groupe du pharmacien clinicien et celui de l’infirmière praticienne, à raison d’une moyenne de 2,2 visites en clinique. Les patients ayant atteint le dosage ciblé de sacubitril-valsartan, par rapport à ceux ne l’ayant pas atteint, étaient considérablement plus jeunes et leur tension artérielle systolique moyenne de base était plus élevée. Une amélioration de la classification NYHA a été observée chez 29 % des patients (35/119) entre le début de la prise de sacubitril-valsartan et l’atteinte du dosage ciblé ou de la dose maximale tolérée. Des effets secondaires ont été observés chez 85 patients (66 %), principalement une hypotension, et 12 d’entre eux (9 %) ont dû réduire la dose. Seuls 9 patients (7 %) ont dû abandonner la thérapie.
Conclusions
Cette étude démontre l’innocuité et le seuil de tolérance en contexte réel du sacubitril-valsartan pour le traitement de l’insuffisance cardiaque. Elle renforce le fait que les pharmaciens cliniciens peuvent efficacement évaluer et titrer des médicaments au sein d’une clinique d’insuffisance cardiaque multidisciplinaire.
MOTS CLÉS: sacubitril-valsartan, inhibiteur des récepteurs de l’angiotensinenéprilysine, insuffisance cardiaque, pharmaciens cliniciens, médecine clinique
Heart failure–associated mortality has improved over the past 30 years, which is attributable in part to several pharmacologic therapies, including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), β-blockers, and mineralocorticoid receptor antagonists.1–6 However, substantial morbidity and mortality remain, with an estimated 5-year mortality rate of approximately 50%.7 In the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure), sacubitril/valsartan, a first-in-class angiotensin receptor–neprilysin inhibitor, reduced cardiovascular deaths and heart failure hospitalizations relative to enalapril in patients with heart failure with reduced ejection fraction.8 On the basis of this trial, contemporary North American heart failure guidelines now recommend sacubitril/valsartan in place of ACEIs/ARBs for patients who remain symptomatic despite appropriate guideline-directed medical therapy.9,10
The PARADIGM-HF trial had relatively strict inclusion criteria, specifically patients with heart failure with reduced ejection fraction, left ventricular ejection fraction (LVEF) less than or equal to 40%, New York Heart Association (NYHA) class 2–4 symptoms, and elevated serum B-type natriuretic peptide (BNP).8 Furthermore, the PARADIGM-HF trial had an extensive run-in period, whereby only patients who tolerated target doses of both sacubitril/valsartan and enalapril underwent randomization. Thus, the results of the PARADIGM-HF trial may overestimate the tolerability of sacubitril/valsartan and patients’ ability to achieve the target dose in a real-world setting. These factors highlight the need for observational studies to evaluate the use of sacubitril/valsartan in practice.
Studies have shown that medication management at multidisciplinary heart failure clinics reduces the risk of all-cause and heart failure hospitalizations, as well as all-cause mortality.11,12 In addition, pharmacists have been shown to play an integral role in the care of patients with heart failure, including assessment and titration of guideline-directed medical therapy.13 The purpose of the current study was to evaluate the utilization, tolerability, and safety of sacubitril/valsartan at a heart failure clinic with a multi-disciplinary approach (clinical pharmacist or nurse practitioner) to assessment and titration.
This retrospective cohort study was conducted at a tertiary heart failure clinic located at the Abbotsford Regional Hospital and Cancer Centre in Abbotsford, British Columbia. The study included all adult patients (≥ 18 years of age) with a clinical diagnosis of heart failure of any type who were currently or formerly taking sacubitril/valsartan. Patients with missing baseline data and those who died during sacubitril/valsartan titration were excluded.
Sacubitril/valsartan was approved by Health Canada in October 2015 and became eligible for publicly funded drug coverage in British Columbia in May 2018. Data for this study were collected retrospectively for the period from October 2015 to February 2019, with data collection occurring between July 2017 and February 2019. It was not possible to identify patients who were taking sacubitril/valsartan and who were discharged from the clinic before July 2017, because their paper-based outpatient medical records were unavailable. The 2 authors (C.L. from July 2017 to March 2018; A.R.B. from January 2019 to February 2019) collected the data from both paper-based and electronic medical records using a standardized data collection form. The study protocol was submitted to the Fraser Health Research Ethics Board, which deemed it to be a quality improvement project and thus exempt from review.
The heart failure clinic provides specialized cardiac care to an active roster of approximately 400 patients with heart failure in the Abbotsford region. It is staffed by a rotating group of 5 cardiologists and 1 internist, as well as 1 nurse practitioner, 2 registered nurses, 1 dietician, and 1 clinical pharmacist (A.R.B.). The clinical pharmacist provides consultative services, based on referrals, to assess and titrate all heart failure medications. For each patient, sacubitril/valsartan therapy was initiated by either a physician (cardiologist or internist) or the nurse practitioner. All patients whose sacubitril/valsartan was initiated by a cardiologist or internist were referred to the clinical pharmacist (if available) for assessment and titration. These patients were typically scheduled to see the clinical pharmacist every 4–8 weeks (depending on availability) until they achieved the target or maximally tolerated dose of sacubitril/valsartan. For patients whose sacubitril/valsartan was initiated by the nurse practitioner, as well as those with initiation by a physician but for whom timely review (e.g., > 8 weeks) by the pharmacist could not be scheduled, the nurse practitioner performed assessment and titration. For each clinic visit, the clinical pharmacist or nurse practitioner performed a comprehensive patient assessment, including functional status (i.e., NYHA classification), medication review, laboratory monitoring, and physical assessment. Because the clinical pharmacist did not have prescribing privileges, all medication changes were briefly discussed with a cardiologist, internist, or the nurse practitioner to generate a verbal order. Once the target or maximally tolerated dose of sacubitril/valsartan was achieved, patients whose therapy was managed by the clinical pharmacist were referred back to the cardiologist or internist for further management of heart failure. The maximally tolerated dose was defined at the clinician’s discretion, but was typically based on the patient experiencing an intolerable adverse effect at a higher dose or being considered to be at high risk of an adverse effect if the dose was increased. Patients who were not deemed to be receiving the maximally tolerated dose but were not at the target dose at the time of data collection were classified as being in the titration phase. Patients’ tolerance of the target dose was assessed at a final follow-up clinic visit after the dose was increased.
For eligible patients, the following baseline data were collected: age, sex, cause of heart failure, NYHA classification, LVEF, comorbid medical conditions, blood pressure, serum potassium, serum creatinine, estimated glomerular filtration rate, serum BNP (within the preceding 12 months), concurrent heart failure medications (ACEI/ARB, β-blocker, mineralocorticoid receptor antagonist), and starting dose of sacubitril/valsartan. The LVEF was recorded as the most recent assessment via echocardiography (as a mean if a range was provided), multigated acquisition radionuclide angiography, or magnetic resonance imaging. The following data were collected for each clinic visit: sacubitril/valsartan dose, NYHA classification, presence of adverse effects, sacubitril/valsartan discontinuation (if applicable), and reason for discontinuation (if applicable). The dose of sacubitril/valsartan was reported as the combined total of sacubitril and valsartan (i.e., 49/51 mg was reported as 100 mg). Symptomatic adverse effects were assessed by questioning patients about common adverse effects (e.g., light-headedness) or by self-reporting, and the patient’s blood pressure and bloodwork (e.g., serum creatinine, serum potassium) were reviewed at each clinic visit at the clinician’s discretion. Predefined adverse effects included mild hyperkalemia (defined as serum potassium 5.1–5.5 mmol/L), moderate hyperkalemia (defined as serum potassium > 5.5 mmol/L), hypotension (defined as systolic blood pressure < 100 mm Hg, diastolic blood pressure < 60 mm Hg, or symptoms of light-headedness associated with a reduction in blood pressure), and acute renal impairment (defined as ≥ 30% increase in serum creatinine from baseline). Any other potential adverse effects reported by the patient were also collected. Each adverse effect was counted only once for each patient. After the final clinic visit, the number of clinic visits (excluding the initial visit when sacubitril/valsartan was initiated), the sacubitril/valsartan dose, and the NYHA classification were collected.
The primary outcome was the proportion of patients for whom sacubitril/valsartan was prescribed who met modified PARADIGM-HF trial inclusion criteria (defined as NYHA class 2–4 symptoms, LVEF ≤ 40%, serum BNP ≥ 150 pg/mL, and ACEI/ARB and β-blocker before initiation). Secondary outcomes were the proportion of patients who achieved the sacubitril/valsartan target dose (200 mg twice daily), number of clinic visits, rate and type of adverse effects, rate and reason for sacubitril/valsartan discontinuation, and change in NYHA classification from before sacubitril/valsartan initiation to achievement of target or maximally tolerated dose. As well, the following variables were compared between patients whose care was managed by the clinical pharmacist and those with care managed by the nurse practitioner: patient characteristics, proportion of patients who achieved the target dose of sacubitril/valsartan, number of clinic visits, rate and type of adverse effects, and rate of sacubitril/valsartan discontinuation.
The analysis was based on descriptive statistics. Categorical variables are expressed as frequencies with percentages. Continuous variables are expressed as means with standard deviations or medians with interquartile ranges (IQRs). Comparisons were made with an unpaired, 2-sided Student t test for continuous variables and a χ2 test for categorical variables. All statistical analyses were performed with IBM SPSS Statistics (version 21, IBM Corporation, Armonk, New York). A 2-sided p value of less than 0.05 was considered statistically significant.
After review of approximately 700 medical records, 140 patients were identified as currently or formerly taking sacubitril/valsartan. Baseline data were unavailable for 9 of these patients, and 3 patients died during sacubitril/valsartan titration. Therefore, 128 patients were included in the analysis. Patient characteristics are summarized in Table 1. The mean total daily starting dose of sacubitril/valsartan was 149 (standard deviation [SD] 55) mg; of the 128 patients, 67 (52%) were started on 50 mg twice daily, 60 (47%) on 100 mg twice daily, and 1 (1%) on 200 mg twice daily. Forty-one patients (32%) met the modified PARADIGM-HF inclusion criteria (Table 2). The most common reason for not meeting the PARADIGM-HF criteria was lack of baseline serum BNP assessment.
Table 1 Baseline Patient Characteristics
Table 2 Comparison with Modified Inclusion Criteria* for the PARADIGM-HF Trial8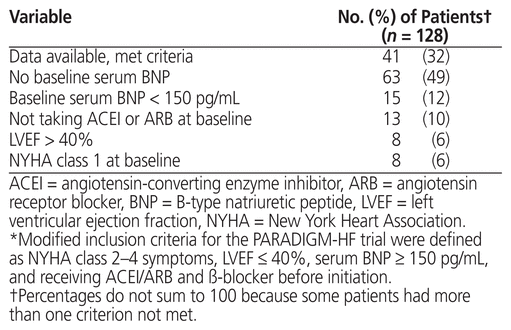
The sacubitril/valsartan regimens are summarized in Table 3. In total, 85 patients (66%) achieved the target dose of sacubitril/valsartan. The mean total daily dose of sacubitril/valsartan achieved was 331 (SD 114) mg. The mean number of follow-up clinic visits was 2.2 (SD 1.0). Paired data for NYHA classification were available for 119 patients (93%). The median NYHA classification was 2 (IQR 2–3) before sacubitril/valsartan initiation and 2 (IQR 2–2) after achievement of the target or maximally tolerated dose. Eighty (67%) of these 119 patients had no change in their NYHA classification, 35 patients (29%) had an improvement in NYHA classification, and 4 patients (3%) had a decline in NYHA classification after achieving the target or maximally tolerated dose of sacubitril/valsartan.
Table 3 Dosing of Sacubitril/Valsartan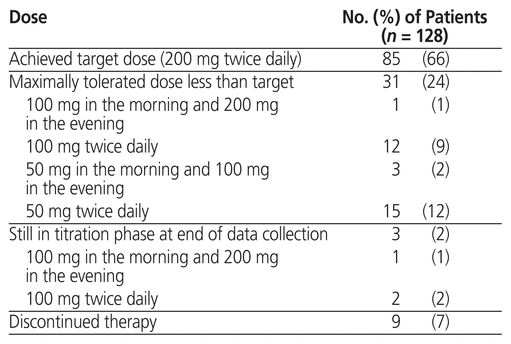
Adverse effects are summarized in Table 4. The most common adverse effect was hypotension. Twelve patients (9%) required a dose reduction of sacubitril/valsartan because of an adverse effect: 10 patients with hypotension (1 of whom was admitted to hospital) and 2 patients with hyperkalemia. Nine patients (7%) discontinued sacubitril/valsartan: 3 because of gastrointestinal issues (diarrhea, bloating, and/or constipation), 3 because of hypotension, and 3 for unknown reasons (for 1 patient, sacubitril/valsartan was discontinued in hospital; the other 2 self-discontinued the therapy). No cases of angioedema were observed.
Table 4 Adverse Effects with Sacubitril/Valsartan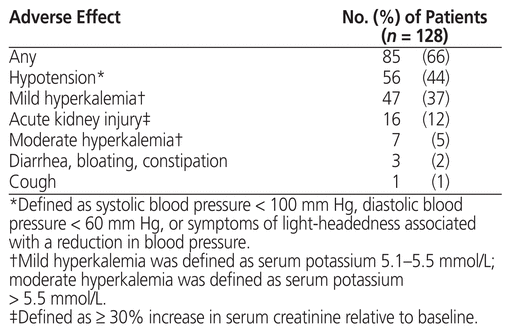
Patients who achieved the target dose of sacubitril/valsartan (n = 85), relative to those who did not (n = 34), were significantly younger (68.2 years versus 73.6 years, p = 0.03) and had a higher mean baseline systolic blood pressure (123.8 mm Hg versus 113.3 mm Hg, p = 0.004). Furthermore, patients who achieved the target dose, relative to those who did not, had a lower rate of overall adverse effects (54% [46/85] versus 94% [32/34], p < 0.001), hypotension (26% [22/85] versus 85% [29/34], p < 0.001), and acute kidney injury (8% [7/85] versus 24% [8/34], p = 0.02). There was no significant difference between groups in the rate of mild hyperkalemia (36% [31/85] versus 35% [12/34], p = 0.90) or moderate hyperkalemia (4% [3/85] versus 12% [4/34], p = 0.09).
Sacubitril/valsartan assessment and titration was managed by the clinical pharmacist for 78 patients (61%) and by the nurse practitioner for 50 patients (39%). There were no statistically significant differences in baseline characteristics between the groups (Table 1). The mean number of clinic visits per patient was 2.1 (SD 1.0) for those in the clinical pharmacist group and 2.3 (SD 1.1) for those in the nurse practitioner group (p = 0.37). Of the 9 patients who discontinued sacubitril/valsartan, 4 had care managed by the clinical pharmacist and 5 had care managed by the nurse practitioner. Among the patients who continued sacubitril/valsartan therapy, 66% (49/74) of those with care managed by the clinical pharmacist achieved the target dose of sacubitril/valsartan, compared with 80% (36/45) of those with care managed by the nurse practitioner (p = 0.11). There were no significant differences in the rates of adverse effects between groups.
In this study, sacubitril/valsartan was generally well tolerated and safe for a select, real-world cohort of patients with heart failure. There were some differences between the present study population and patients in the PARADIGM-HF trial8—older age (70 versus 64 years), higher proportion of patients with NYHA class 3 heart failure (31% versus 23%), and higher median serum BNP (401 pg/mL versus 255 pg/mL)—which is consistent with other observational studies.14–16 In other respects, patients were similar between the present study and the PARADIGM-HF trial: proportion of women (23% versus 21%), systolic blood pressure (121 mm Hg versus 122 mm Hg), serum creatinine (113 μmol/L versus 100 μmol/L), LVEF (29% versus 30%), and proportion with hypertension (74% versus 71%). Baseline use of β-blockers and mineralocorticoid receptor antagonists was higher in the present study (100% versus 83% and 69% versus 54%, respectively). Only one-third of patients in the present study met the modified PARADIGM-HF criteria; however, this was primarily due to a lack of assessment of baseline serum BNP, which is not listed as a criterion for clinical use in the Canadian monograph for sacubitril/valsartan.17 Therefore, it could be argued that use of sacubitril/valsartan in these patients was appropriate. Eight patients (6%) had LVEF over 40% and would not have been enrolled in the PARADIGM-HF trial. Notably, the recently published PARAGON-HF trial (Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction) demonstrated that among patients with LVEF of 45% or higher, sacubitril/valsartan improved NYHA classification but did not reduce the composite outcome of hospitalization for heart failure and death from cardiovascular causes.18 Therefore, sacubitril/valsartan should be recommended only for patients with heart failure with reduced ejection fraction, which was the case for most of the patients in the present study.
Overall, approximately two-thirds of patients achieved the target dose, which is comparable to or higher than results in other observational studies.14,15,19–21 One possible explanation is that the present study was conducted at a multidisciplinary heart failure clinic with titration of heart failure medication led primarily by a clinical pharmacist. As well, both the clinical pharmacist and the nurse practitioner provided frequent follow-up (typically every 4–8 weeks) with a specific focus on sacubitril/valsartan assessment and titration. Inability to achieve the target dose of sacubitril/valsartan was likely secondary to the presence of adverse effects, as opposed to other factors such as status quo bias, inertia of previous practice, or lack of self-efficacy. Patients who experienced an adverse effect, particularly hypotension and acute kidney injury, were less likely to achieve the target dose. Accordingly, older patients and those with lower systolic blood pressure were at a higher risk of experiencing an adverse effect. A greater proportion of patients with care managed by the nurse practitioner, relative to those with care managed by the clinical pharmacist, achieved the sacubitril/valsartan target dose (80% versus 66%), although the difference was not statistically significant. This difference may have been due to variation in the baseline characteristics; specifically, more patients in the clinical pharmacist group were taking a mineralocorticoid receptor antagonist at baseline (72% versus 64%).
This study reinforces the concept that clinical pharmacists can effectively assess and titrate heart failure pharmacotherapy and supports the creation of pharmacist-led titration clinics to achieve guideline-directed medical therapy. The benefit of pharmacist involvement as part of a multidisciplinary team in the management of heart failure is well established. Studies have shown that medication management at multidisciplinary heart failure clinics reduces the risk of all-cause and heart failure hospitalizations, as well as all-cause mortality.11,12 More specifically, pharmacist care of patients with heart failure has been shown to reduce both all-cause and heart failure–related hospitalizations.13 In addition, pharmacist-led titration of heart failure medications in outpatient settings has been shown to increase the rate of achievement of target doses of ACEIs/ARBs and β-blockers.22–24 Pogge and Davis19 showed that among 52 heart failure patients for whom sacubitril/valsartan was prescribed in a pharmacist-led clinic, 45 patients (87%) achieved the target dose.
In the present study, 29% of patients who were taking the target or maximally tolerated dose of sacubitril/valsartan had an improvement in their NYHA classification. However, the overall median NYHA classification did not change from baseline to achievement of the target or maximally tolerated dose. In other observational studies, sacubitril/valsartan has been associated with lower NYHA classification, as well as increases in LVEF and peak oxygen consumption and reductions in diuretic use, serum BNP, and hospitalizations.14,20,25–28 Although 66% of patients in the present study experienced an adverse effect while taking sacubitril/valsartan, it did not typically lead to discontinuation, which is consistent with other literature.15,20,28 Hypotension was markedly higher in the present study compared with the PARADIGM-HF trial (44% versus 14%), but was consistent with other observational studies.15,20,28,29 Furthermore, the rate of hypotension was relatively high, despite a mean baseline blood pressure of roughly 121/72 mm Hg. Conversely, the rate of moderate hyperkalemia (serum potassium > 5.5 mmol/L) was approximately 5% in the present study, as opposed to 17% in the PARADIGM-HF trial. Although angioedema was not observed in the present study, this result was unsurprising, given that the incidence in the PARADIGM-HF trial was only 0.3%.8
This study had limitations that warrant discussion. It was a single-centre medical record review that relied on the completeness and accuracy of documentation. Because the study was primarily descriptive, no formal sample size calculation was performed for the comparison of patients with care managed by the clinical pharmacist versus the nurse practitioner. The observed improvement in NYHA classification for a small proportion of patients is compelling, because this result was based on a paired sample. However, other factors, such as fluid and sodium restriction and exercise, may have contributed to the observed improvement. Despite having objective criteria, the NYHA classification is a subjective assessment that is at risk of inter-user variability; however, this limitation may have been minimized by having the same clinician (clinical pharmacist or nurse practitioner) perform the assessment at each follow-up visit. Patients were followed only until they achieved the target or maximally tolerated dose of sacubitril/valsartan. Thus, further studies are warranted to evaluate the long-term safety and tolerability of sacubitril/valsartan in practice.
This study has demonstrated the real-world safety and tolerability of sacubitril/valsartan in the management of heart failure. More than two-thirds of patients achieved the target dose of the drug. Although the overall incidence of adverse effects (particularly hypotension) was common, these effects rarely necessitated discontinuation of therapy. This study reinforces that clinical pharmacists are effective in assessing and titrating heart failure medications in a multidisciplinary heart failure clinic.
1 SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302.
2 Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, et al.; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194–9.
3 MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001–7.
4 CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13.
5 Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al; Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709–17.
6 Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shil H, et al.; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
7 Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603.

8 McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al.; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11): 993–1004.
9 Ezekowitz JA, O’Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017; 33(11):1342–433.
10 Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017; 136:e137–e161.
11 Gandhi S, Mosleh W, Sharma UC, Demers C, Farkouh ME, Schwalm JD. Multidisciplinary heart failure clinics are associated with lower heart failure hospitalization and mortality: systematic review and meta-analysis. Can J Cardiol. 2017;33(10):1237–44.
12 Wijeysundera HC, Trubiani G, Wang X, Mitsakakis Nm, Austin PC, Ko DT, et al. A population-based study to evaluate the effectiveness of multi-disciplinary heart failure clinics and identify important service components. Circ Heart Fail. 2013;6(1):68–75.
13 Koshman SK, Charrois TL, Simpson SH, McAlister FA, Tsuyuki RT. Pharmacist care of patients with heart failure: a systematic review of randomized trials. Arch Intern Med. 2008;168(7):687–94.
14 Wachter R, Viriato D, Klebs S, Grunow SS, Schindler M, Engelhard J, et al. Early insights into the characteristics and evolution of clinical parameters in a cohort of patients prescribed sacubitril/valsartan in Germany. Postgrad Med. 2018;130(3):308–16.
15 Martens P, Lambeets S, Lau CW, Dupont M, Mullens W. Impact of sacubitril/valsartan on heart failure admissions: insights from real-world patient prescriptions. Acta Cardiol. 2019;74(2):115–22.
16 Norberg H, Bergdahl E, Lindmark K. Eligibility of sacubitril-valsartan in a real-world heart failure population: a community-based single-centre study. ESC Heart Fail. 2018;5(2):337–43.

17 Entresto [product monograph including patient medication information]. Novartis Pharmaceuticals Canada Inc; 2017 Oct [cited 2019 Dec 20]. Available from: https://pdf.hres.ca/dpd_pm/00041846.PDF
18 Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–20.
19 Pogge EK, Davis LE. Evaluating the safety and tolerability of sacubitril/valsartan for HFrEF managed within a pharmacist clinic. Am J Cardiovasc Drugs. 2018;18(2):143–51.
20 Antol DD, Casebeer AW, DeClue RW, Stemkowski S, Russo PA. An early view of real-world patient response to sacubitril/valsartan: a retrospective study of patients with heart failure with reduced ejection fraction. Adv Ther. 2018;35(6):785–95.
21 Senni M, McMurray JJV, Wachter R, McIntyre HF, Anand IS, Duino V, et al. Impact of systolic blood pressure on the safety and tolerability of initiating and up-titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail. 2018;20(3):491–500.
22 Lowrie R, Mair FS, Greenlaw N, Forsyth P, Jhund PS, McConnachie A, et al.; Heart Failure Optimal Outcomes from Pharmacy Study (HOOPS) Investigators. Pharmacist intervention in primary care to improve outcomes in patients with left ventricular systolic dysfunction. Eur Heart J. 2012; 33(3):314–24.
23 Hickey A, Suna J, Marquart L, Denaro C, Javorsky G, Munns A, et al. Improving medication titration in heart failure by embedding a structured medication titration plan. Int J Cardiol. 2016;224:99–106.
24 Bhat S, Kansal M, Kondos GT, Groo V. Outcomes of a pharmacist-managed heart failure medication titration assistance clinic. Ann Pharmacother. 2018;52(8):724–32.
25 Palau P, Mollar A, Domínguez E, Sanchis J, Bayes-Genís A, Núñez J. Early sacubitril/valsartan-driven benefit on exercise capacity in heart failure with reduced ejection fraction: a pilot study. Rev Esp Cardiol (Engl Ed). 2019; 72(2):167–9.
26 Almufleh A, Marbach J, Chih S, Stadnick E, Davies R, Liu P, et al. Ejection fraction improvement and reverse remodeling achieved with sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am J Cardiovasc Dis. 2017;7(6):108–13.
27 Kałuzna-Oleksy M, Kolasa J, Migaj J, Pawlak A, Lelonek M, Nessler J, et al. Initial clinical experience with the first drug (sacubitril/valsartan) in a new class - angiotensin receptor neprilysin inhibitors in patients with heart failure with reduced left ventricular ejection fraction in Poland. Kardiol Pol. 2018;76(2):381–7.
28 Martens P, Beliën H, Dupont M, Mullens W. Insights into implementation of sacubitril/valsartan into clinical practice. ESC Heart Fail. 2018;5(3):275–83.

29 De Vecchis R, Ariano C, Di Biase G, Noutsias M. Sacubitril/valsartan for heart failure with reduced left ventricular ejection fraction: a retrospective cohort study. Herz. 2019;44(5):425–32.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors thank Gordon Klammer and Dale Toews of the Abbotsford Regional Hospital and Cancer Centre for their assistance with study design and data interpretation.
Canadian Journal of Hospital Pharmacy, VOLUME 73, NUMBER 3, May-June 2020