Nationwide Trends in Dispensing of Sodium Glucose Cotransporter 2 Inhibitors
Michael
Fralick
,
Diana
Martins
,
Mina
Tadrous
,
Tara
Gomes
ABSTRACT
Background
Three large cardiovascular outcome trials have investigated the safety of sodium glucose cotransporter 2 (SGLT2) inhibitors.
Objective
To analyze the nationwide dispensing of SGLT2 inhibitors before and after the publication of these trials.
Methods
A cross-sectional study was conducted of monthly prescription dispensing of SGLT2 inhibitors from May 23, 2014, to April 30, 2019, using nationwide data for Canada. An autoregressive integrated moving average (ARIMA) model was fitted to the monthly number of tablets dispensed for each SGLT2 inhibitor; the model included a ramp intervention function at the publication dates of interest to estimate the impact on SGLT2 inhibitor dispensing patterns.
Results
The rate of canagliflozin and dapagliflozin dispensing declined after publication of results of the empagliflozin cardiovascular trial in September 2015. After publication of results of the canagliflozin trial in June 2017, which indicated a reduction in cardiovascular events and an increase in the risk of lower-limb amputation, canagliflozin remained the most commonly dispensed SGLT2 inhibitor, but its rate of dispensing declined further. In contrast, the rate of empagliflozin dispensing increased, while the rate of dapagliflozin dispensing was unchanged. After publication of the dapagliflozin trial in November 2018, which indicated no clear reduction in cardiovascular events, short-term trends in dispensing of canagliflozin, empagliflozin, and dapagliflozin were largely unaffected.
Conclusions
The cardiovascular outcome trials appeared to have an important impact on the dispensing of SGLT2 inhibitors in Canada.
KEYWORDS: diabetes, sodium glucose cotransporter 2 (SGLT2) inhibitors, health policy, epidemiology
RÉSUMÉ
Contexte
Trois grands essais portant sur les résultats cardiovasculaires avaient pour objet l’étude de l’innocuité des inhibiteurs du cotransporteur sodium-glucose de type 2 (SGLT2).
Objectif
Analyser la délivrance nationale des inhibiteurs du SGLT2 avant et après la publication de ces essais.
Méthodes
Une étude transversale a été menée sur la délivrance d’ordonnances mensuelles d’inhibiteurs du SGLT2 du 23 mai 2014 au 30 avril 2019, à l’aide de données nationales pour le Canada. Un modèle de moyenne mobile intégrée autorégressive (ARIMA) a été adapté au nombre mensuel de comprimés distribués pour chaque inhibiteur du SGLT2; le modèle comprenait une fonction d’intervention progressive aux dates de publication d’intérêt pour estimer l’effet sur les schémas de délivrance d’inhibiteurs du SGLT2.
Résultats
Le taux de délivrance de canagliflozine et de dapagliflozine a diminué après la publication des résultats de l’essai cardiovasculaire empagliflozine en septembre 2015. Après la publication des résultats de l’essai cardiovasculaire canagliflozine en juin 2017, qui indiquaient une réduction des événements cardiovasculaires et une augmentation du risque d’amputation des membres inférieurs, la canagliflozine est restée l’inhibiteur du SGLT2 le plus couramment délivré, mais son taux de délivrance a encore diminué. En revanche, le taux d’empagliflozine délivré a augmenté, tandis que le taux de délivrance de dapagliflozine est resté identique. Après la publication de l’essai sur la dapagliflozine en novembre 2018, qui n’indiquait aucune réduction nette des événements cardiovasculaires, les tendances à court terme de la délivrance de la canagliflozine, de l’empagliflozine et de la dapagliflozine n’ont pratiquement pas changé.
Conclusions
Les essais portant sur les résultats cardiovasculaires semblaient avoir un effet important sur la délivrance des inhibiteurs du SGLT2 au Canada.
MOTS CLÉS: diabète, inhibiteurs du cotransporteur sodium-glucose de type 2 (SGLT2), politique de santé, épidémiologie
INTRODUCTION
Sodium glucose cotransporter 2 (SGLT2) inhibitors are an effective class of medications for adults with type 2 diabetes mellitus.1–3 Canagliflozin was the first SGLT2 inhibitor to be approved in Canada (in May 2014), followed by dapagliflozin (December 2014) and then empagliflozin (July 2015). Although their initial listing on the drug formulary varied by province, canagliflozin was listed on most formularies by October 2015, dapagliflozin was listed on most formularies by January 2017, and empagliflozin was listed on most formularies by May 2016. Cardiovascular outcome trials have demonstrated that, compared with placebo, empagliflozin reduced cardiovascular events and all-cause mortality (trial published on September 17, 2015),1 canagliflozin reduced cardiovascular events but not all-cause mortality and increased the risk of lower-limb amputation and bone fracture (trial published on June 12, 2017),2 and dapagliflozin did not reduce the risk of cardiovascular events or increase the risk of lower-limb amputation or bone fracture (trial published on November 10, 2018).3 Our objective was to analyze nationwide dispensing of SGLT2 inhibitors before and after these 3 trials were published.
METHODS
We conducted a cross-sectional study of monthly dispensing of prescriptions for SGLT2 inhibitors in Canada from May 23, 2014 (the date of Health Canada approval for the first SGLT2 inhibitor), to April 30, 2019 (date of last available data), using nationwide IQVIA Geographic Prescription Monitor data.4 The IQVIA database contains prescription transactions from Canadian pharmacies for all dispensed products. At the national level, more than 79% of total prescriptions dispensed are captured by a panel composed of approximately 6600 pharmacies. The monthly estimates are created using IQVIA’s patented geospatial projection methodology to report on total prescriptions dispensed across Canada at various levels of geography.4 Although health care in Canada is publicly funded for all residents, drug coverage is not publicly funded and therefore varies by province5 (for additional details about coverage, see Appendix 1, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/208).
We fit an autoregressive integrated moving average (ARIMA) model to the monthly number of tablets dispensed for each SGLT2 inhibitor. We used an ARIMA model because it is a common method for interrupted time series analysis. We added a ramp intervention function to the models, which allowed us to estimate gradual slope changes in the trends for dispensing of SGLT2 inhibitors. For additional details about the modelling, see Appendix 1.
RESULTS
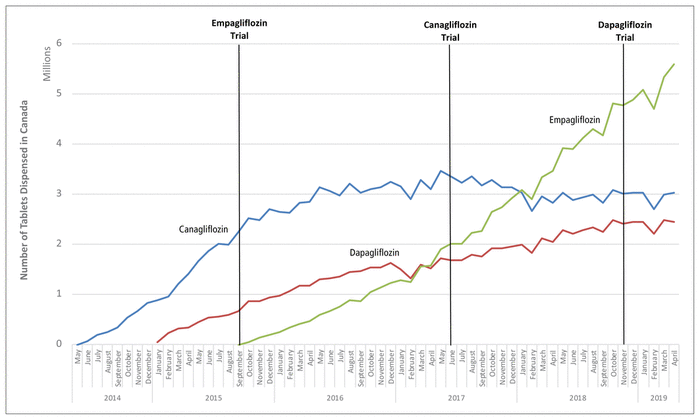
Before September 2015, the most commonly dispensed SGLT2 inhibitor was canagliflozin (14 865 886 tablets; 82.9% of all SGLT2 tablets), followed by dapagliflozin (3 065 436 tablets; 17.1%) (Figure 1). During that period, most dispensing of SGLT2 inhibitors was paid for with cash or by private insurance (for canagliflozin, 78.2%; for dapagliflozin, 89.9%). The results of the empagliflozin cardiovascular trial, published in September 2015, showed a reduction in cardiovascular events and all-cause mortality, which coincided with empagliflozin availability and uptake in Canada. Because empagliflozin was not available before this period, we were unable to quantify the impact of trial results on empagliflozin dispensing. However, we were able to quantify the impact on the other two SGLT2 inhibitors, which were already available in Canada. There was a reduction in the rate of dispensing of both canagliflozin (
p
< 0.001) and dapagliflozin (
p
= 0.033).
| |
|
|
|
FIGURE 1
Nationwide dispensing of sodium glucose cotransporter 2 (SGLT2) inhibitors in Canada between 2014 and 2019. All of the trials were placebo-controlled and showed the following: empagliflozin reduced cardiovascular events and all-cause mortality (study published September 17, 2015); canagliflozin reduced cardiovascular events (but not all-cause mortality) and increased the risk of lower-limb amputation and bone fracture (study published June 12, 2017); and dapagliflozin did not reduce the risk of cardiovascular events or increase the risk of lower-limb amputation or bone fracture (study published November 10, 2018). Canagliflozin (blue line) was the first SGLT2 inhibitor approved in Canada (May 2014), followed by dapagliflozin (red line; December 2014) and then empagliflozin (green line; July 2015).
|
The results of the canagliflozin trial, published in June 2017, indicated a reduction in cardiovascular events but not all-cause mortality and an increase in the risk of lower-limb amputation. After publication of this study, canagliflozin remained the most commonly dispensed SGLT2 inhibitor, but its rate of dispensing decreased further (
p
< 0.001). In contrast, the rate of dispensing of empagliflozin increased (
p
< 0.001), and the rate of dispensing was unchanged for dapagliflozin (Table A1 in Appendix 1). In January 2018, empagliflozin (3 079 165 tablets; 38.1%) became the most commonly dispensed SGLT2 inhibitor (canagliflozin, 37.4%; dapagliflozin, 24.5%).
The results of the dapagliflozin trial, published in November 2018, indicated no clear reduction in cardiovascular events or all-cause mortality, and short-term dispensing trends were largely unaffected for canagliflozin (
p
= 0.28), empagliflozin (
p
= 0.41), and dapagliflozin (
p
= 0.52) (see Table A1 in Appendix 1). By the end of our study period, in April 2019, empagliflozin remained the most commonly dispensed SGLT2 inhibitor (5 591 477 tablets, 50.5%; canagliflozin, 3 018 130, 27.3%; dapagliflozin, 2 452 022, 22.2%), and approximately half (51.2%) of all dispensing of SGLT2 inhibitors was paid for with cash or by private insurance.
DISCUSSION
In this nationwide study of dispensing of SGLT2 inhibitors over a 5-year period, the cardiovascular outcome trials appeared to have an important impact on the dispensing of SGLT2 inhibitors in Canada. For example, there was a reduction in the rate of dispensing of canagliflozin following publication of the empagliflozin cardiovascular outcome trial. However, the publication date coincided with when empagliflozin became available in Canada, and it is therefore difficult to determine the relative contribution of each event.
Following publication of results from the cardiovascular outcome trial for canagliflozin, there was a significant reduction in the rate of canagliflozin dispensing, although it remained the most commonly dispensed SGLT2 inhibitor for the next 7 months (until January 2018). This finding was surprising, because canagliflozin was the only SGLT2 inhibitor with an increased risk of amputation and bone fracture, and 2 alternative SGLT2 inhibitors were approved for use in Canada.1 However, canagliflozin was the first SGLT2 inhibitor to become available in Canada. Similar findings have been observed for other classes of medications (e.g., direct oral anticoagulants, antiplatelet agents), whereby the first medication approved within a class is often the most commonly prescribed, even after alternative options become available.6 This pattern may reflect prescribing inertia or simply familiarity with the first available medication.7
One limitation of this study was the lack of patient-level and provider-level data, which meant we were unable to estimate how often patients were directly switched from one SGLT2 inhibitor to another. Furthermore, we had data only up to April 2019; as such, we were able to assess only the short-term impacts of the dapagliflozin trial (5 months of data) and were unable to analyze the impact of the positive renal outcomes trial for canagliflozin.8 Similarly, the results of trials evaluating heart failure outcomes with dapagliflozin or empagliflozin were published after April 2019.9,10
CONCLUSION
Our study provides a contemporary example of the lasting impact of being the first medication approved within a class and also shows how dispensing patterns change in response to updated clinical evidence.
References
1 Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Crossref PubMed
2 Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Crossref PubMed
3 Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Crossref
4 Xu Y, Gomes T, Mamdani MM, Juurlink DN, Cadarette SM, Tadrous M. Analysis of trends in insulin utilization and spending across Canada from 2010 to 2015. Can J Diabetes. 2019;43(3):179–185.e1.
Crossref
5 Brandt J, Shearer B, Morgan SG. Prescription drug coverage in Canada: a review of the economic, policy and political considerations for universal pharmacare. J Pharm Policy Pract. 2018;11(1):Article 28.
Crossref PubMed PMC
6 Schulze U, Ringel M. What matters most in commercial success: first-in-class or best-in-class. Nat Rev Drug Discov. 2013;12(6):419–20.
Crossref PubMed
7 Roumie CL, Elasy TA, Wallston KA, Pratt S, Greevy RA, Liu X, et al. Clinical inertia: a common barrier to changing provider prescribing behavior. Jt Comm J Qual Patient Saf. 2007;33(5):277–85.
PubMed
8 Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Crossref PubMed
9 McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Crossref PubMed
10 Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
Crossref PubMed
Michael Fralick, MD, PhD, is with the Sinai Health System and the Department of Medicine, University of Toronto, Toronto, Ontario
Diana Martins, MSc, was, during the period of the study, with Unity Health Toronto, Toronto, Ontario
Mina Tadrous, PharmD, MS, PhD, is with the Women’s College Research Institute, Women’s College Hospital; ICES; and the Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Ontario
Tara Gomes, PhD, MHSc, is with Unity Health Toronto; ICES; and the Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Ontario
Acknowledgements
The authors appreciate feedback received from Afsaneh Raissi on earlier versions of this manuscript. The authors thank IQVIA for the use of their data. The statements, findings, conclusions, views, and opinions expressed in this report are based in part on data obtained under license from IQVIA Solutions Canada Inc., concerning the following information services: GPM, data period May 1, 2014 – April 30, 2019. All rights reserved. The statements, findings, conclusions, views, and opinions expressed herein are not necessarily those of IQVIA Inc. or any of its affiliated or subsidiary entities.
Address correspondence to: Dr Michael Fralick, Sinai Health System, 60 Murray Street, Toronto ON M5T 3L9,
email:
mike.fralick@mail.utoronto.ca
(Return to Top)
Competing interests:
Other than study funding (described below), no competing interests were declared. (
Return to Text
)
Funding:
This project was supported by the Ontario Drug Policy Research Network, which is funded by grants from the Ontario Ministry of Health (MOH). The sponsors had no role in the design or conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by the Ontario MOH is intended or should be inferred. (
Return to Text
)
Canadian Journal of Hospital Pharmacy
, VOLUME
75
, NUMBER
2
,
Spring
2022
