Amanda Leong, BScPharm, ACPR, Alison Gordon, BScPharm, Belal Alshaikh, MD, MSc, Kamran Yusuf, MD, Deonne Dersch-Mills, BScPharm, ACPR, PharmD
The most recent recommendations regarding enteral feeding solutions for newborns state that they should have a maximum osmolality of 450 mOsm/kg (400 mOsm/L) and that the use of hyperosmolar feeding solutions may be a factor in the development of necrotizing enterocolitis.1,2 These recommendations are based on historical consensus,3 and there is little other evidence to guide this practice. For preterm newborns at risk of necrotizing enterocolitis, or infants at risk of osmotic diarrhea because of gastrointestinal abnormalities, enteral medications are often diluted in small amounts of breast milk or formula (usual osmolality about 300 mOsm/kg), both for ease of administration and to reduce the osmolar challenge of the medications. Unfortunately, for many compounded and commercially available oral liquid medications, published osmolality values are not available to clinicians to aid in assessing the osmolar load and the risks associated with enteral administration. The purpose of this study was to measure the osmolality of several proprietary and compounded medications commonly used in the neonatal intensive care unit.
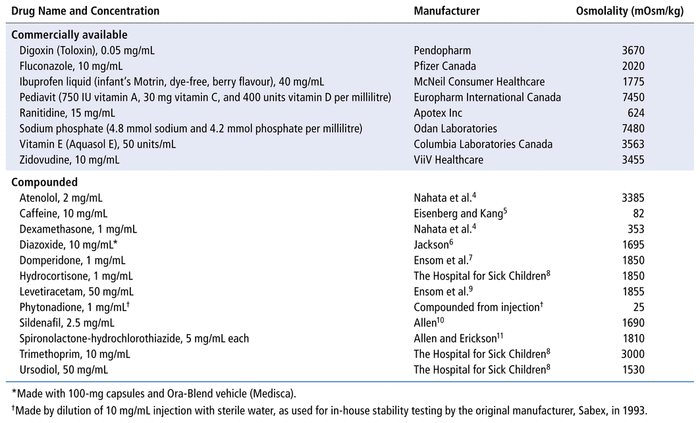
We performed an analytically controlled laboratory study to measure the osmolality of 23 medications, specifically 8 proprietary and 15 compounded medications (Table 1).4–11 Osmolality was measured with a Micro OSMETTE II model 6002 osmometer (Precision Systems, Natick, Massachusetts), calibrated with aqueous normal sodium chloride in triplicate. The maximum measurable osmolality was 2000 mOsm/kg; medications with higher osmolality were diluted 1:1 or 1:2 with distilled water before measurement, and the resulting osmolality was multiplied by 2 (for 1:1 dilutions) or 3 (for 1:2 dilutions). Osmolality was measured in 2 aliquots of each medication (from the same lot). We planned to measure a third time if 2 osmolality measurements differed by more than 10 mOsm/kg, but this did not occur.
TABLE 1 Osmolality of Commercially Available and Compounded Medications for Neonates
The measured osmolality of the proprietary medications ranged from 624 mOsm/kg to 7480 mOsm/kg, and that of the compounded medications ranged from 25 mOsm/kg to 3385 mOsm/kg (Table 1). Only 3 of the 23 medications had osmolality below the recommended maximum 450 mOsm/kg: phytonadione and dexamethasone (both of which were injectable products that are given enterally in our setting), as well as caffeine (which is specifically compounded for preterm neonates). Compounds made from injection solutions or formulated specifically for neonates may have lower osmolalities than those made with sweetened and preserved diluents; however, this supposition would need confirmation through further osmolality measurements of multiple compounded oral liquid medications.
Our results demonstrate the lack of appropriate neonatal medication formulations available from manufacturers and a lack of appropriate compounding recipes for neonates.
1 Chandran S, Chua MC, Lin W, Min Wong J, Saffari SE, Rajadurai VS. Medications that increase osmolality and compromise the safety of enteral feeding in preterm infants. Neonatology. 2017;111(4):309–16.
2 Radmacher PG, Adamkin MD, Lewis ST, Adamkin DH. Milk as a vehicle for oral medications: hidden osmoles. J Perinatol. 2012;32(3):227–9.
3 Barness LA, Mauer AM, Holliday MA, Anderson AS, Dallman PR, Forbes GB, et al. Commentary on breast-feeding and infant formulas, including proposed standards for formulas. Pediatrics. 1976; 57(2):278–85.
4 Nahata MC, Pai VB, Hipple TF. Pediatric drug formulations. 5th ed. Harvey Whitney Books Company; 2003.
5 Eisenberg MG, Kang N. Stability of citrated caffeine solutions for injectable and enteral use. Am J Hosp Pharm. 1984;41(11):2405–6.
6 Jackson M. Handbook of extemporaneous preparation: a guide to pharmaceutical compounding. Pharmaceutical Press; 2010.
7 Ensom MH, Decair D, Hamilton DP. Stability of domperidone in extemporaneously compounded suspensions. J Inform Pharmacother. 2002;8:100.
8 Compounding Service [compounding recipes]. The Hospital for Sick Children; [cited 2018 Oct]. Available from: http://www.sickkids.ca/Pharmacy/Compounding-Service/index.html
9 Ensom MHH, Decarie D, Rudolph S. Stability of levetiracetam in extemporaneously compounded suspensions. Can J Hosp Pharm. 2011; 64(3):207–11.
10 Allen LV Jr. Sildenafil citrate 2.5 mg/mL oral liquid. Int J Pharm Compound. 2006 Jul–Aug;:306.
11 Allen LV Jr, Erickson MA 3rd. Stability of labetolol hydrochloride, metoprolol tartrate, verapamil hydrochloride, and spironolactone with hydrochlorothiazide in extemporaneously compounded oral liquids. Am J Health Syst Pharm. 1996;53(19):2304–9.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 73, NUMBER 4, Fall 2020