Stability of Generic Formulations of Bortezomib 1.0 and 2.5 mg/mL in Vials and Syringes Stored at 4°C and Room Temperature (23°C or 25°C)
Shirley Law, Flay Charbonneau, John Iazzetta, William Perks, Nathan H Ma, Scott E Walker
ABSTRACT
Background
The availability of generic versions of bortezomib raises questions about the reliability of extrapolating stability data from one brand to another.
Objective
To evaluate the stability of bortezomib formulations available from Janssen, Teva Canada, Actavis Pharma, Dr. Reddy’s Laboratories, Apotex, and MDA, reconstituted with 0.9% sodium chloride (normal saline) to produce solutions of either 1.0 or 2.5 mg/mL and stored over at least 21 days under refrigeration (4°C) or at room temperature (either 23°C or 25°C) in the manufacturer’s original glass vials or in polypropylene syringes.
Methods
On study day 0, solutions with concentration 1.0 mg/mL or 2.5 mg/mL of the Teva, Actavis, Dr. Reddy’s, Apotex, and MDA generic formulations were prepared. Three units of each type of container (glass vials and syringes) were stored at 4°C and 3 units at room temperature. Concentration and physical inspection were completed on at least 8 study days (including day 0) over a 21- to 84-day study period. Bortezomib concentrations were determined by a validated stability-indicating liquid chromatographic method with ultraviolet detection. The end point of these studies was the time to reach 90% of the initial concentration (T-90) with 95% confidence, which is expressed as “T-9095%CI”, where CI refers to the confidence interval. In addition to estimating the T-9095%CI, differences in stability among products from all manufacturers were compared using multiple linear regression. Previously published data for the Janssen product were included in the overall comparisons.
Results
In all of the studies, the analytical method separated degradation products from bortezomib, such that the concentration of bortezomib was measured specifically, accurately (deviations < 2.5%), and reproducibly (average replicate error 2.5%). During all studies, solutions retained more than 94% of the initial concentration at 4°C. The T-9095%CI exceeded the study period for all formulations under all combinations of concentration, container, and temperature, except the 84-day study for the MDA product. Multiple linear regression showed no significant differences among manufacturers (p = 0.57).
Conclusions
In this study, formulations of bortezomib currently marketed in Canada (by Janssen, Teva Canada, Actavis Pharma, Dr. Reddy’s Laboratories, Apotex, and MDA) were pharmaceutically equivalent and interchangeable. Given that there was no difference in stability related to manufacturer, nominal concentration, or container, we conclude that these formulations are physically and chemically stable for at least 35 days under refrigeration and at least 25 days at room temperature.
KEYWORDS: bortezomib, stability, generic formulation stability, beyond-use date
RÉSUMÉ
Contexte
La disponibilité de versions génériques de bortezomib soulève des questions relatives à la fiabilité de l’extrapolation des données concernant la stabilité d’une marque à l’autre.
Objectif
Évaluer la stabilité des formules de bortezomib de Janssen, de Teva Canada, d’Actavis Pharma, des Laboratoires du Dr Reddy, d’Apotex et de MDA, reconstituées avec 0,9 % de chlorure de sodium (solution saline normale) pour produire des solutions de 1 ou de 2,5 mg/mL et réfrigérées au moins 21 jours à 4 °C ou à température ambiante (23 °C ou 25 °C), dans des fioles en verre du fabricant ou dans des seringues en polypropylène.
Méthodes
La préparation des solutions avec une concentration de 1 mg/mL ou 2,5 mg/mL des formules génériques de Teva, d’Actavis, du Dr Reddy, d’Apotex et de MDA a eu lieu le jour 0 de l’étude. Trois unités de chaque contenant (fioles en verre et seringues) étaient stockées à 4 °C et 3 unités, à température ambiante. L’inspection de la concentration et l’inspection physique ont été réalisées pendant au moins 8 jours (y compris le jour 0) de l’étude qui a duré de 21 à 84 jours. Les concentrations de bortezomib ont été déterminées par une méthode chromatographique liquide validée, indiquant la stabilité à l’aide d’une détection par rayons ultraviolets. Le point final de ces études était le temps nécessaire pour que le produit atteigne 90 % de la concentration initiale (T-90) avec un seuil de confiance de 95 %, exprimé par T-90IC 95 %, IC indiquant l’intervalle de confiance. En plus de l’estimation du T-90IC 95 %, les différences de stabilité des produits de tous les fabricants ont été comparées à l’aide d’une régression linéaire multiple. Les données publiées précédemment sur le produit Jansen sont incluses dans les comparaisons globales.
Résultats
La méthode analytique de toutes les études qui ont été menées a séparé les produits de dégradation du bortezomib de telle manière que la concentration était mesurée de manière spécifique, précise (déviations < 2,5 %) et reproductible (erreur de réplique 2,5 %). Tout au long des études, les solutions ont retenu plus de 94 % de la concentration initiale à 4 °C. Le T-90IC 95 % de toutes les formules dans toutes les combinaisons de concentration, de contenant et de température, dépassait la durée des études, à l’exception du produit MDA dans l’étude de 84 jours. La régression linéaire multiple n’a indiqué aucune différence importante parmi les fabricants (p = 0,57).
Conclusions
Dans cette étude, les formules de bortezomib actuellement commercialisées au Canada (par Janssen, Teva Canada, Actavis Pharma, les Laboratoires du Dr Reddy, Apotex et MDA) étaient équivalentes et interchangeables d’un point de vue pharmaceutique. Puisqu’aucune différence de stabilité, de concentration nominale ou de contenant liée à l’un ou l’autre des fabricants n’a été révélée, nous concluons que ces formules sont physiquement et chimiquement stables pendant au moins 35 jours sous réfrigération et au moins 25 jours à température ambiante.
MOTS CLÉS: bortezomib, stabilité, stabilité de formule générique, date limite d’utilisation
INTRODUCTION
Bortezomib is indicated for the treatment of patients with previously untreated multiple myeloma for whom stem cell transplant is unsuitable, for the treatment of progressive multiple myeloma in patients who have received at least 1 prior therapy, and for the treatment of patients with mantle cell lymphoma who have experienced relapse or whose disease was refractory to at least 1 prior therapy.1–6 It is available in Canada from multiple manufacturers as 3.5 mg of sterile lyophilized powder in a 10-mL clear glass vial for reconstitution with 0.9% sodium chloride (normal saline [NS]).1–6
Product monographs from 6 manufacturers of this drug—Janssen, Teva Canada, Actavis Pharma, Dr. Reddy’s Laboratories, Apotex, and MDA—state that the total storage time of a reconstituted solution with concentration 1 mg/mL or 2.5 mg/mL, in the manufacturer’s original vial or after transfer to a syringe, must not exceed 8 h at room temperature with exposure to normal indoor lighting.1–6 A study published in this Journal in 2008 demonstrated that 1 mg/mL solutions of bortezomib prepared from the Janssen formulation (Velcade), intended for IV administration, retained more than 95% of the initial concentration for up to 42 days when stored at either 4°C or 23°C.7 A study reported in Lancet Oncology in 2011 demonstrated that in 222 patients, there was no significant difference in time to progression or 1-year overall survival with subcutaneous (SC) or IV bortezomib, although adverse events were significantly fewer with SC administration. SC injections are administered as 2.5 mg/mL (3.5 mg bortezomib reconstituted with 1.4 mL of NS) to limit the volume injected.8 Given that SC administration achieved equal efficacy with a reduction in adverse events, the SC route has become the preferred method of administration. A study published in 2014 demonstrated that a 2.5 mg/mL solution of bortezomib in the original manufacturer’s vial (Velcade, Janssen), intended for SC administration, retained more than 94% of the initial concentration for up to 21 days when stored at either 4°C or 23°C.9
In 2015, Teva launched a generic version of bortezomib, followed by the release of other generics by Actavis in late 2015 and Dr. Reddy’s in early 2017.10 Other formulations received a Notice of Compliance in Canada in 2019, including those manufactured by Apotex, Marcan, MDA, PharmaScience, Pfizer, and Sandoz.10 Many of the manufacturers have requested stability studies of their respective formulations to provide evidence for use beyond the expiry time identified in the respective product monographs (i.e., 8 h at 23°C).1–6
The objective of the research reported here was to evaluate the stability of 5 generic bortezomib products. Each study was conducted separately in the same laboratory, near the time of launch for each formulation. Each formulation was reconstituted in accordance with the manufacturer’s recommendations with either 1.4 mL of NS to produce a 2.5 mg/mL solution or 3.5 mL of NS to produce a 1.0 mg/mL solution. The reconstituted solutions were stored in the original manufacturer’s glass vial or polypropylene syringes, and the stability was evaluated after storage under refrigeration (4°C) or at room temperature (either 23°C or 25°C).
Some pharmacists have interpreted the guidelines of the National Association of Pharmacy Regulatory Authorities (NAPRA)11 as requiring that each institution conduct separate evaluations of the stability of the formulation or manufacturer’s product used in that institution. Therefore, a secondary objective was to compare the results of these studies to determine if there were any differences in stability among manufacturers’ formulations and, if the products were found to be similar, to recommend that the products be considered pharmaceutically equivalent and interchangeable. Such a finding may be important, especially in the event of a drug shortage. Two different brands of polypropylene syringes were used in the course of the study. Differences in stability attributable to differences in the storage container were evaluated as part of the assessment of drug formulations from different manufacturers.
METHODS
Materials
Each of the 6 available formulations of bortezomib for injection contains 3.5 mg of bortezomib as a mannitol boronic ester. The only nonmedicinal ingredient is mannitol. The products do not contain any preservatives, buffers, or antioxidants.1–6
Chromatographic Analysis
The stability-indicating method of André and others12 was modified and revalidated in our laboratory before the initial 2008 study.7 All subsequent investigations were conducted using the same analytical method, according to accepted criteria.13–15 The liquid chromatographic system consisted of a solvent delivery pump (model P4000, Thermo Fisher Scientific Separation Products), which pumped a mixture of 30% acetonitrile and 70% 0.05 M potassium phosphate dibasic (high-performance liquid chromatography [HPLC] grade, catalogue no. P3786, Sigma Aldrich). The pH of the buffer was adjusted to 6.8 with concentrated phosphoric acid (HPLC grade, catalogue no. A260–500, Fisher Scientific) before mixing with acetonitrile. On each analysis day, the mobile phase was prepared to achieve a retention time for bortezomib of about 6.6 min through a 15 cm × 4.6 mm reversed-phase C-18 5-μm column (Supelco ABZ+, Waters Scientific) at 1.0 mL/min. A 2-μL quantity of each prepared sample, quality control solution, and standard was injected directly onto the liquid chromatographic column using an autoinjector (Ultra WISP 715, Waters Scientific), in duplicate.
The column effluent was monitored with a variable- wavelength ultraviolet detector (UV6000, Thermo Fisher Scientific) at 270 nm. The signal from the detector was integrated and recorded with a chromatography data system (ChromQuest, version 5.0, Thermo Fisher Scientific). The area under the bortezomib peak at 270 nm was subjected to least-squares linear regression, and the actual bortezomib concentration in each sample was determined by interpolation from the standard curve.
Assay Validation
Following the set-up of the chromatographic system for bortezomib as described in the 2008 article,7 the suitability of this method for use as a stability-indicating assay was tested by accelerating the degradation of bortezomib with various concentrations of sodium hypochlorite. The contents of a 3.5-mg vial of bortezomib (bortezomib mannitol boronic ester for injection, Velcade, Janssen Ortho Inc; lot 4CBS301, expiry March 2006) was dissolved in 3.5 mL of distilled water to prepare a 1 mg/mL solution.7 The mixture was vortex-mixed and chromatographed immediately. Chromatograms from all samples were inspected for the appearance of additional peaks, and the bortezomib peak was compared between samples for changes in concentration, retention time, and peak shape (electronic overlay and numeric calculation of tailing). UV spectral purity (200–365 nm, 6-nm bandwidth, deuterium lamp; determined with UV6000 system, Thermo Fisher Scientific) of the bortezomib peak in a chromatogram of a degraded sample produced by sodium hypochlorite was compared with the spectrum of the authentic, undegraded sample of bortezomib in water obtained at time 0.
To revalidate the specificity of the system before each study, a 2.5 mg/mL solution of bortezomib was intentionally degraded using 5 μL of 0.3% sodium hypochlorite (sodium hypochlorite 0.5%, PCS 5000 oxidizing disinfectant, Process Cleaning Solutions, Peterborough, Ontario; lot 096133, expiry September 30, 2019; diluted with distilled water).
After this first phase of evaluation and validation, the accuracy and reproducibility of standard curves were tested over 5 days, and system suitability criteria (theoretical plates, tailing, and retention time) were developed to ensure consistent chromatographic performance on each study day.16
Stability Study
Similar to the method used in the prior studies of the Janssen formulation,7,9 on study day 0 of each of the generic bortezomib studies, each of twelve 3.5-mg vials of bortezomib mannitol boronic ester for injection (Teva, lot 1590615, expiry June 2018; Actavis, lot EF16005C, expiry June 2018; Dr. Reddy’s, lot H7005, expiry December 2019; Apotex, lot BORAC1048, expiry November 2020; MDA, lot 1802580G, expiry July 2021) was reconstituted with 3.5 mL of NS to prepare 1.0 mg/mL solutions. The contents of 6 vials of each company’s formulation were drawn into 3-mL polypropylene Becton-Dickinson syringes (Teva, Actavis, and Dr. Reddy’s formulations) or 3-mL polypropylene Equashield closed system transfer syringes (Apotex and MDA formulations); the remaining 6 reconstituted solutions were left in the manufacturers’ glass vials. In each study, 3 of the 6 vials and 3 of the 6 syringes were stored at room temperature (23°C ± 2°C or 25°C ± 2°C), protected from fluorescent room light; the other 3 syringes and 3 vials were stored in the refrigerator (4°C) without exposure to fluorescent lighting.
Similarly, on study day 0 of each of the generic bortezomib studies, each of twelve 3.5-mg vials was reconstituted with 1.4 mL of NS to prepare 2.5 mg/mL solutions. The contents of 6 vials of each company’s formulation were drawn into 3-mL polypropylene Becton-Dickinson syringes (Teva, Actavis, and Dr. Reddy’s formulations) or 3-mL polypropylene Equashield closed system transfer syringes (Apotex and MDA formulations); the remaining 6 reconstituted solutions were left in the manufacturers’ glass vials. In each study, 3 of the 6 vials and 3 of the 6 syringes were stored at room temperature (23°C ± 2°C or 25°C ± 2°C), protected from fluorescent room light; the other 3 syringes and 3 vials were stored in the refrigerator (4°C) without exposure to fluorescent lighting.
Study Days
Sampling days were slightly different during each study, according to when the study was completed and the manufacturer’s desire to replicate the original study design, as reported in 2008.7 For the Actavis and Dr. Reddy’s formulations, 8 sampling days occurred over a 21-day study period (days 0, 1, 2, 5, 7, 11, 14, and 21). For the Teva formulation, 10 sampling days occurred over a 42-day study period (0, 1, 3, 7, 10, 14, 22, 28, 34, and 42). For the Apotex formulation, 11 sampling days occurred over a 42-day period (0, 1, 4, 8, 11, 15, 18, 21, 28, 35, and 42). For the MDA formulation, 11 sampling days occurred over 84 days (0, 1, 2, 3, 7, 10, 14, 21, 35, 62, and 84).
Bortezomib Analysis
On each study day, a 3.5-mg vial of bortezomib from each manufacturer (Teva, lot 1590615, expiry June 2018; Actavis, lot EF16005C, expiry June 2018; Dr. Reddy’s, lot H7005, expiry December 2019; Apotex, lot BORAC1048, expiry November 2020; MDA, lot 1802580G, expiry July 2021) was reconstituted with 1.167 mL of distilled water to make a 3 mg/mL solution. On each study day, this stock solution was further diluted to prepare standards with final concentrations of 3.000, 2.250, 1.125, 0.563, and 0.375 mg/mL. When combined with a blank, these standards served to construct a standard curve. In addition, 2 quality control samples with bortezomib concentrations of 0.75 and 1.5 mg/mL were prepared from this same stock solution. A 2-μL quantity of each standard, sample, or quality control solution was chromatographed in duplicate without further dilution. Intraday and interday errors were assessed by the coefficients of variation (CVs) of the peak areas of both the quality control samples and the standards.
On each study day for each manufacturer, samples drawn from each of the 3 vials and 3 syringes stored at each of the 2 temperatures were assayed for bortezomib content. All samples initially contained a nominal concentration of 1.0 mg/mL or 2.5 mg/mL of bortezomib. A 2-μL quantity of each sample was injected directly onto the liquid chromatographic system without further preparation, in duplicate, to ensure the ability to distinguish concentrations in vials with concentrations that differed by 10% or more.17,18
Physical Stability
On each study day, samples drawn for concentration analysis were inspected visually for changes in colour and particulate matter against a white and a black background.
Data Reduction and Statistical Analysis
After determining the CV of the assay, a power calculation showed that duplicate injection had the ability to distinguish between concentrations that differed by at least 10% within each type of container.17,19 Means were calculated for replicate analyses. Mean results from different days for each test were compared statistically to determine whether an association existed between the observed result and time. The percent remaining was analyzed by linear regression, and a 95% confidence interval (CI) was constructed around the slope of percent remaining versus study days. The time to reach 90% of the initial concentration (T-90) with 95% confidence (expressed as “T-9095%CI”) was calculated from the time (in days) for the lower limit of the 95% CI to reach 90%. Analysis of variance was used to test differences in concentration on different study days, with different initial nominal concentrations, different containers, and different storage temperatures. The 5% level was used as the a priori cut-off for significance.
Bortezomib concentrations were considered “acceptable” or “within acceptable limits” if the lower limit of the 95% confidence limit of concentration remaining was greater than 90% (T-9095%CI) of the initial (day 0) concentration.
Manufacturer Comparison
Identifying potential differences in stability between manufacturers was also an objective of this study. Bortezomib stability data for the Janssen formulation (the innovator product) for the 1.0 mg/mL concentration (published in 2008)7 and the 2.5 mg/mL concentration (published in 2014)9 were included in the evaluation. The primary end point of all of the individual studies was the time to reach 90% of the initial concentration, with 95% confidence (T-9095%CI). This end point involves the construction of a confidence interval; therefore, although the value of T-9095%CI is based on the change in percent remaining (degradation rate, expressed as percentage per day), it is also a function of variability in the data (standard deviation of regression) and number of study days (ranging between 8 and 11). To ensure homogeneity of the data across all 6 formulations, the standard deviation of regression observed for each combination of initial nominal concentration, container type, storage temperature, and manufacturer was compared using analysis of variance and linear regression.
In the evaluation of manufacturer, a variable for manufacturer was added to the same multiple linear regression model (IBM SPSS Statistics, version 20.0.0) used in individual studies with a constant (effectively time 0 of 100%). In this analysis, study data for percent remaining from all formulations on each study day were pooled and analyzed using the variables study day, initial nominal concentration, storage temperature, type of container, and manufacturer. Other potential factors, such as number of study days and study duration, were not included in this analysis because of their correlation with manufacturer. The 5% level was used as the a priori cut-off for significance.
RESULTS
Accelerated Degradation and Assay Validation
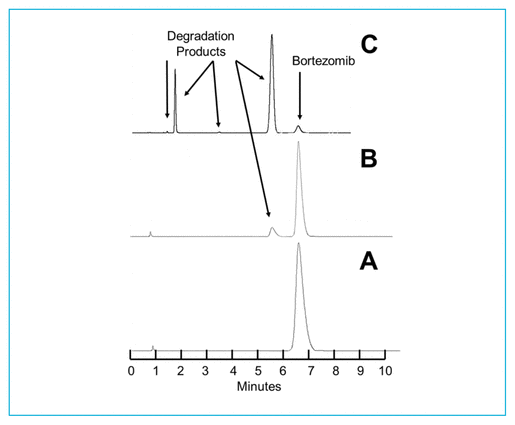
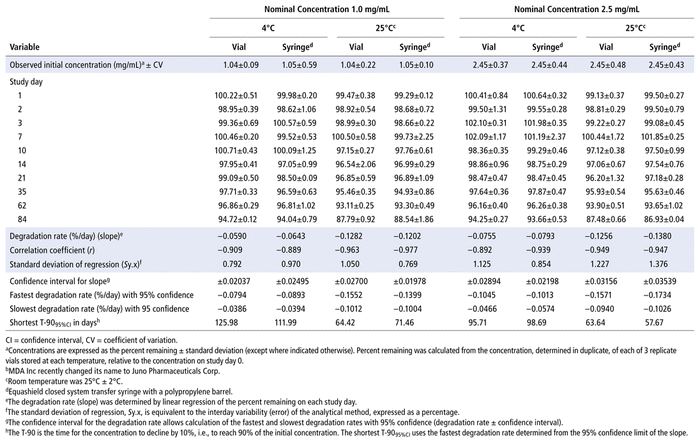
As reported previously,7,9 degradation of bortezomib with sodium hypochlorite occurs quickly. At 23°C, addition of 5 μL of a 0.5% solution of sodium hypochlorite to a 1.0 mg/mL solution of bortezomib in water led to immediate degradation, with 6.32% of the original concentration remaining. Solutions containing lower concentrations of sodium hypochlorite degrade bortezomib more slowly. When 5 μL of a 0.25% solution of sodium hypochlorite was added to bortezomib (2.5 mg/mL), 42.38% of the original concentration remained when the sample was chromatographed immediately. The treated solutions contained degradation products of bortezomib, which eluted at 2.0 and 5.5 min (Figure 1). Additional peaks were observed to elute at 14.5 min and between 2 and 4.5 min when solutions of sodium hypochlorite with concentration above 0.4% were added. None of these degradation products interfered with quantification of bortezomib, and the UV spectrum of the bortezomib peak (200–365 nm) in a degraded sample was no different than the spectrum of the authentic, undegraded standard. The retention times reported in this study are slightly different from retention times reported in previous articles7,9; however, all validation studies showed separation of the degradation products from bortezomib, and none of the degradation products in any study interfered with quantification of bortezomib. When compared with the chromatograms published by André and others,12 the chromatograms are virtually identical to those produced with hydrogen peroxide. Hydrogen peroxide and sodium hypochlorite generate all of the degradation products produced by acid, base, and/or heat, as well as 2 additional degradation products, which eluted in our system at 6.5 and 14.5 min.
| |
|
|
|
FIGURE 1 Chromatograms of bortezomib 2.5 mg/mL in water under various degradation conditions. (A) Before addition of sodium hypochlorite. (B) Immediately after addition of 5 μL of 0.25% sodium hypochlorite; 42.38% of the original compound remains. (C) Immediately after addition of 5 μL of 0.5% sodium hypochlorite; 6.32% of the original compound remains. Visually evident degradation products appeared at 2.0 and 5.5 min.
|
As a result of the chromatographic separation of these degradation products from bortezomib, and the similarity of the UV spectrum (200–365 nm) between an authentic standard and bortezomib in a degraded sample, it was concluded that this was a stability-indicating analytical method.13–15
Analysis of standard curves and quality control samples during each study showed an average absolute deviation from the expected concentration of 2.50% for the Teva product, 2.18% for the Actavis product, 1.91% for the Dr. Reddy’s product, 2.17% for the Apotex product, and 2.10% for the MDA product. The standard deviation of regression was 1.02% for the Teva product, 0.82% for the Actavis product, 0.76% for the Dr. Reddy’s product, 1.06% for the Apotex product, and 1.02% for the MDA product. Analytical reproducibility, within a day (as measured by the CV), averaged 1.02% for the Teva product, 0.90% for the Actavis product, 0.62% for the Dr. Reddy’s product, 0.44% for the Apotex product, and 0.36% for the MDA product.
These results indicate that analytical performance was similar for each of the separate studies and that differences in concentration of 10% or more could be confidently detected within individual containers with acceptable error rates.17,18
Bortezomib Stability Studies
In all studies, all solutions stored in either the original manufacturer’s glass vials or the polypropylene syringes were initially clear and colourless and remained unchanged for the duration of the study period. No visible particles were observed in any solution in any of the studies.
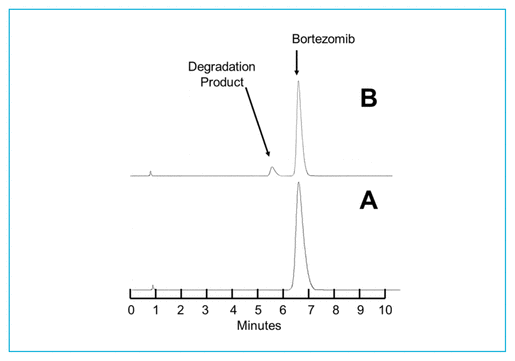
Concentrations observed during each study of the bortezomib solutions with nominal concentrations of 1.0 mg/mL and 2.5 mg/mL are presented in Table 1 (Teva), Table 2 (Actavis), Table 3 (Dr. Reddy’s), Table 4 (Apotex), and Table 5 (MDA). Bortezomib concentrations were considered “acceptable” or “within acceptable limits” if the lower limit of the 95% confidence interval of concentration remaining was greater than 90% of the initial (day 0) concentration (T-9095%CI). Using this criterion, the shortest time to reach the lowest acceptable concentration for the 5 generic products, with storage at 4°C, was calculated to be 60.27 days for the Teva formulation (Table 1), 35.42 days for the Actavis formulation (Table 2), 37.21 days for the Dr. Reddy’s formulation (Table 3), 64.85 days for the Apotex formulation (Table 4), and 95.71 days for the MDA formulation (Table 5). For each formulation, the shortest time exceeded the study duration for that formulation and averaged about 30% longer than the time to reach the lowest acceptable concentration for the 5 generic products with storage at 23°C or 25°C. At room temperature, the T-9095%CI was 46.45 days for the Teva formulation (Table 1), 25.72 days for the Actavis formulation (Table 2), 31.41 days for the Dr. Reddy’s formulation (Table 3), 56.70 days for the Apotex formulation (Table 4), and 57.67 days for the MDA formulation (Table 5). For all but one of the formulations, the shortest time exceeded the study duration for that formulation; the exception was the MDA formulation, which had the longest study duration (84 days). In that study, after 84 days of storage at room temperature, approximately 87%–89% of the initial concentration remained, and a degradation product, observed during the accelerated study with elution at 5.5 min, was observed in chromatograms (Figure 2).
TABLE 1 Measured Concentrationsa of Bortezomib from Teva and Percent Remaining on Each Study Day
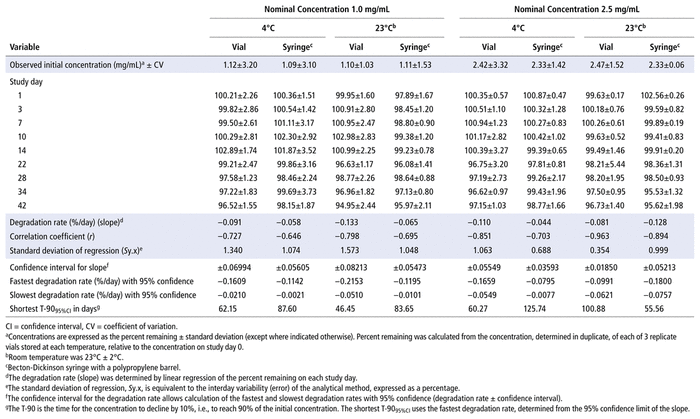
TABLE 2 Measured Concentrationsa of Bortezomib from Actavis and Percent Remaining on Each Study Day
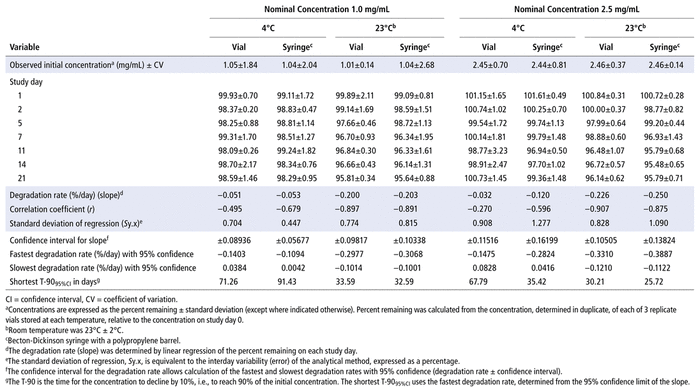
TABLE 3 Measured Concentrationsa of Bortezomib from Dr. Reddy’s and Percent Remaining on Each Study Day
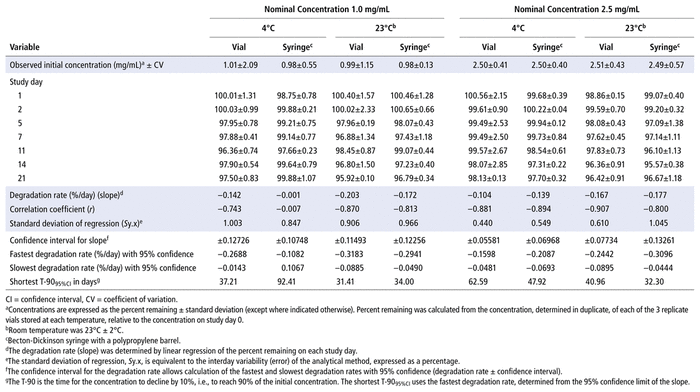
TABLE 4 Measured Concentrationsa of Bortezomib from Apotex and Percent Remaining on Each Study Day
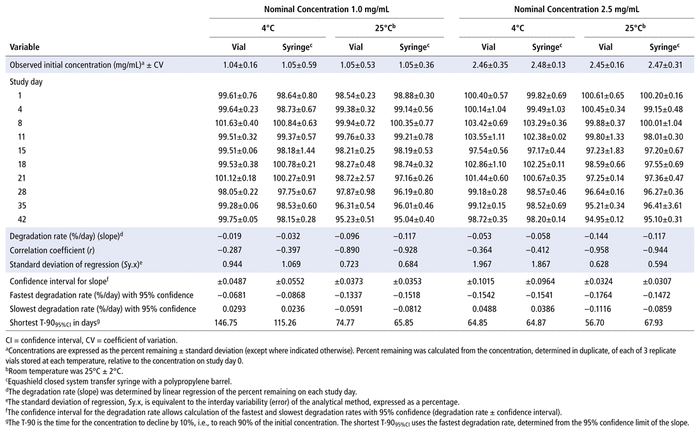
TABLE 5 Measured Concentrationsa of Bortezomib from MDAb and Percent Remaining on Each Study Day
| |
|
|
|
FIGURE 2 Chromatograms of bortezomib 2.5 mg/mL, reconstituted in normal saline and stored in syringes at room temperature (25°C) during the MDA stability study. (A) On study day 0. (B) After 84 days of storage at room temperature. Very small amounts of a degradation product originally observed during the accelerated degradation study were observed in solutions stored at room temperature.
|
Analysis of variance detected differences in the percent remaining of 1% or less. In every study, significant changes in concentration due to study day (p < 0.001) and temperature (p < 0.001) were detected. Only the Actavis study demonstrated a difference related to either container or concentration, and in both cases the difference was less than 1%.
Manufacturer Comparisons
Data from the 5 studies of generic formulations and the previously published studies of the Janssen product7,9 are summarized in Table 6. All of these studies, including the original studies of the Janssen formulation, were completed in the same laboratory over an 11-year period, using the same analytical method. The primary end point of each study was an evaluation of the degradation rate and the shortest time to reach 90% of the initial concentration with 95% confidence (T-9095%CI). Because confidence intervals are involved in calculation of the T-9095%CI, this end point is dependent on variability in the data. To ensure homogeneity within the data set, the standard deviations of regression observed for each combination of concentration, container type, storage temperature, and manufacturer were compared. The standard deviation of regression varied from 0.354% to 1.967%. Analysis of variance detected no significant differences in the standard deviation of regression due to the factors of manufacturer (p = 0.81), storage temperature (p > 0.99), concentration (p = 0.64), type of container (p = 0.17), or study duration (p = 0.31). Furthermore, there was no correlation between the standard deviation of regression and the time to reach 90% of the initial concentration with 95% confidence (T-9095%CI) (r2 = 0.0009, n = 46, p = 0.84). These results indicate that the standard deviation of regression is effectively a random variable in the analysis.
TABLE 6 Summary of Bortezomib Stability Studies: Shortest Time to Reach 90% Remaining (with 95% Confidence)
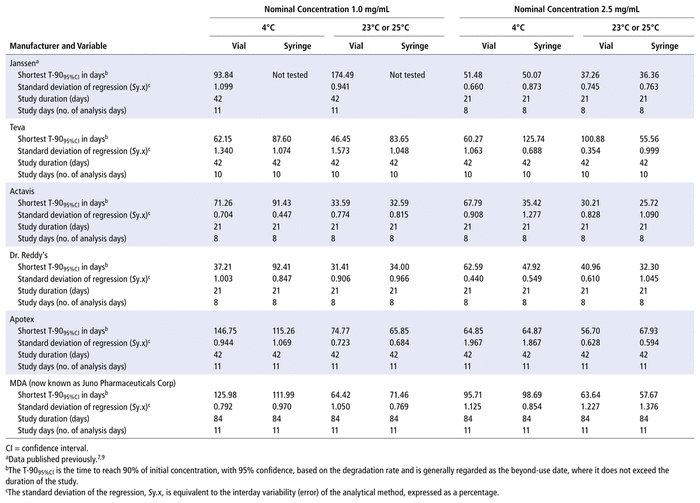
In the pooled analysis of study data for percent remaining, using multiple linear regression, the results were similar to those seen in the individual formulation studies: only study day (p < 0.001) and temperature (p < 0.001) were identified as significant variables affecting the percent remaining. Manufacturer (p = 0.57) did not significantly affect the percent remaining. Nominal initial concentration (p = 0.34) and container (p = 0.38) were also not identified as significant factors. Given than the pooled analysis represented glass vials from 6 manufacturers and 2 different brands of polypropylene syringes (Becton-Dickinson and Equashield), this evaluation demonstrates that differences in container manufacturers also do not affect bortezomib stability.
Inspection of Table 6 shows that the shortest T-9095%CI occurred in studies evaluating stability over 21 days, where the estimated value of T-9095%CI averaged 47.1 days (range 25.7–92.4 days). In contrast, the average value of T-9095%CI was 86 days for studies lasting 42 and 84 days (range 36.4–174.5 days). This should not be interpreted as indicating a difference in stability among the manufacturers. Because confidence intervals widen as they extend beyond the last study day, studies of shorter duration will generally intersect with a “90% remaining” limit earlier, even when the degradation rate (stability) is similar. Manufacturer was not identified as significantly affecting the percent remaining (p = 0.57) or the degradation rate (p = 0.56).
DISCUSSION
In each of the 5 studies of the stability of generic formulations of bortezomib, the solutions stored in manufacturers’ vials and syringes, at the 2 concentrations tested (1 mg/mL and 2.5 mg/mL) and under 2 storage temperatures (4°C and room temperature), retained more than 90% of the initial concentration over the respective study period, except for solutions of the MDA formulation stored at room temperature. Similarly, the value of T-9095%CI exceeded the study period for all formulations, except the MDA formulation stored at room temperature.
When the innovator stability data were pooled with data from the stability studies of the 5 generic formulations to evaluate the effect of manufacturer on the T-9095%CI, multiple linear regression detected no significant differences related to manufacturer (p = 0.57), type of container (p = 0.38), or initial nominal concentration (p = 0.34). As was the case in all of the individual studies, temperature (p < 0.001) and study day (p < 0.001) were significant factors in the pooled analysis. This brings into question the need for stability data specific to the manufacturer’s formulation or container used in each institution. To obtain such data is a formidable and costly task. Most institutions are unable to conduct their own studies and must rely on published data or manufacturers’ in-house data. When there are no published data demonstrating differences in stability between products from different manufacturers, it would appear to be unnecessary, financially burdensome, and contrary to the principle of evidence-based medicine to demand such data. In fact, we are aware of only 2 studies that compare stability of a particular product between manufacturers.19,20 Both of these studies investigated the stability of vancomycin and reported no difference in stability due to the manufacturer, following dilution with NS or dextrose 5% in water.19,20 Although some publications have reported that generic products are of inferior quality, these studies are frequently biased or poorly designed. Similarly, no differences due to manufacturer were observed in the current evaluation and previously published bortezomib studies.7,9 Furthermore, every study generated a T-9095%CI greater than 25 days, which is longer than any beyond-use date (BUD) permitted by the current (November 2016) NAPRA guidelines.11
Health Canada declares a new drug to be the “pharmaceutical equivalent” of another drug if it contains “identical amounts of the identical medicinal ingredients, in comparable dosage forms, but that does not necessarily contain the same non-medicinal ingredients”.21 Even so, most manufacturers of generic IV formulations develop their respective formulations following analysis of the innovators’ formulations, thereby achieving some degree of pharmaceutical equivalence. In this study, all 5 generic products had the same medicinal and nonmedicinal ingredients and were described virtually identically in the product monographs as follows: “bortezomib for injection is supplied in … vials containing 3.5 mg of bortezomib as a mannitol boronic ester, as a white to off-white cake or powder. The only nonmedicinal ingredient is mannitol.”1–6 The differences among the products seem to be limited to the vial size (Teva is marketed in a 13.5-mL vial, whereas all others are supplied in 10-mL vials), with stoppers being specified as free of natural rubber latex for all except the Dr. Reddy’s and Apotex products.
In many Canadian provinces, including Ontario, similarity in formulation that results in similar physical and chemical properties can form the grounds for a waiver of bioequivalence data.22 When pharmaceutical equivalence results in similar physical and chemical properties (including pH and concentration), it is very likely to result in similar stability, as demonstrated by this study. Therefore, for drugs for which pharmaceutical equivalence has been demonstrated, with known chemical stability exceeding BUDs established by USP General Chapter <797>23 and NAPRA,11 extrapolating the BUD across manufacturers would seem reasonable, provided that within a particular institution, pharmacy practitioners can answer questions related to sterility and have knowledge of the institutional contamination rate.
CONCLUSION
We conclude that formulations of bortezomib currently marketed in Canada (manufactured by Janssen, Teva Canada, Actavis Pharma, Dr. Reddy’s, Apotex, and MDA) are pharmaceutically equivalent and interchangeable. Based on the observation that there is no effect of manufacturer or nominal concentration on stability, using the shortest time to reach 90% of the initial concentration (with 95% confidence, T-9095%CI), we conclude that these formulations are physically and chemically stable for at least 35 days at 4°C and at least 25 days at room temperature.
References
1 Velcade® [product monograph]. Toronto (ON): Janssen Inc; 2016 Mar 16.
2 Bortezomib for injection [product monograph]. Toronto (ON): Teva Canada; 2016 May 26.
3 ACT bortezomib [product monograph]. Toronto (ON): Actavis Pharma Company; 2016 May 9.
4 Bortezomib for injection [product monograph]. Bachupally (India): Dr. Reddy’s Laboratories Limited; 2016 Apr 28.
5 Bortezomib [product monograph]. Toronto (ON): Apotex Inc; 2019 Feb 15.
6 Bortezomib [product monograph]. Oakville (ON): MDA Inc; 2019 Jan 29.
7 Walker SE, Milliken D, Law S. Stability of bortezomib reconstituted with 0.9% sodium chloride at 4°C and room temperature (23°C). Can J Hosp Pharm. 2008;61(1):14–20.
8 Moreau P, Pylypenko H, Grosicki S, Karamanesht L, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–40.
Crossref PubMed
9 Walker SE, Charbonneau LF, Law S. Stability of bortezomib 2.5 mg/mL in vials and syringes stored at 4°C and room temperature (23°C). Can J Hosp Pharm. 2014;67(2):102–7.
PubMed PMC
10 Health Canada drug product database. Health Canada; [cited 2017 Jul 31]. Available from: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
11 Model standards for pharmacy compounding of hazardous sterile preparations. National Association of Pharmacy Regulatory Authorities; 2016 [cited 2020 Nov 30]. Available from: https://napra.ca/general-practice-resources/model-standards-pharmacy-compounding-hazardous-sterile-preparations
12 André P, Cisternino S, Chiadmi F, Toledano A, Schlatter J, Fain O, et al. Stability of bortezomib 1-mg/mL solution in plastic syringe and glass vial. Ann Pharmacother. 2005;39(9):1462–6.
Crossref PubMed
13 Trissel LA. Avoiding common flaws in stability and compatibility studies of injectable drugs. Am J Hosp Pharm. 1983;40(7):1159–60.
PubMed
14 Trissel LA, Flora KP. Stability studies: five years later. Am J Hosp Pharm. 1988;45(7):1569–71.
PubMed
15 Policy for publication of chemical stability study manuscripts. Can J Hosp Pharm. 1990;43(1):3–4.
16 Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, et al. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies [conference report]. J Pharm Sci. 1992;81(3):309–12.
Crossref
17 Frieman JA, Chalmers TC, Smith H, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial—survey of 71 negative trials. N Engl J Med. 1978;299(13):690–4.
Crossref
18 Stolley PD, Strom BL. Sample size calculations for clinical pharmacology studies. Clin Pharmacol Ther. 1986;39(5):489–90.
Crossref PubMed
19 Lewis PO, Kirk LM, Brown SD. Comparison of three generic vancomycin products using liquid chromatography-mass spectrometry and an online tool. Am J Health Syst Pharm. 2014;71(12):1029–38.
Crossref PubMed
20 Huvelle S, Godet M, Hecq JD, Gillet P, Jamart J, Galanti LM. Long-term stability of vancomycin hydrochloride in oral solution: the brand name versus a generic product. Int J Pharm Compound. 2016;20(4):347–50.
21 Food and Drug Regulations; C.R.C., c. 870 [cited 2020 Jan 26]. Available from: http://laws-lois.justice.gc.ca/PDF/C.R.C.,_c._870.pdf
22 Drug submission screening checklist for aqueous solutions. Ministry of Health and Long-Term Care (Ontario); [cited 2020 Nov 30]. Available from: www.health.gov.on.ca/en/pro/programs/drugs/dsguide/didfa_templates/aqueous_solution_checklist.pdf
23 General chapter <797>: pharmaceutical compounding — sterile preparations. In: USP 36. United States Pharmacopeial Convention (USP); 2013.
Shirley Law, DipPharmTech, is a Research Assistant in Quality Control in the Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, Ontario
Flay Charbonneau, RPh, BSc(Pharm), is the Manager, Pharmacy of the Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, Ontario
John Iazzetta, PharmD, was, at the time of study execution, the Coordinator of Clinical Trials in the Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, Ontario
William Perks, RPh, BSc(Pharm), EMBA, is the Manager of Pharmacy Compounding in the Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, Ontario
Nathan H Ma, PharmD, ACPR, MSc, is the Clinical Trials Pharmacist in the Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, Ontario
Scott E Walker, MScPhm, is a Pharmacist in the Department of Pharmacy, Sunnybrook Health Sciences Centre, and a Professor in the Faculty of Pharmacy, University of Toronto, Toronto, Ontario
Competing interests: Other than support from various manufacturers of bortezomib for the individual studies, as outlined in the “Funding” paragraph below, no competing interests were declared. (
Return to Text
)
Address correspondence to: Scott E Walker, Sunnybrook Health Sciences Centre, 2075 Bayview Avenue, Toronto ON M4N 3M5, email:scott.walker@sunnybrook.ca
(Return to Top)
Funding: The studies of individual generic formulations were each funded separately through research support agreements or unrestricted educational grants from Teva Canada Ltd, Dr. Reddy’s Laboratories Ltd, Apotex Inc, and Juno Pharmaceuticals Corp (formerly MDA Inc). (
Return to Text
)
Canadian Journal of Hospital Pharmacy, VOLUME 74, NUMBER 1, Winter 2021







