Rachel Cormier , Tim MacLaggan , Daniel Landry , Rachel Harris , Andrew FlewellingABSTRACT
Background
Prevalence surveys are useful tools for assessing the appropriateness of antimicrobial therapy.
Objectives
The primary objective was to assess patterns of antimicrobial utilization and appropriateness in New Brunswick hospitals. The secondary objective was to assess the impact of hospital size and the presence of a penicillin allergy label on antimicrobial appropriateness.
Methods
A point prevalence survey was conducted of inpatients taking 1 or more systemic antimicrobials during admission to hospitals in New Brunswick. A structured protocol and web-based data collection tool (National Antimicrobial Prescribing Survey) were used for this survey. Data regarding hospital size and presence of a penicillin allergy label were also collected. Antimicrobial utilization was assessed in terms of guideline compliance and appropriateness. Results were summarized descriptively. A χ2 analysis was performed to describe secondary outcomes.
Results
Ten hospitals participated, and a total of 2200 patients were admitted at the time of the survey. The overall prevalence of antimicrobial use was 22.7% (500/2200). A total of 648 antimicrobials were ordered. The most frequently prescribed antimicrobials by class were first-generation cephalosporins (14.0%, 91/648), third-generation cephalosporins (11.3%, 73/648), and piperacillin–tazobactam (10.2%, 66/648). The most common indications for antimicrobial therapy were respiratory tract infections (27.3%, 177/648), urinary tract infections (12.2%, 79/648), and intra-abdominal infections (11.4%, 74/648). Compliance with local or regional treatment guidelines, where applicable, was 66.2% (188/284). Provincially, 68.1% (441/648) of the antimicrobial orders were deemed appropriate. Larger centres had substantially higher rates of appropriateness ( p < 0.001). The presence of a penicillin allergy label had no impact on appropriateness ( p = 0.21).
Conclusions
Several opportunities for targeted interventions were identified to improve antimicrobial prescribing, including decreasing the use of broad-spectrum antimicrobials, increasing guideline compliance, and ensuring documentation of antimicrobial duration by prescribers.
KEYWORDS: antimicrobial, prevalence survey, stewardship, antimicrobial utilization, appropriateness
RÉSUMÉ
Contexte
Les enquêtes de prévalence sont des outils utiles permettant d’évaluer la pertinence de la thérapie antimicrobienne.
Objectifs
L’objectif principal consistait à évaluer les modèles d’utilisation des antimicrobiens et leur pertinence dans les hôpitaux du Nouveau-Brunswick. L’objectif secondaire consistait, quant à lui, à évaluer l’effet de la taille de l’hôpital et de la présence d’une étiquette indiquant une allergie à la pénicilline sur la pertinence des antimicrobiens.
Méthodes
Une enquête ponctuelle a été menée auprès de patients hospitalisés prenant un ou plusieurs antimicrobiens systémiques lors de leur admission dans des hôpitaux du Nouveau-Brunswick. Un protocole structuré et un outil de collecte de données en ligne (National Antimicrobial Prescribing Survey, ou enquête nationale sur la prescription d’antimicrobiens ) ont été utilisés pour cette enquête. Des données concernant la taille de l’hôpital et la présence d’une étiquette indiquant une allergie à la pénicilline ont aussi été recueillies. L’utilisation des antimicrobiens a été évaluée sur le plan de la pertinence et de la conformité aux lignes directrices. Les résultats ont été résumés de manière descriptive. Une analyse χ2 a été effectuée pour décrire les résultats secondaires.
Résultats
Dix hôpitaux ont participé, et un total de 2200 patients ont été admis au moment de l’enquête. La prévalence globale de l’utilisation d’antimicrobiens était de 22,7 % (500/2200). Au total, 648 antimicrobiens ont été prescrits. Les antimicrobiens les plus fréquemment prescrits (par classe) étaient les céphalosporines de première génération (14,0 %, 91/648); les céphalosporines de troisième génération (11,3 %, 73/648); et la pipéracilline-tazobactam (10,2 %, 66/648). Les indications les plus courantes de l’antibiothérapie étaient les infections des voies respiratoires (27,3 %, 177/648), les infections des voies urinaires (12,2 %, 79/648) et les infections intra-abdominales (11,4 %, 74/648). Le respect des directives de traitement locales ou régionales, le cas échéant, était de 66,2 % (188/284). À l’échelle provinciale, 68,1 % (441/648) des ordonnances d’antimicrobiens ont été jugées appropriées. Les grands centres avaient des taux de pertinence sensiblement plus élevés ( p < 0,001). La présence d’une étiquette indiquant une allergie à la pénicilline n’a eu aucun effet sur la pertinence ( p = 0,21).
Conclusions
Plusieurs occasions d’interventions ciblées ont été dégagées pour améliorer la prescription d’antimicrobiens, y compris la diminution de l’utilisation d’antimicrobiens à large spectre, une plus grande conformité aux lignes directrices et l’assurance que la durée de l’antimicrobien est consignée par les prescripteurs.
MOTS CLÉS: antimicrobien, enquête de prévalence, gestion responsable, utilisation des antimicrobiens, pertinence
According to the World Health Organization, antimicrobial resistance “threatens the effective prevention and treatment of an ever-increasing range of infections caused by bacteria, parasites, viruses and fungi”.1 Because of the increasing consumption and misuse of antimicrobials globally, the threat of antimicrobial resistance to public health is currently on the rise.1 Infections associated with antimicrobial resistance are concerning because they lead to longer hospital stays, higher medical costs, and increased risk of death.2 According to a 2014 report from the Public Health Agency of Canada, more than 18 000 patients in Canadian hospitals acquire antimicrobial-resistant infections every year.3 Government and societal action are therefore required to combat this problem.1 In 2015, the Government of Canada released a federal action plan to address this issue by increasing both the surveillance of antimicrobial use and the implementation of strong stewardship practices.4 To inform antimicrobial stewardship practices locally, it becomes essential to comprehend patterns of antimicrobial utilization and the threats of antimicrobial resistance that exist.
Prevalence surveys have proven useful tools in determining the appropriateness of antimicrobial therapy.5 Point prevalence surveys have been used to assess antibiotic utilization both internationally and within Canada.6–17 Several such surveys assessing antimicrobial appropriateness have been conducted in New Brunswick to date, including the Global Point Prevalence Survey (from the Netherlands)18 and a modified version of the European Surveillance of Antimicrobial Consumption point prevalence survey.19 Unfortunately, these studies lacked a broad assessment of antimicrobial appropriateness, because they were limited to evaluations of compliance with provincial guidelines or predefined definitions (e.g., therapy duplication, inappropriate route or dose, no documented indication, bug–drug mismatch, opportunity to de-escalate, treatment of asymptomatic bacteriuria), leaving upwards of 50% of antimicrobial orders without an assessment of appropriateness, where neither provincial guidelines nor the predefined definitions were applicable.18,19 As a result, current understanding of antimicrobial usage and appropriateness within New Brunswick is based on an incomplete picture.
Data from previous provincial point prevalence surveys were highly suggestive that antimicrobial guideline compliance varies by hospital size, with substantially higher rates of guideline compliance at referral centres than at nonreferral hospitals.18,19 Several surveys found in the literature also demonstrated an association between the presence of an antimicrobial allergy label and a higher rate of inappropriate antimicrobial prescribing.20,21 Although the effects of allergy labels in general have been investigated, the specific impact of a penicillin allergy label on antimicrobial appropriateness has yet to be established.
The recent introduction of the Australian National Antimicrobial Prescribing Survey (NAPS; https://www.naps.org.au), now available for use in Canada, facilitates a broad assessment of antimicrobial appropriateness, distinct from guideline concordance. In addition, this survey provides an estimate of antimicrobial use.22 With the use of this tool, the present study aimed to assess patterns of antimicrobial utilization and appropriateness within NB hospitals. Furthermore, the study assessed the impact of a penicillin allergy label and hospital type (referral or nonreferral centre) on appropriateness. The results will be used to identify opportunities to improve patient outcomes and safety through the implementation of targeted antimicrobial stewardship initiatives.
This study was a province-wide point prevalence survey of antimicrobial usage. It included inpatients admitted to 10 regional hospitals in New Brunswick, Canada, who were receiving 1 or more antimicrobials. The study was approved by the research ethics boards of Horizon Health Network, on October 2, 2019 (File 100513), and Vitalité Health Network, on October 23, 2019 (File CER-2019–21). This study met the criteria for the secondary use of information under TCPS2 (Tri-Council Policy Statement) article 5.5A, and informed consent was therefore not obtained.
At each participating hospital, a list of all inpatients admitted at 08h00 on each day of the audit was produced, and patients were screened for eligibility. Data were included in the NAPS database for all inpatients who had an active antimicrobial prescription on their medication chart at 08h00 on the audit day, as well as those who had received a stat dose of an antimicrobial within the previous 24 hours, including for surgical prophylaxis. Patients receiving ambulatory care (day stay and outpatients), hospital-in-the-home patients, residential aged care patients (i.e., in veterans’ units and nursing homes), and emergency department patients not yet admitted to the hospital were excluded from the survey.
Survey data were collected between November 2019 and February 2020 and were submitted from participating hospitals to a central database through a web-based interface. A formal training session was provided to all surveyors before the survey began. Most data collection took place in the months of November and December 2019. Once the survey had been initiated within a given site, data collection was completed within a 4-week window. Because it was not feasible to sample all inpatients in the larger health care facilities on 1 calendar day, different wards were surveyed over separate days, with each ward being surveyed only once. More than half of the sites ( n = 6) needed more than 2 days to complete the survey. Patient flow within the hospital was considered, to prevent patients from being counted twice if they were likely to be moved (e.g., from critical care to a step-down unit). Additionally, the surgical wards were surveyed on days after the days when most elective procedures were scheduled (i.e., Tuesday to Friday) to facilitate an assessment of duration of prophylactic therapy in the preceding 24 hours.
Individual patient charts were reviewed. Data were collected using the NAPS standardized structured protocol and web-based data entry tool. The NAPS data set included the following: demographic characteristics, specifically date of survey, hospital, patient identification number, age, sex, and specialty of admission; antimicrobial use, specifically antimicrobial agent, route, dose, frequency, indication (documented or presumed), and documentation of stop date; guideline compliance and assessment of appropriateness; and, if applicable, allergies to antimicrobials, surgical procedure performed, microbiology test results, and clinical notes or comments. Appropriateness was assessed according to the structured algorithm (accessed through https://www.naps.org.au), which consists of 5 categories of appropriateness, defined as 1 = optimal, 2 = adequate, 3 = suboptimal, 4 = inadequate, and 5 = not assessable. The structured algorithm provides a methodical and combined assessment of appropriateness through compliance with guidelines (local), patient allergies, surgical prophylaxis duration (less than or greater than 24 hours), microbiology, route, dose or frequency, duration, antimicrobial spectrum (too broad or too narrow), indication, and any restrictions that applied. If patients’ cases were too complex (due to multiple comorbidities, allergies, or microbiology results), if there was insufficient documentation, or if notes were not comprehensive enough, appropriateness was considered “not assessable” (score of 5). A score of 1 or 2 was considered to represent “appropriate” prescribing and a score of 3 or 4 was considered to represent “inappropriate” prescribing.22
In addition to the standard data set, information about a patient’s penicillin allergy label, including the type of reaction (high-risk reaction, not a high-risk reaction, or unknown) and the presence or absence of appropriate documentation of the reaction, was collected. A predefined tool, with definitions, was provided to guide the investigators in answering these additional survey questions. The hospital from which a patient’s data were collected was entered into the NAPS database, which then categorized the data according to the bed count of the particular hospital site. The hospital site was considered to be a referral centre if the bed count was 250 or more; nonreferral centres encompassed hospitals with fewer than 250 beds. Although the definitions based on bed count were arbitrary, this was considered a reasonable division based upon hospital sizes in the province of New Brunswick and their provision of clinical services.
The data collection and assessments of antimicrobial appropriateness were undertaken by the auditing team in accordance with the definitions outlined by the NAPS (Appendix 1, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/208). The team consisted of 1 or more clinical pharmacists based at each of the 10 sites (including T.M., R.H., D.L.) and a pharmacy resident (R.C.). Two of the clinical pharmacist team members (T.M, D.L.) were part of the infectious disease service at their respective hospitals; they also served on the provincial antimicrobial stewardship team. Investigators at each site collected the data and assessed guideline compliance and appropriateness. All antimicrobial entries were then jointly validated by 2 infectious disease pharmacists (alternate investigators). If the alternate investigators had difficulty interpreting the assessment, they contacted the investigator who did the initial data collection or assessment, and a conclusion was reached by consensus. Additionally, if an investigator deemed an antimicrobial order as “not assessable”, an alternate investigator was consulted for confirmation or further evaluation.
Data acquired during the point prevalence survey were presented using descriptive statistics. Continuous variables (such as patient age, antimicrobial dose, and frequency) were described using means, standard deviations, and ranges, whereas categorical variables (such as guideline compliance, appropriateness, and indication) were described using frequencies and percentages. Assessments of antimicrobial appropriateness in relation to hospital type (referral versus nonreferral) and in relation to presence or absence of a penicillin allergy label were compared using a 2×2 χ2 test. For the purposes of these χ2 tests, the unit of analysis was the antimicrobial. All tests were assessed using an α level of 0.05. Cramer V effect sizes were reported for χ2 tests (95% confidence intervals [CIs] for effect sizes are also reported). An a priori power analysis, performed using G*Power software (α = 0.05, power = 0.8, df = 1, effect size = 0.3, minimum sample size = 88), indicated sufficient power for these analyses, given the sample size of this study.
Ten hospitals within the province of New Brunswick participated in the survey. Included were regional and major sites from each zone: 5 from Horizon Health Network and 5 from Vitalité Health Network. Four of these sites were considered to be referral centres (≥ 250 beds), and 6 sites were considered to be nonreferral centres (< 250 beds). Three of the 4 referral sites employed infectious disease physicians certified by the Royal College of Physicians and Surgeons of Canada.
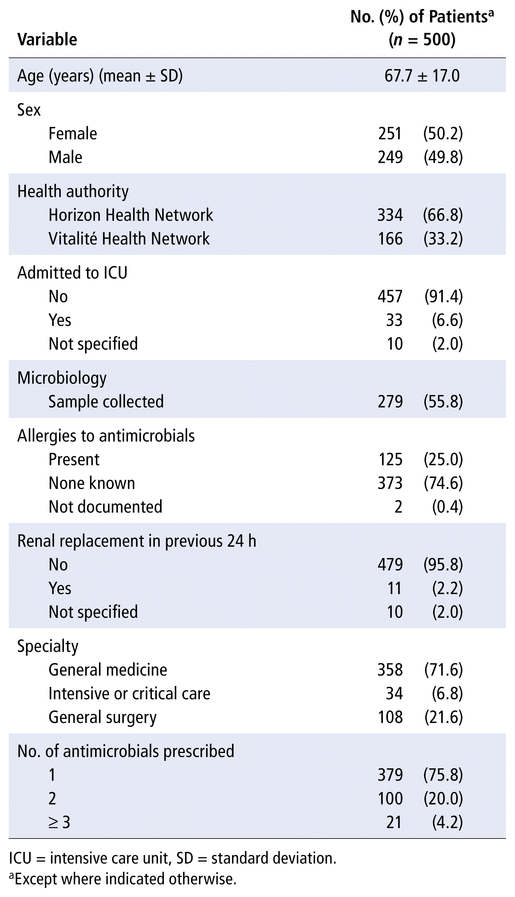
A total of 2220 patients were admitted at the time of the survey, of whom 500 (22.7%) were receiving systemic antimicrobial therapy. Approximately two-thirds of the patients included in the analysis (i.e., receiving an antimicrobial) were admitted within Horizon Health Network and one-third from Vitalité Health Network. Approximately 50% of these inpatients were male, and roughly 75% were receiving only 1 antimicrobial agent, as summarized in the baseline characteristics presented in Table 1. A total of 648 antimicrobial agents were prescribed for 76 types of indications. The most common types of indications were respiratory tract infections (27.3%, 177/648), urinary tract infections (UTIs) (12.2%, 79/648), and intra-abdominal infections (11.4%, 74/648), as illustrated in Figure 1.
TABLE 1
Baseline Characteristics of Patients Receiving Antimicrobial Agents at New Brunswick Hospitals

|
|
||
|
FIGURE 1 Top 10 indications for antimicrobial use in New Brunswick hospitals. |
||
Overall, 52.3% (339/648) of the prescriptions were for parenteral antimicrobial agents, and 47.7% (309/648) were for oral antimicrobial agents. The most commonly prescribed antimicrobials were first-generation cephalosporins (14.0%, 91/648), third-generation cephalosporins (11.3%, 73/648), piperacillin–tazobactam (10.2%, 66/648), and fluoroquinolones (9.1%, 59/648), as shown in Figure 2.
|
|
||
|
FIGURE 2 Antimicrobial agents most frequently prescribed in New Brunswick hospitals. |
||
Documentation of the indication for antimicrobial prescribing occurred for 90.3% (585/648) of the prescriptions. The intended duration of antimicrobial therapy (i.e., stop or review date) was documented for only 67.1% (435/648) of the antimicrobial orders.
A total of 54 (8.3%) antimicrobials were prescribed for surgical prophylaxis. Of these, 77.8% (42/54) were ordered for a duration up to 24 hours and 22.2% (12/54) for longer than 24 hours. Cefazolin was the most frequently prescribed antimicrobial (79.6%, 43/54) for this indication. Metronidazole (7.4%, 4/54) and ciprofloxacin (3.7%, 2/54) were the next mostly commonly used.
Of the 648 antimicrobial orders assessed, local, regional, or provincial guidelines were applicable in 284 (43.8%) cases. Of these 284 antimicrobial orders with an applicable guideline, 66.2% (188/284) were deemed compliant and 33.8% (96/284) were deemed noncompliant. No guidelines were available for 161 (24.8%) of the 648 antimicrobial orders assessed. Antimicrobial therapy was directed toward the causative pathogen (based on available microbiology results) in 192 (29.6%) of the 648 cases. The remaining 11 (1.7%) orders were deemed not assessable with regard to guideline applicability.
Across the 10 hospitals surveyed, antimicrobial prescriptions were deemed optimal in 53.4% (346/648) of cases and adequate in 14.7% (95/648) of cases. Therefore, 68.1% (441/648) of antimicrobial orders provincially were deemed appropriate. Conversely, 17.4% (113/648) of prescriptions were considered suboptimal, and 13.6% (88/648) were considered inadequate. As such, 31.0% (201/648) of all antimicrobial orders were deemed inappropriate. A total of 6 (0.9%) antimicrobial entries were regarded as not assessable because of lack of documentation or notes or the heightened complexity of the case. Figure 3 shows the overall provincial assessment of appropriateness.
|
|
||
|
FIGURE 3 Summary of level of antimicrobial appropriateness in New Brunswick hospitals. |
||
In terms of antimicrobial appropriateness according to specified indications, concerning trends were noted for antimicrobials prescribed for UTIs: more specifically, 54.4% (43/79) of these entries were deemed inappropriate. Ciprofloxacin was the most commonly prescribed antimicrobial for this indication (25.3%, 20/79).
Fluoroquinolones were the most inappropriately prescribed antimicrobial class, as illustrated by the appropriateness ratings for the top 4 broad-spectrum antimicrobial agents (Figure 4). Fluoroquinolones were predominantly prescribed for UTIs (cystitis, pyelonephritis, asymptomatic bacteriuria, and catheter-associated UTI). UTIs accounted for more than half (55.2%, 16/29) of inappropriate fluoroquinolone prescriptions.
|
|
||
|
FIGURE 4 Assessment of appropriateness for the top 4 broad-spectrum antimicrobial agents. Numbers of antimicrobial orders: n = 22 for carbapenems, n = 63 for ceftriaxone, n = 59 for fluoroquinolones, and n = 66 for piperacillin–tazobactam. |
||
The distinct medical specialties appeared to have similar rates of inappropriate prescribing. In general medicine (e.g., medical oncology and family medicine), 32.9% (151/459) of antimicrobial orders were deemed inappropriate; the proportions were 28.1% (38/135) in general surgery (e.g., vascular, gastroenterological) and 25.0% (12/48) in intensive or critical care.
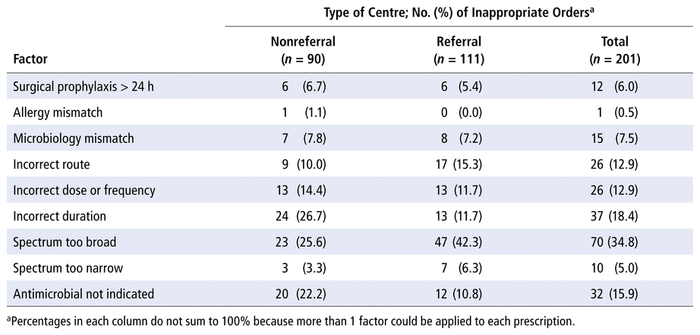
Factors driving inappropriateness were collected to inform the NAPS appropriateness assessment, and more than 1 factor could be applied to each prescription. For the 201 antimicrobial orders deemed inappropriate, the top 3 factors driving inappropriateness were as follows: the spectrum of the antimicrobial was “too broad” for the given indication (34.8%, 70/201), the duration of therapy was incorrect (18.4%, 37/201), and an antimicrobial was “not indicated” (15.9%, 32/201) (Table 2).
TABLE 2
Factors Driving Inappropriateness in Nonreferral (< 250 Beds) and Referral (≥ 250 Beds) Centres
The top 3 factors associated with inappropriate prescribing of fluoroquinolones were as follows: the spectrum of activity was unnecessarily broad (51.7%, 15/29), the dose or frequency was incorrect (20.7%, 6/29), and the route of administration was incorrect (17.2%, 5/29). The primary factor leading to inappropriateness of fluoroquinolone prescribing for UTIs was the spectrum of activity being deemed “too broad” for this indication (68.8%, 11/16).
For a given antimicrobial, factors driving inappropriateness at nonreferral centres (< 250 beds) included incorrect duration (26.7%, 24/90), spectrum too broad (25.6%, 23/90), and antimicrobial not indicated (22.2%, 20/90). In comparison, spectrum too broad (42.3%, 47/111) was the most common factor associated with an inappropriate rating for a given antimicrobial prescription at referral centres (≥ 250 beds).
There was a substantially higher rate of appropriate prescribing in referral centres than in nonreferral centres (75.0% versus 54.0%; χ2 [df = 1, n = 642] = 28.0; p < 0.001; effect size 0.21 [95% CI 0.14–0.29]).
A penicillin allergy label was identified for 14.2% (71/500) of the patients surveyed. There was no significant difference in appropriateness rating of antimicrobial therapy for patients with and without a penicillin allergy label (69.9% versus 63.0%; χ2 [df = 1, n = 642] = 1.60; p = 0.21; effect size 0.05 [95% CI 0.00–0.13]).
In this study, conducted in 2019–2020, approximately one-fourth (22.7%) of the hospital inpatient population in New Brunswick was receiving 1 or more systemic antimicrobial agents. These findings are in keeping with previous point prevalence surveys completed in New Brunswick in 2012 and 2018.18,19 Slightly higher rates of antimicrobial usage have been reported in other provinces across Canada and internationally, with upwards of 30% of inpatients receiving antimicrobial therapy in those jurisdictions.18,23–25
First-generation cephalosporins were the most frequently prescribed antimicrobials in NB hospitals, with cefazolin being the most frequently ordered of this class. It is postulated this result was secondary to the implementation of provincial surgical prophylaxis and β-lactam allergy guidelines.26,27 Cefazolin is recommended as first-line therapy for most surgical procedures, irrespective of penicillin allergy status.26 The most frequently ordered antimicrobials provincially had broad spectra of activity. According to the NB guidelines, these antimicrobials are usually indicated for nosocomial infections or severe community-acquired infections. Given the results of this survey, overuse of broad-spectrum antimicrobials should be considered as an area of potential improvement in antimicrobial utilization. The results of the current survey are consistent with previous Canadian literature, where high use of piperacillin–tazobactam and ceftriaxone has also been reported.18,23,24 Similarly, data from other Canadian surveys have indicated high usage of fluoroquinolones, specifically ciprofloxacin.16,18,24
Concerning utilization patterns and appropriateness for specific indications were identified during this study, in particular the frequent use of fluoroquinolones (specifically ciprofloxacin) for patients with UTIs. The appropriate use of fluoroquinolones represents an important target to optimize provincial prescribing patterns (especially in the context of UTIs). Prescribers may benefit from education about the risks associated with excessive and inappropriate use of fluoroquinolones, such as risk of resistance to this class and to other antimicrobial classes.28 Fluoroquinolones are unnecessarily broad, in terms of their spectrum of activity, for empiric treatment of most community-acquired infections, and they are associated with several adverse drug reactions (such as Clostridioides difficile infection, tendinitis, tendon rupture, peripheral neuropathy, QTc prolongation, and dysglycemia), particularly among older adults.29
A need to improve documentation was observed in the participating hospitals. Patient charts in both paper and electronic form were reviewed, and approximately 1 in 3 charts had no documentation of planned duration of therapy or review date, and 1 in 10 charts had no documentation of the indication for antimicrobial therapy. Data from previous point prevalence surveys conducted in Nova Scotia and New Brunswick reported documentation of indication in approximately 80% of cases and documentation of duration in only half of all cases.6,19 Higher rates (ideally 100%) of documentation should be targeted, as these indicators are considered essential components of antimicrobial prescribing. Documentation of the indication for and duration of antimicrobial therapy in the patient’s medical record facilitates informed assessment and reassessment of therapy, supports seamless transfer of care between medical teams, enables institutional antimicrobial audits, and promotes accountability.30
Lack of compliance with local guidelines was observed in this study. Approximately 1 in 3 antimicrobial entries were deemed noncompliant with local guidelines, when an applicable guideline was present. The antimicrobial stewardship committee should conduct further investigations to determine the reasons for noncompliance with local guidelines. Potential reasons for physicians’ noncompliance to guidelines identified in the literature include lack of awareness, lack of familiarity and disagreement with developed guidelines, inertia, contrasting patient and physician goals of care, and environmental constraints.31,32
Referral centres had substantially higher rates of appropriate antimicrobial prescribing than nonreferral centres. A similar trend was observed in previous provincial surveys.18,19 Greater access to resources at referral centres, such as infectious disease physicians, microbiologists, and clinical pharmacists, may contribute to the observed significant findings, but further investigation is warranted.
Notably, this study revealed that the presence of a penicillin allergy label had no significant impact on the appropriateness of antimicrobial therapy. This result conflicts with one earlier study, which demonstrated that higher rates of inappropriate prescribing (odds ratio 1.68) and increased use of broad-spectrum antimicrobials were associated with antimicrobial allergy labels.20 Advancement in prescribers’ knowledge of β-lactam allergy and cross-reactivity due to implementation of β-lactam allergy guidelines27 may have contributed to the current study’s finding.
This study had several strengths. To the authors’ knowledge, it is the first provincial point prevalence study based on the NAPS method. The data provide a baseline measurement of current antimicrobial utilization and appropriateness for the province of New Brunswick as a whole, in addition to data for individual hospital sites. The study grants insight as to where opportunities exist to improve patient outcomes and safety through targeted antimicrobial stewardship initiatives. The point prevalence survey can be repeated in the future to measure the impact of such interventions on antimicrobial utilization and appropriateness. Researchers considering this type of study in other parts of Canada will be able to replicate the current study using the standardized NAPS protocol in both referral and nonreferral centres. Because the NAPS method allows for a certain degree of subjectivity in ratings, we employed an independent second check by 2 infectious disease pharmacists to standardize assessment and reduce potential bias. In addition, to the authors’ knowledge, this is the first point prevalence study in Canada to report the association between antimicrobial appropriateness and hospital size and presence of a penicillin allergy label.
Although the results of this survey provide valuable insights into antimicrobial utilization by NB hospitals, several limitations should be considered. Point prevalence surveys are limited to a moment in time (i.e., a single day) and may not reflect overall prescribing trends within provincial health networks. The data for this study were not collected on the same day for all sites; therefore, it is possible (though unlikely) that seasonal variation affected the results. The findings may reflect or may have been influenced by select individuals’ case loads or practice sites, especially for smaller sites. Generalizability to other regions in Canada may be limited, given that prescribing trends and antimicrobial resistance rates vary across the country. Even though definitive conclusions cannot be drawn from a point prevalence survey such as this, the trends observed can help to indicate where future antimicrobial stewardship efforts should be focused.
This study contributes to knowledge about the prevalence of antimicrobial utilization, compliance with guidelines, level of appropriateness, and documentation in Canada and can be used locally as a benchmark to identify targets for future antimicrobial stewardship interventions. Key targets for quality improvement initiatives include decreasing the use of broad-spectrum antimicrobials, especially fluoroquinolone (ciprofloxacin) for UTIs; increasing guideline compliance; and ensuring documentation of antimicrobial duration by prescribers. There is also a need to address higher rates of inappropriate antimicrobial prescribing among smaller rural hospitals in New Brunswick. Regular repetition of such surveys (every 2 or 3 years) would be an effective tool to evaluate the effectiveness of future planned interventions.
1 Antimicrobial resistance. World Health Organization; 2021 Nov 17 [cited 2019 Aug 2]. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
2 Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42 Suppl 2:S82–9.
Crossref
3 Antimicrobial resistance and use in Canada: a federal framework for action. Public Health Agency of Canada; 2014 Oct [cited 2019 Aug 2]. Available from: https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/antimicrobial-resistance-use-canada-federal-framework-action.html
4 Federal action plan on antimicrobial resistance and use in Canada: building on the federal framework for action. Public Health Agency of Canada; 2015 Mar [cited 2019 Aug 1]. Available from: http://healthycanadians.gc.ca/alt/pdf/publications/drugs-products-medicaments-produits/antibiotic-resistance-antibiotique/action-plan-daction-eng.pdf
5 Willemsen I, Groenhuijzen A, Bogaers D, Stuurman A, Van Keulen P, Kluytmans J. Appropriateness of antimicrobial therapy measured by repeated prevalence surveys. Antimicrob Agents Chemother. 2007;51(3):864–7.
Crossref PubMed PMC
6 Black E, Neville H, Losier M, Harrison M, Abbass K, Slayter K, et al. Antimicrobial use at acute care hospitals in Nova Scotia: a point prevalence survey. Can J Hosp Pharm. 2018;71(4):234–42.
PubMed PMC
7 Tribble AC, Lee BR, Flett KB, Handy LK, Gerber JS, Hersh AL, et al.; Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS) Collaborative. Appropriateness of antibiotic prescribing in United States children’s hospitals: a national point prevalence survey. Clin Infect Dis. 2020;71(8):e226–e234.
Crossref PubMed
8 Gharbi M, Doerholt K, Vergnano S, Bielicki JA, Paulus S, Menson E, et al. Using a simple point-prevalence survey to define appropriate antibiotic prescribing in hospitalised children across the UK. BMJ Open. 2016;6(11):e012675.
Crossref PubMed PMC
9 Akhloufi H, Streefkerk RH, Melles DC, de Steenwinkel JEM, Schurink CAM, Verkooijen RP, et al. Point prevalence of appropriate antimicrobial therapy in a Dutch university hospital. Eur J Clin Microbiol Infect Dis. 2015;34(8):1631–7.
Crossref PubMed PMC
10 Aldeyab MA, Kearney MP, McElnay JC, Magee FA, Conlon G, MacIntyre J, et al. A point prevalence survey of antibiotic use in four acute-care teaching hospitals utilizing the European Surveillance of Antimicrobial Consumption (ESAC) audit tool. Epidemiol Infect. 2012;140(9):1714–20.
Crossref
11 Aldeyab MA, Kearney MP, McElnay JC, Magee FA, Conlon G, Gill D, et al. A point prevalence survey of antibiotic prescriptions: benchmarking and patterns of use. Br J Clin Pharmacol. 2011;71(2):293–6
Crossref PubMed PMC
12 Seaton RA, Nathwani D, Burton P, McLaughlin C, MacKenzie AR, Dundas S, et al. Point prevalence survey of antibiotic use in Scottish hospitals utilising the Glasgow Antimicrobial Audit Tool (GAAT). Int J Antimicrob Agents. 2007;29(6):693–9.
Crossref PubMed
13 Al-Taani GM, Scott M, Farren D, Gilmore F, McCullagh B, Hibberd C, et al. Longitudinal point prevalence survey of antibacterial use in Northern Ireland using the European Surveillance of Antimicrobial Consumption (ESAC) PPS and Global-PPS tool. Epidemiol Infect. 2018;146(8):985–90.
Crossref PubMed
14 Zarb P, Amadeo B, Muller A, Drapier N, Vankerckhoven V, Davey P, et al. Identification of targets for quality improvement in antimicrobial prescribing: the web-based ESAC point prevalence survey 2009. J Antimicrob Chemother. 2011;66(2):443–9.
Crossref
15 Ansari F, Erntell M, Goossens H, Davey P. The European Surveillance of Antimicrobial Consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis. 2009; 49(10):1496–504.
Crossref PubMed
16 Lee C, Walker SAN, Daneman N, Elligsen M, Palmay L, Coburn B, et al. Point prevalence survey of antimicrobial utilization in a Canadian tertiary-care teaching hospital. J Epidemiol Glob Health. 2015;5(2):143–50.
Crossref PubMed PMC
17 Blinova E, Lau E, Bitnun A, Cox P, Schwartz S, Atenafu E, et al. Point prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unit. Pediatr Crit Care Med. 2013;14(6):e280–8.
Crossref PubMed
18 Brideau-Laughlin D, Girouard G, Levesque M, MacLaggan T, Murray J, Salmon J. A point prevalence survey of antimicrobial use: benchmarking and patterns of use to support antimicrobial stewardship efforts [abstract]. Can J Hosp Pharm. 2013;66(4):265.
19 MacLaggan T, Landry D. New Brunswick hospital 2018 global point prevalence survey (an internal quality improvement initiative of the New Brunswick Antimicrobial Stewardship Committee). New Brunswick Antimicrobial Stewardship Committee; 2018. Unpublished internal report.
20 Trubiano JA, Chen C, Cheng AC, Grayson ML, Slavin MA, Thursky KA. Antimicrobial allergy “labels” drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother. 2016;71(6): 1715–22.
Crossref PubMed
21 Knezevic B, Sprigg D, Seet J, Trevenen M, Trubiano J, Smith W, et al. The revolving door: antibiotic allergy labelling in a tertiary care centre. Intern Med J. 2016;46(11):1276–83.
Crossref PubMed
22 NAPS—National Antimicrobial Prescribing Survey [website]. Melbourne Health (Australia); [cited 2019 Aug 13]. Available from: https://www.naps.org.au/
23 Frenette C, Sperlea D, German GJ, Afra K, Boswell J, Chang S, et al. The 2017 global point prevalence survey of antimicrobial consumption and resistance in Canadian hospitals. Antimicrob Resist Infect Control. 2020;9(1):104.
Crossref PubMed PMC
24 Taylor G, Gravel D, Saxinger L, Bush K, Simmonds K, Matlow A, et al. Prevalence of antimicrobial use in a network of Canadian hospitals in 2002 and 2009. Can J Infect Dis Med Microbiol. 2015;26(2):85–9.
Crossref PubMed PMC
25 Versporten A, Zarb P, Caniaux I, Gros MF, Drapier N, Miller M, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619–29.
Crossref PubMed
26 Surgical prophylaxis guidelines. NB Provincial Health Authorities Anti-Infective Stewardship Committee; 2018 [cited 2020 Feb 10]. Available from: https://en.horizonnb.ca/media/951204/surgical_prophylaxis.pdf
27 Management of penicillin and beta-lactam allergy. NB Provincial Health Authorities Anti-Infective Stewardship Committee; 2017 Sep [cited 2019 Sep 20]. Available from: https://www.horizonnb.ca/media/927867/management_of_penicillin_and_beta_lactam_allergy.pdf
28 Hooper D. Jacoby G. Mechanism of drug resistance: quinolone resistance. Ann N Y Acad Sci. 2015;1354(1):12–3.
Crossref PubMed PMC
29 Mandell L, Tillotson G. Safety of fluoroquinolones: an update. Can J Infect Dis. 2002;13(1):54–61.
30 Cooke FJ, Holmes AH. The missing care bundle: antibiotic prescribing in hospitals. Int J Antimicrob Agents. 2007;30(1):25–9.
Crossref PubMed
31 Barth JH, Misra S, Aakre KM, Langlois MR, Watine J, Twomey PJ, et al. Why are clinical practice guidelines not followed? The European Federation of Clinical Chemistry and Laboratory Medicine and European Union of Medical Specialists joint working group on guidelines. Clin Chem Lab Med. 2016;54(7):1133–9.
32 Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PAC, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–65.
Crossref PubMed
Competing interests: For activities outside the scope of the study published here, Daniel Landry has received a research grant and reimbursement of travel expenses from Baxter. No other competing interests were declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy , VOLUME 75 , NUMBER 2 , Spring 2022