Stability of
N
-Acetylcysteine 60 mg/mL in Extemporaneously Compounded Injectable Solutions
M Mihaela
Friciu
,
Anaëlle
Monfort
,
Pierre-André
Dubé
,
Grégoire
Leclair
ABSTRACT
Background
N
-Acetylcysteine (NAC) administered by the IV route is the current treatment of choice for acetaminophen overdose. However, the protocol approved by health authorities in most countries has a complex dosing regimen, which leads to dosage errors in one-third of cases. Therefore, the Canadian Antidote Guide in Acute Care Toxicology and individual poison centres have begun to recommend a simplified regimen using continuous IV infusion. Unfortunately, no study has demonstrated the stability of IV solutions of NAC at concentrations above 30 mg/mL or in solutions other than 5% dextrose.
Objective
To evaluate the stability of solutions of NAC 60 mg/mL in 0.9% sodium chloride, 0.45% sodium chloride, or 5% dextrose, stored for up to 72 hours in polyvinyl chloride (PVC) bags at 25°C.
Methods
Solutions of the desired concentration were prepared from a commercial solution of NAC 200 mg/mL, with dilution in 0.9% sodium chloride, 0.45% sodium chloride, or 5% dextrose, and were then stored at room temperature in PVC bags for up to 72 hours. At predetermined time points (0, 16, 24, 40, 48 and 72 h), samples were collected and analyzed using a stability-indicating high-performance liquid chromatography method. A solution was considered stable if it maintained at least 90.0% of its initial concentration. Particulate matter count was also evaluated to confirm chemical stability. Finally, organoleptic properties, such as odour and colour, were evaluated to assess the stability of the solutions.
Results
All solutions maintained at least 98.7% of their initial concentration. No obvious changes in odour or colour were observed. Moreover, particle counts remained acceptable throughout the study, according to the criteria specified in United States Pharmacopeia (USP) General Chapter <788>.
Conclusions
NAC 60 mg/mL, diluted in 0.9% sodium chloride, 0.45% sodium chloride, or 5% dextrose and stored in PVC bags at 25°C, was chemically and physically stable for a period of at least 72 hours.
KEYWORDS:
N
-acetylcysteine,
stability
,
antidote
,
acetaminophen overdose
RÉSUMÉ
Contexte
La N-acétylcystéine (NAC) administrée par IV est actuellement le traitement de choix en cas de surdose d’acétaminophène. Cependant, le protocole approuvé par les autorités sanitaires de la plupart des pays s’accompagne d’un schéma posologique complexe qui entraîne des erreurs de dosage dans un tiers des cas. C’est pourquoi, le
Guide canadien des antidotes en toxicologie d’urgence
et les centres antipoison ont commencé à recommander un schéma simplifié utilisant des perfusions IV. Malheureusement, aucune étude n’a permis de démontrer la stabilité des solutions IV de NAC à des concentrations supérieures à 30 mg/mL ou dans des solutions autres que 5 % de dextrose.
Objectif
Évaluer la stabilité des solutions de 60 mg/mL de NAC dans 0,9 % de chlorure de sodium, 0,45 % de chlorure de sodium ou 5 % de dextrose, stockées jusqu’à 72 heures dans des pochettes de chlorure de polyvinyle (PVC) à 25 °C.
Méthodes
Les solutions ont été préparées à partir d’une solution commerciale de 200 mg/mL de NAC, avec une dilution dans 0,9 % de chlorure de sodium, dans 0,45 % de chlorure de sodium ou dans 5 % de dextrose. Elles ont ensuite été stockées à température ambiante dans des pochettes en PVC pendant une période allant jusqu’à 72 h. À des instants prédéterminés (0, 16, 24, 40, 48 et 72 h), des échantillons étaient recueillis et analysés à l’aide d’une méthode de chromatographie en phase liquide à haute performance indiquant la stabilité. Si la solution préservait au moins 90 % de sa concentration initiale, elle était jugée stable. Un comptage de particules a aussi permis de confirmer la stabilité chimique. Finalement, les propriétés organoleptiques, comme l’odeur et la couleur, ont été examinées pour évaluer la stabilité des solutions de NAC.
Résultats
Toutes les solutions préservaient au moins 98,7 % de leur concentration initiale. Aucun changement manifeste d’odeur ou de couleur n’a été observé. De plus, le nombre de particules est resté acceptable pendant toute la durée de l’étude selon les critères indiqués dans le chapitre général de la
Pharmacopée américaine
(USP) <788>.
Conclusions
La solution de 60 mg/mL de NAC, diluée dans 0,9 % de chlorure de sodium, dans 0,45 % de chlorure de sodium ou dans 5 % de dextrose et stockée dans des pochettes en PVC à 25 °C était chimiquement et physiquement stable pendant au moins 72 h.
MOTS CLÉS:
N-acétylcystéine
,
stabilité
,
antidote
,
surdose d’acétaminophène
INTRODUCTION
For more than 40 years,
N
-acetylcysteine (NAC) administered by the IV route has been used as the treatment of choice for acetaminophen (paracetamol) overdose. However, the 21-hour protocol approved by the health authorities of most countries has a complex dosing regimen: 150 mg/kg (maximum 15 g) of NAC in 3 mL/kg (up to 200 mL) of 5% dextrose in water (D5W) over 1 hour, 50 mg/kg (maximum 5 g) of NAC in 7 mL/kg (up to 500 mL) of D5W over 4 hours, and then 100 mg/kg (maximum 10 g) of NAC in 7 mL/kg (up to 1000 mL) of D5W over 16 hours.1 This means that nurses must administer 3 bags, each with a different concentration, to each patient. In a study by Hayes and others,2 one-third of patients experienced medication errors related to the 3-bag protocol. Recently, many poison centres and toxicologists have begun to recommend a 1-bag or 2-bag regimen to simplify the prescription and administration of the antidote and to minimize the potential for medication errors.3–5
The Canadian Antidote Guide in Acute Care Toxicology, which brings together the recommendations of Canada’s 5 poison centres, states that continuous IV infusions of NAC should be administered using a volumetric pump.6 In 2016, the US Food and Drug Administration contracted the American Society of Health-System Pharmacists to develop and implement national standardized concentrations for IV medications, in an effort called the Standardize 4 Safety Initiative.7 Antidotes are high-risk medications used to treat medication errors and are typically administered to critically ill patients. Thus, antidotes should be considered in the development of standardized concentrations.
Little stability data are available in the literature concerning IV solutions of NAC. Dribben and others8 showed that NAC at a concentration of 30 mg/mL in polyvinyl chloride (PVC) bags containing 5% dextrose is physically and chemically stable at room temperature (25°C) for a 60-hour period. In that study, less than 10% of the NAC was lost over 60 hours, and 10% to 15% was lost over 72 hours. The US manufacturer’s package insert states that NAC (concentration unspecified) is physically and chemically stable for 24 hours at room temperature in 0.45% sodium chloride (container unspecified).9 This information is not provided in the monographs approved by Health Canada.10 The results of other published studies have not been specific to IV solutions and therefore cannot be extrapolated.11,12
When a patient is poisoned, clinicians always aim to give the most concentrated solutions of any required antidotes. The rationale is to limit the volume administered, given that patients in this situation are often intoxicated with multiple agents, and fluid overload must be avoided (e.g., to prevent accumulation of fluid in the lungs).13,14 The objective of the current study was to evaluate the physical and chemical stability of NAC 60 mg/mL in various diluents with storage in PVC bags at 25°C; as such, we tested twice the maximum concentration that is known to be stable in diluents that are currently used in clinical practice but for which data are not yet available. The results of this analysis could be used by pharmacists and toxicologists to establish a standard NAC concentration for IV administration according to established procedures in their hospitals.
METHODS
Extemporaneous Preparations
Multiple solutions of NAC 60 mg/mL were prepared from a 200 mg/mL commercial solution (Teligent OÜ; lot 185061, expiry May 2020). Sixty-millilitre samples of the commercial solution (equivalent to 12 000 mg of NAC) were transferred into 200-mL volumetric flasks using a 100-mL graduated cylinder and brought to volume with either 0.9% sodium chloride (Baxter; lot W8K16M0, expiry May 2020), 0.45% sodium chloride (Baxter; lot W8J31A1, expiry April 2020), or 5% dextrose (Baxter; lot W8F26A1, expiry December 2019). The solutions all had a final concentration of 60 mg/mL.
Design of Stability Study
Each 60 mg/mL solution of NAC was packaged into three 150-mL PVC bags (Baxter; lot DR18I20066), such that each bag contained 60 mL of solution. The remaining quantity of each solution (20 mL) was discarded. All of the bags were incubated at 25°C ± 2°C and 60% ± 5% relative humidity for up to 72 hours. At each time point (0, 16, 24, 40, 48, and 72 hours), the bags were shaken for 10 seconds, and a 1-mL aliquot was then retrieved from each bag using a 1-mL sterile syringe. From this 1-mL aliquot, only 20 μL was precisely measured for further analyses. For each test sample, the organoleptic properties (odour and colour) were inspected, and the NAC concentration was assayed by high-performance liquid chromatography with ultraviolet detection (HPLC-UV). Finally, at 0 and 72 hours, the particle count was evaluated by light obscuration using 25 mL from each bag.
Liquid Chromatography
HPLC-UV Method
The HPLC system (Prominence UFLC, Shimadzu) was equipped with an LC-20AD binary pump operating at a flow rate of 0.6 mL/min, a DGU-20A5 solvent degasser, an SPD-M20A multiple-wavelength photodiode array detector set at 220 nm for NAC, an SIL-20AC HT refrigerated autosampler at 5°C, and a CTO-20AC column oven at 25°C. A C18 with polar end capping Hydro-RP Synergi 4 column (3.0 × 100 mm, 3 μm, Phenomenex) was used for this study. The isocratic mobile phases consisted of 97% aqueous solution of o-phosphoric acid 14.6 mmol/L (solution from 85% o-phosphoric acid, Fisher Scientific; lot 173953) and 3% methanol (Fisher Scientific; lot 144689). The total run time for each injection was 15 minutes. Quantification was performed using the area under the peak eluting at approximately 3.1 minutes.
Preparation of Stocks and Standard Curve Solutions
A stock solution of NAC 10 mg/mL was prepared from bulk powder (Sigma Aldrich; lot WXBC7926V) by dissolving 67.9 mg of NAC in 6.79 mL of a 10% methanol aqueous solution. Five standards with nominal concentrations of 0.25, 0.50, 0.75, 1.0, and 1.5 mg/mL were prepared by diluting the stock solution with the 10% methanol aqueous solution to create a calibration curve. These standards were analyzed in triplicate using HPLC. The precision of this method was assessed by evaluating the intraday coefficients of variation of each standard’s peak area. The acceptable limit for the coefficient of variation was defined as less than 1%.
Sample Preparation for HPLC Injection
For the HPLC analysis, 20 μL of each test sample was first diluted with 1 mL of 10% methanol aqueous solution in a 1.5-mL centrifuge tube, vortex-mixed for 10 seconds, and then transferred to a sealed 96-well plate (VWR; lot 25114129). These injection solutions had a nominal concentration of 1.176 mg/mL and were analyzed in duplicate immediately after preparation.
Forced Degradation of
N
-Acetylcysteine
A stock solution of NAC 10 mg/mL was prepared from bulk powder by dissolving 67.9 mg of NAC in 6.79 mL of a 10% methanol aqueous solution. A 0.5-mL volume of this solution was mixed with either 0.5 mL of water, 0.5 mL of aqueous hydrochloric acid 0.1 mol/L, 0.5 mL of aqueous sodium hydroxide 0.1 mol/L, or 0.5 mL of 3% aqueous hydrogen peroxide. These 4 solutions were then stored at 60°C for 3 hours. A 100-μL volume of the acidic solution was neutralized using 50 μL of aqueous sodium hydroxide 1 mol/L and then diluted with 850 μL of 10% methanol aqueous solution. Similarly, 100 μL of the alkaline solution was neutralized using 50 μL of aqueous hydrochloric acid 1 mol/L and diluted with 850 μL of 10% methanol aqueous solution. The water and peroxide solutions were directly diluted with 900 μL of 10% methanol aqueous solution. Finally, all of the solutions were prepared as described above and analyzed by HPLC. The chromatograms obtained from these analyses were compared with the chromatograms obtained from the NAC standard solutions and from a 1 mg/mL solution of NAC in 3% aqueous hydrogen peroxide that was not subjected to heat (because NAC in 3% aqueous hydrogen peroxide subjected to heating was completely degraded).
Particle Count by Light Obscuration
Particle count was evaluated in 25 mL of each bag of NAC 60 mg/mL (
n
= 9) at 0 and 72 hours using a light obscuration particle counter (LS-20 particle counter, Lighthouse Worldwide Solutions). Each sample was run through the instrument 3 times, in accordance with United States Pharmacopeia (USP) General Chapter <788>,15 which recommends using a sample with a minimum volume of 20 mL spread across four 5-mL aliquots, with the results for the first aliquot being discarded. To ensure that the preparation meets the USP criteria, the average number of particles present in each test unit should not exceed 25 per millilitre for particles equal to or greater than 10 μm in diameter and should not exceed 3 per millilitre for particles equal to or greater than 25 μm in diameter.15
RESULTS
No notable changes in odour (sulphur smell) or colour (transparent) were observed in any of the solutions after 72 hours of storage under various conditions.
Regression analysis of the peak area of NAC versus concentration of the NAC standard demonstrated linearity over the range of concentrations tested, with a coefficient of determination (
R
2) of 1.00. The coefficient of determination is used to evaluate the linearity of the method within a specific concentration range. The closer this value is to 1, the better the linear model is able to predict the concentration of a sample. The intraday coefficients of variation calculated for the triplicate injection samples were considered acceptable (no greater than 1.0%), falling between 0.05% and 0.18% for all standards of the calibration curve.
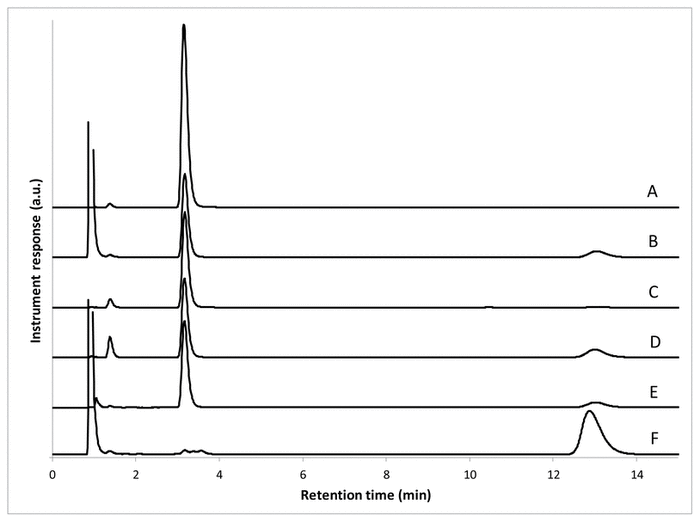
No peak overlap of NAC with excipients, impurities, or degradation products was observed. The NAC peak impurity index calculated between 190 and 250 nm was not less than 0.9999 in any case. The impurity index is considered valid when it is close to 1. Therefore, in this case, the impurity index was acceptable. The following recoveries were observed after degradation: 88% in 3% aqueous hydrogen peroxide at 25°C, 100% in water, 83% in aqueous hydrochloric acid 0.1 mol/L, 91% in aqueous sodium hydroxide 0.1 mol/L, and 5% in 3% aqueous hydrogen peroxide at 60°C (Figure 1).
| |
|
|
|
FIGURE 1
Representative chromatograms of
N
-acetylcysteine, at nominal concentration of 1 mg/mL, in various diluents with various storage conditions. A: In 10% methanol aqueous solution. B: In 3% aqueous hydrogen peroxide not subjected to heating. C: In water, at 60°C for 3 hours. D: In hydrogen chloride 0.1 mol/L, at 60°C for 3 hours. E: In sodium hydroxide 0.1 mol/L, at 60°C for 3 hours. F: In 3.0% aqueous hydrogen peroxide, at 60°C for 3 hours.
|
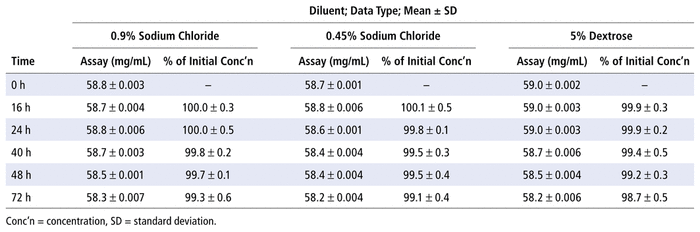
Moreover, the concentration of NAC was at least 90.0% of the initial concentration in all preparations stored in PVC infusion bags at 25°C for up to 72 hours (Table 1).
TABLE 1
Concentration of
N
-Acetylcysteine and Percent of Initial Concentration Remaining at Each Time Point after Preparation in Various Diluents and Storage in Polyvinyl Chloride Bags at 25°C
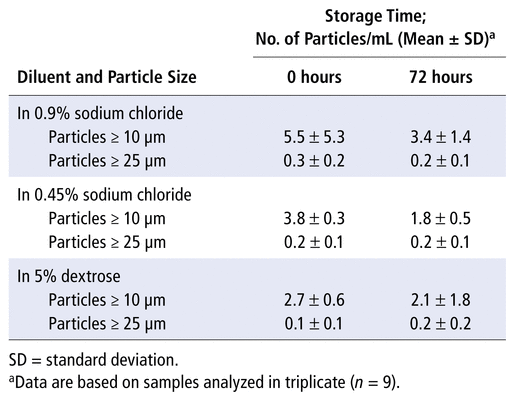
Finally, the results obtained in the evaluation of particulate matter fell within the criteria for particulate matter in injection solutions specified by UPS General Chapter <788> Test 1A for all samples, both at the initial time point and after 72 hours of storage at 25°C and 60% relative humidity (Table 2).
TABLE 2
Particulate Matter in
N
-Acetylcysteine 60 mg/mL Solutions Stored in Polyvinyl Chloride Bags at 25°C for 72 Hours
DISCUSSION
According to the HPLC analyses, solutions of NAC 60 mg/mL prepared in 0.9% sodium chloride, 0.45% sodium chloride, and 5% dextrose maintained at least 98.7% of their initial concentrations for up to 72 hours when stored at 25°C in PVC infusion bags. In addition, the particulate matter counts demonstrated that these NAC solutions fell within USP specifications for particulate matter in solutions for injection, with fewer than 25 particles of diameter 10 μm or larger per millilitre and fewer than 3 particles of diameter 25 μm or larger per millilitre. The results also indicated that no physical or chemical changes occurred under the defined storage conditions.
To date, the only study that has evaluated the stability of NAC in PVC infusion bags showed that 30 mg/mL solutions in 5% dextrose were stable at 25°C for a maximum of 60 hours.8 In addition, the US manufacturer’s package insert states that NAC is physically and chemically stable for a maximum of 24 hours at 25°C in 0.45% sodium chloride and 5% dextrose.9 However, this information is not provided in the monographs approved by Health Canada.10 Therefore, to our knowledge, the current study is the first to demonstrate the stability of NAC 60 mg/mL diluted in 0.9% sodium chloride, 0.45% sodium chloride, and 5% dextrose solutions and stored in PVC infusion bags for up to 72 hours at room temperature.
When determining the most suitable standardized solution of NAC for IV administration, clinicians should consider the physical and chemical stability of the admixture (as demonstrated in this study), as well as the most concentrated solution (for critically ill patients needing fluid restriction) suitable for peripheral IV administration, given that most acetaminophen-intoxicated patients will not have central line access.
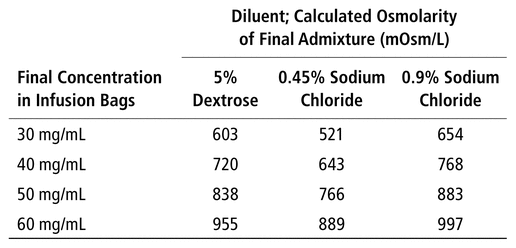
Table 3 summarizes the calculated osmolarities of final admixtures, considering the limits of commercially available products. The osmolarities of the diluents used to prepare these admixtures were 250 mOsm/L for 5% dextrose, 154 mOsm/L for 0.45% sodium chloride, and 310 mOsm/L for 0.9% sodium chloride,16–18 whereas the osmolarity of the 200 mg/mL solution of NAC was 2600 mOsm/L.9 Calculation of osmolarity for NAC 60 mg/mL in 5% dextrose is given as an example:
Caution should be applied with NAC 60 mg/mL solutions in 5% dextrose and 0.9% sodium chloride, because the osmolarity may be above the maximum osmolarity tolerated for peripheral IV administration. In addition, there is no firm consensus within the scientific community concerning maximum osmolarity. However, the latest guidelines published by the American Society for Parenteral and Enteral Nutrition state that “an osmolarity up to 900 mOsm/L can be safely infused peripherally”.19
TABLE 3
Calculated Osmolarity of
N
-Acetylcysteine in IV Infusion Bags at Different Concentrations, Based on Commercial Solution of NAC 200 mg/mL for IV Administration
Multiple adverse events and safety issues have been reported with the standard 3-bag IV regimen, consisting of a loading dose of 150 mg/kg (maximum 15 g) of NAC in 3 mL/kg (up to 200 mL) of D5W infused over 60 minutes, followed by 50 mg/kg (maximum 5 g) of NAC in 7 mL/kg (up to 500 mL) of D5W infused over 4 hours, followed by 100 mg/kg (maximum 10 g) of NAC in 7 mL/kg (up to 1000 mL) of D5W infused over 16 hours.1 For example, Ferner and others20 prospectively analyzed 186 NAC infusion bags prepared on site and found that only one-third of the bags contained within about 10% of the anticipated dose of NAC. In addition, about one-tenth of the bags contained 50% more NAC than expected. Smart infusion pumps are now widely used in hospitals for safety reasons.21 In its latest guidelines for optimizing safe implementation and use of smart infusion pumps (published in 2020), the US Institute for Safe Medication Practices recommended, among other things, to standardize and limit the number of drug concentrations for continuous and intermittent infusions and to use commercially prepared solutions when available.22 The standard IV regimen for NAC cannot be programmed into smart infusion pumps, as there may be too many different concentrations required. With many hospitals and poison control centres seeking to use simpler alternative IV regimens for NAC,3–5,23,24 this study provides health care professionals and the pharmaceutical industry with data previously not available concerning the stability of concentrated NAC.
CONCLUSION
Solutions of NAC 60 mg/mL prepared with 0.9% sodium chloride, 0.45% sodium chloride, or 5% dextrose and stored in PVC bags at 25°C were stable for up to 72 hours. All of the solutions, which were prepared from a commercial 200 mg/mL solution, maintained at least 90.0% of their initial concentration under all tested storage conditions.
References
1 Hendrickson RG, Howland MA. Chapter A3: N-Acetylcysteine. In: Nelson LS, Howland MA, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, editors. Goldfrank’s toxicologic emergencies. 11th ed. McGraw-Hill Education; 2019.
2 Hayes BD, Klein-Schwartz W, Doyon S. Frequency of medication errors with intravenous acetylcysteine for acetaminophen overdose. Ann Pharmacother. 2008;42(6):766–70.
Crossref
3 McNulty R, Lim JME, Chandru P, Gunja N. Fewer adverse effects with a modified two-bag acetylcysteine protocol in paracetamol overdose. Clin Toxicol. 2018;56(7):618–21.
Crossref
4 Wong A, Graudins A. N-Acetylcysteine regimens for paracetamol overdose: time for a change? Emerg Med Australas. 2016;28(6):749–51
Crossref
5 Wong A, Graudins A. Simplification of the standard three-bag intravenous acetylcysteine regimen for paracetamol poisoning results in a lower incidence of adverse drug reactions. Clin Toxicol. 2016;54(2):115–9.
Crossref
6 Bailey B, Blais R, Dubé PA, Friesen M, Gaudreault P, Gosselin S, et al. Acetylcysteine In: Canadian antidote guide in acute care toxicology. Centre antipoison du Québec, CIUSSS de la Capitale-Nationale; 2018 [cited 2020 Jun 30]. Available from: https://www.ciusss-capitalenationale.gouv.qc.ca/node/4848?lang=en
7 Standardize 4 Safety Initiative overview. American Society of Health-System Pharmacists; 2019 [cited 2020 June 30]. Available from: https://www.ashp.org/Pharmacy-Practice/Standardize-4-Safety-Initiative
8 Dribben WH, Porto SM, Jeffords BK. Stability and microbiology of inhalant N-acetylcysteine used as an intravenous solution for the treatment of acetaminophen poisoning. Ann Emerg Med. 2003;42(1):9–13.
Crossref
9 Acetadote [prescribing information]. Cumberland Pharmaceuticals; 2016 [cited 2020 Jun 30]. Available from: http://www.acetadote.com/acetadote-prescribing-information-11-2016.pdf
10 Acetylcysteine solution USP product monograph. Teligent Canada Inc; 2016 Dec 19.
11 Fohl AL, Johnson CE, Cober MP. Stability of extemporaneously prepared acetylcysteine 1% and 10% solutions for treatment of meconium ileus. Am J Health Syst Pharm. 2011;68(1):69–72.
Crossref
12 Kiser TH, Oldland AR, Fish DN. Stability of acetylcysteine solution repackaged in oral syringes and associated cost savings. Am J Health Syst Pharm. 2007;64(7):762–6.
Crossref
13 Malbrain MLNG, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):36180.
Crossref
14 Alobaidi R, Morgan C, Basu RK, Stenson E, Featherstone R, Majumdar SR, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(3):25768.
Crossref
15 General Chapter <788>: Particulate matter in injections. In: United States pharmacopeia 42 – national formulary 37. United States Pharmacopeial Convention; 2018.
16 5% dextrose injection USP product monograph. Baxter Corporation; 2014 Jul 30.
17 0.45% sodium chloride injection USP product monograph. Baxter Corporation; 2018 Dec 3.
18 0.9% sodium chloride injection USP product monograph. Baxter Corporation; 2016 Nov 3.
19 Boullata JI, Gilbert K, Sacks G, Labossiere RJ, Crill C, Goday P, et al. A.S.P.E.N. clinical guidelines: parenteral nutrition ordering, order review, compounding, labeling, and dispensing. J Parenteral Enteral Nutr. 2014;38(3):33477.
Crossref
20 Ferner RE, Langford NJ, Anton C, Hutchings A, Bateman DN, Routledge PA. Random and systematic medication errors in routine clinical practice: a multicentre study of infusions, using acetylcysteine as an example. Br J Clin Pharmacol. 2001;52(5):573–7.
Crossref
21 Smart pumps in practice: survey results reveal widespread use, but optimization is challenging. Institute for Safe Medication Practices; 2018 [cited 2021 Mar 12]. Available from: https://www.ismp.org/resources/smart-pumps-practice-survey-results-reveal-widespread-use-optimization-challenging
22 Guidelines for optimizing safe implementation and use of smart infusion pumps. Institute for Safe Medication Practices; 2020 [cited 2021 March 12]. Available from: https://www.ismp.org/guidelines/safe-implementation-and-use-smart-pumps
23 Bateman DN, Dear JW, Thomas SHL. New regimens for intravenous acetylcysteine, where are we now? Clin Toxicol 2016;54(2):758.
Crossref
24 Isbister GK, Downes MA, Mcnamara K, Berling I, Whyte IM, Page CB. A prospective observational study of a novel 2-phase infusion protocol for the administration of acetylcysteine in paracetamol poisoning. Clin Toxicol 2016;54(2):1206
Crossref
M Mihaela Friciu
, MSc, is a Research Associate with the Faculty of Pharmacy, Université de Montréal, Montréal, Quebec
Anaëlle Monfort
, BSc, is a PhD student with the Faculty of Pharmacy, Université de Montréal, Montréal, Quebec
Pierre-André Dubé
, BPharm, PharmD, MSc, FOPQ, is a Pharmacist and Toxicologist with the Institut national de santé publique du Québec, Québec, Quebec
Grégoire Leclair
, BPharm, PhD, is a Full Professor with the Faculty of Pharmacy, Université de Montréal, Montréal, Quebec
Competing interests:
None declared. (
Return to Text
)
Address correspondence to: Grégoire Leclair, Faculté de pharmacie, Université de Montréal, PO Box 6128, Downtown Station, Montréal QC H3C 3J7,
email:
gregoire.leclair@umontreal.ca
(Return to Top)
Funding:
This work was supported, through an unrestricted grant, by the Institut national de santé publique du Québec (INSPQ). The funder was not involved in the design or conduct of the study, including the collection, management, or interpretation of the stability data obtained. (
Return to Text
)
Canadian Journal of Hospital Pharmacy
, VOLUME
74
, NUMBER
4
,
Fall
2021



