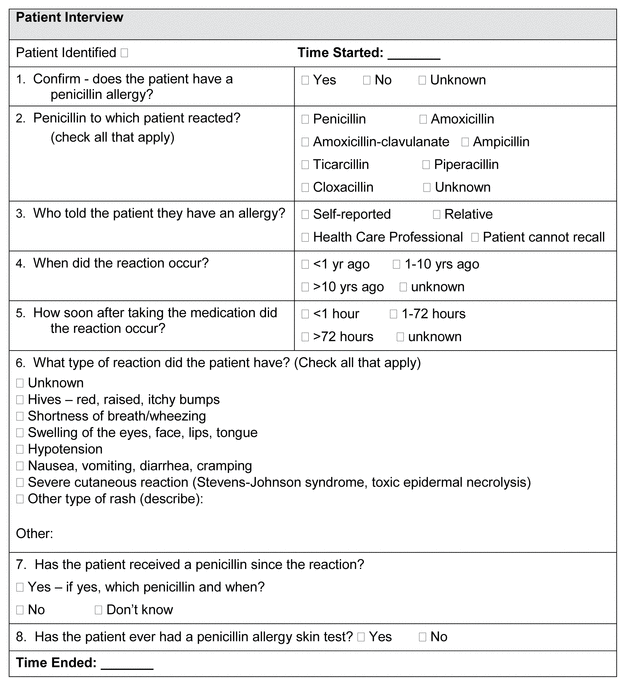
FIGURE 1 Allergy history questionnaire, adapted from the Providence Health Care penicillin allergy questionnaire.
Jessica Manning, Robert T Pammett, Abu Obeida Hamour, Aleisha Enemark, Barret BarrABSTRACT
Background
Inappropriate allergy labelling is associated with significant clinical and pharmacoeconomic implications. Detailed antimicrobial allergy assessments represent a key component of antimicrobial stewardship and aid in identifying true type I (immediate hypersensitivity) reactions. The allergy history form currently used at the University Hospital of Northern British Columbia (UHNBC), in Prince George, relies on the assessor’s ability to ask appropriate prompting questions to obtain a thorough history, but it may not be sufficient to accurately identify true allergies.
Objective
To compare a standardized allergy history questionnaire and the current allergy history form in terms of the quality and quantity of documentation gathered.
Methods
This prospective observational study involved patients who were admitted to medical and surgical services at UHNBC from November 2018 to January 2019 with a penicillin-class allergy reported on their electronic medical record (EMR). A list of patients with EMR-reported allergies was generated by the hospital’s health information software system, and these patients were interviewed using the standardized allergy history questionnaire.
Results
A total of 48 patients were assessed during the study period. Nineteen (40%) of the patients had an inappropriate allergy label on their EMR. Only 36 (75%) had an allergic reaction described on their EMR. Furthermore, only 36 (75%) of the 48 patients had the same allergy recorded on the EMR and on the allergy history form contained in their paper chart, of whom 22 had a documented reaction. The mean time to complete the standardized allergy history questionnaire was 2 minutes.
Conclusions
At the study institution, documentation of allergy histories was often incomplete. Detailed allergy assessments are the first step in identifying true immunoglobulin E–mediated hypersensitivity reactions. Utilization of a standardized allergy history questionnaire is feasible and may serve to improve documentation and overall antimicrobial stewardship.
KEYWORDS: allergy, label, standardized, documentation
RÉSUMÉ
Contexte
L’étiquetage inapproprié de l’allergie est associé à des conséquences cliniques et pharmacoéconomiques importantes. Les évaluations détaillées des allergies antimicrobiennes sont une composante-clé de la gestion antimicrobienne: elles contribuent à déterminer les réactions d’hypersensibilité véritables de type 1 (immédiates). Le formulaire des antécédents d’allergies actuellement utilisé à l’University Hospital of Northern British Columbia (UHNBC), à Prince George, s’appuie sur la capacité de l’évaluateur à poser les questions appropriées pour obtenir un historique détaillé, mais il ne suffit pas de déterminer précisément les véritables allergies.
Objectif
Comparer la qualité et la quantité des informations recueillies au moyen d’un questionnaire normalisé sur les antécédents d’allergies avec celles recueillies au moyen des formulaires.
Méthodes
Cette étude d’observation prospective portait sur des patients admis dans les services médicaux et chirurgicaux à l’UHNBC de novembre 2018 à janvier 2019, dont les dossiers médicaux électroniques (DME) indiquaient une allergie à des médicaments de la classe de la pénicilline. Le logiciel des informations sur la santé a généré une liste des patients présentant les allergies indiquées et ces patients ont été interrogés à l’aide d’un questionnaire normalisé des antécédents d’allergies.
Résultats
Un total de 48 patients a été évalué pendant la période de l’étude. Le DME de dix-neuf (40 %) patients portait une étiquette inappropriée. Seuls 36 DME des patients (75 %) décrivaient une réaction allergique. De plus, seulement 36 (75 %) des 48 patients avaient la même réaction allergique enregistrée à la fois au DME et dans le formulaire des antécédents d’allergies de leur dossier papier, et la réaction de 22 d’entre eux était documentée. Le temps de réponse moyen au questionnaire normalisé sur les antécédents d’allergies était de 2 minutes.
Conclusion
Dans cette étude, la description des antécédents d’allergies était souvent incomplète. Les évaluations détaillées des allergies sont la première étape permettant de déterminer les réactions véritables d’hypersensibilité à l’immunoglobuline E. L’utilisation d’un questionnaire normalisé des antécédents d’allergies est faisable et pourrait servir à améliorer la documentation ainsi que la gestion globale des antimicrobiens.
MOTS CLÉS: allergie, étiquette, normalisé, documentation
Inappropriate antibiotic allergy labelling is a significant issue, contributing to increased antibiotic resistance, longer hospital stays, and increased health care costs.1–3 Penicillin-class allergies are among the most commonly reported medication allergies, with a prevalence of approximately 10% in the general population and up to 15% in hospitalized patients.4,5 Numerous factors may contribute to the high prevalence of reported penicillin allergies, including vague allergy histories, inaccurate documentation, and attribution of non-allergic reactions (e.g., amoxicillin rash confounded by viral illness).6,7 It has been shown that up to 90% of patients with a reported allergy can safely tolerate penicillins, and true penicillin-induced anaphylaxis is rare, with a reported incidence of 0.02% to 0.04%.5,8,9 Additionally, immunoglobulin E (IgE) antibodies dissipate over time, and approximately 80% of patients with a previous true penicillin allergy no longer react to penicillin after 10 years.10
Penicillins and other β-lactam antibiotics are the drugs of choice for many infectious indications and come with a well-established safety profile and relatively low cost.9 Penicillin allergy labelling has significant clinical and pharmacoeconomic implications. For example, patients with presumed allergy to penicillin may alternatively receive suboptimal antibiotics with broader spectrums of activity and potentially poorer safety profiles (greater chance of toxicity).1,11 These patients are at higher risk of colonization with resistant pathogens such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus, as well as greater risk of morbidity and complications such as Clostridioides difficile infection.11 Additionally, it has been demonstrated that penicillin allergies are associated with increased cost of antibiotic treatment in hospital (by up to 63%) relative to non-allergic patients.5,12
Skin testing for penicillin allergy is a well-validated confirmatory method that has been implemented by many antimicrobial stewardship programs.9 Skin testing is indicated for those with a history of type I (immediate hypersensitivity) reactions and is the gold standard for clinical “delabelling”; this type of testing has been shown to refute more than 80% of allergy labels.5 The negative predictive value of skin testing is below 100%, so those with a negative response to the initial skin test should proceed to an oral dose challenge4,6 A true type I reaction manifests as urticaria, angioedema, wheezing, dyspnea, and/or hypotension within 72 hours of administration.13 Reactions that occur more than 72 hours after drug administration are termed late hypersensitivity reactions and are classified as type II, III, or IV. These are not IgE-mediated reactions, and therefore skin testing does not play a role in their evaluation.6,13
The 2016 guideline of the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America recommends that antimicrobial stewardship programs conduct allergy assessments for patients with a history of β-lactam allergy, as well as penicillin skin testing when appropriate.14 Detailed allergy assessment alone is a key component of antimicrobial stewardship and should be implemented by institutions to accurately identify patients with a true immune-mediated response to penicillins.15–17 Previous studies have shown that standardized allergy history questionnaires support the acquisition of clinically relevant information18 and can lead to interventions that are economically feasible.19 Our organization currently uses a paper allergy history form, which relies on the assessor’s ability to ask appropriate prompting questions to obtain a thorough history. The current form is formatted to document the substance to which the patient is allergic and the reaction experienced, but does not collect key information such as when the reaction occurred, the temporal relationship of the reaction to the medication use, details of the reaction itself, and whether the patient has been re-exposed to the medication since the initial reaction. Reliance upon the interviewer to remember to ask these key questions often results in incomplete documentation, as recognized by hospital pharmacists within the organization.
The primary aim of this study was to compare a standardized allergy history questionnaire and the current allergy history form in terms of the quantity and quality of documentation gathered. The secondary aims were to determine the number of potential candidates for clinical delabelling (via penicillin skin test or oral penicillin challenge) and to measure the time required to complete a thorough allergy history using the standardized questionnaire.
This prospective observational study involved patients admitted to medical and surgical services at the University Hospital of Northern British Columbia (UHNBC) from November 18, 2018, to January 11, 2019. UHNBC is a teaching hospital with 219 acute care beds located in Prince George, the “hub” of northern British Columbia. Ethics approval was sought from and provided by the University of British Columbia’s Clinical Research Ethics Board and the organization’s Research Review Committee.
Patients who reported an allergy to a penicillin (penicillin, amoxicillin, amoxicillin-clavulanate, ampicillin, ticarcillin, piperacillin, and cloxacillin) at the time of admission were identified twice weekly by means of reports generated from the organization’s health information software system. These allergy reports were obtained as part of the standard admission process, whereby a health care provider completes a basic allergy history form (on paper), which is then scanned and sent to the pharmacy department for entry into the patient’s electronic medical record (EMR). The weekly software reports were generated by admission date and included all reported allergies, to capture different penicillins as well as uncoded (free-texted) medication allergies. The patients identified in this way were invited to participate in the study and were given a minimum of 24 hours to reflect on their participation and provide consent. Patients were excluded if they were under 19 years of age, had been admitted to a service other than those defined in the inclusion criteria, or had been discharged before enrolment.
Consenting patients were interviewed using the standardized allergy history questionnaire (Figure 1), which was adapted from the penicillin allergy questionnaire used by Providence Health Care in British Columbia. One author (J.M.), a postgraduate year 1 pharmacy practice resident, conducted all of the patient interviews and collected all of the data. The time to conduct each questionnaire was documented, as were any current antibiotic orders for the participant at the time of the interview. For all participants, allergy histories (both on paper forms and within the EMR) were updated, and pharmaceutical care was provided on the basis of this information as appropriate. The data were analyzed by descriptive statistics.
|
|
||
|
FIGURE 1 Allergy history questionnaire, adapted from the Providence Health Care penicillin allergy questionnaire. |
||
Participants with allergies determined to have the potential for clinical delabelling were then classified as having low, medium, or high risk for negative consequences during delabelling; see Table 1 for further details of the risk levels. Risk stratification provides support for clinicians when they are considering whether penicillin skin testing or drug challenges are appropriate. We are not aware of a validated tool available to health care professionals for stratification of patients according to allergy history; therefore, we adopted the allergy classification criteria from an early draft of the delabelling toolkit currently being prepared for British Columbia health authorities by the Provincial Antimicrobial Experts (PACE) group and used these criteria to categorize patients with potential for delabelling. This toolkit determines risk using a risk stratification process similar to that described by Shenoy and others.20
TABLE 1 Classification of Allergies for Purpose of Delabellinga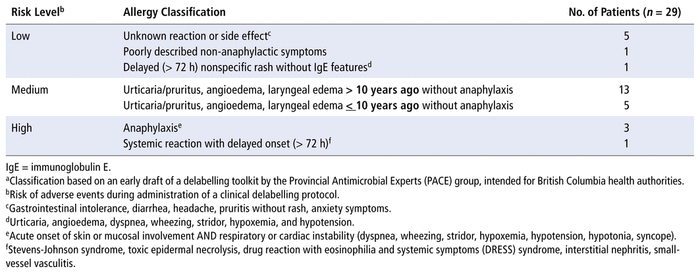
A total of 136 patients with reported allergy to penicillins were identified during the data collection period. Of these, 55 were discharged before consenting to participate, and 33 were excluded from data collection for the following reasons: unable to consent (n = 9), consented to participate but was discharged before participation (n = 7), was transferred to a site outside the data collection area (n = 5), readmission of a person who had already participated in this study (n = 5), refused consent (n = 3), was receiving care from the interviewer (a potential conflict of interest; n = 3), or died before consenting to participate (n = 1).
A total of 48 individuals participated in the study and were interviewed using the standardized allergy history questionnaire. The mean age of participants was 60.4 years (standard deviation 19.3 years), and 28 (58%) were female. The drug to which an allergy was listed in the EMR was penicillin for 40 participants, amoxicillin for 10 patients, and piperacillin for 3 patients, with some patients having more than 1 drug listed as an allergen.
For 36 (75%) of the participants, a description of the reaction was documented on the EMR (e.g., “rash”). In addition, 36 (75%) of the participants had the same allergy recorded on both their EMR and the allergy form in their paper chart; for the other participants, reporting in the EMR and the paper chart was inconsistent.
For 19 participants (40%), the allergy label in the EMR was deemed inappropriate, for the following reasons: re- exposure to penicillin without incident (n = 8); signs or symptoms of penicillin intolerance, not allergy (n = 6); denial of penicillin allergy by the participant during the interview (n = 3); and “other” (n = 2) (Figure 2).
|
|
||
|
FIGURE 2 Reasons for inappropriate allergy labels on patients’ electronic medical records (n = 48). |
||
Twenty-nine (60%) of the participants were identified as candidates for clinical delabelling in accordance with the draft delabelling toolkit. Of those participants, 7 (24%) were classified as having low risk of adverse events during administration of the clinical delabelling protocol, 18 (62%) as having medium risk, and 4 (14%) as having high risk; see Table 1 for more details about the risk levels.
From a feasibility perspective, the mean time to conduct the standardized allergy history questionnaire was 2 minutes (range 1–4 minutes).
The results of this study demonstrate the importance of a comprehensive, standardized allergy assessment and highlight the difference between information recorded in this way and the current standard of practice at the study institution. A substantial proportion of the patients interviewed (40%) had an inappropriate penicillin allergy label on their EMR. Inappropriate labelling is particularly troublesome for allergies to antibiotics, specifically penicillins, because these allergy labels are pervasive and are associated with important clinical and pharmacoeconomic implications.1,2 These results also showed that patients often reported non-allergic reactions as allergies; as such, formal allergy histories represent an opportunity for patient education about the differences between allergies and intolerances. After administering a standardized allergy history questionnaire, secondary sources of information, such as community pharmacy records, prescription dispensing databases, hospital records, and records of the primary care physician, may be consulted to gather more data about a patient’s allergy status; this information may support clinical delabelling without the need for penicillin skin testing or oral challenges. Although skin testing is the gold standard for penicillin delabelling, it is not accessible in all centres, further exemplifying the importance of detailed allergy histories. Only when complete allergy-related information is gathered can informed decision-making occur regarding the use of penicillins.
In addition to serving as a tool for asking appropriate allergy-related questions, a standardized allergy history questionnaire can improve overall documentation. As demonstrated in this study, documentation of allergy histories at UHNBC was often incomplete or incongruent with the various health records being used to provide care. These problems indicate that health care professionals were obtaining suboptimal allergy histories for patients admitted to hospital, which could subsequently affect the quality of care that individuals receive. Anecdotally, allergy history information is sometimes copied from admission forms, ambulance records, medication administration records, or other sources onto the current allergy history form, without verification of the information with a primary source, such as the patient or caregiver. Use of a standardized tool may reduce these practices.
The preferred method of verifying penicillin allergies in patients with features of IgE-mediated reactions is skin testing, which is not currently available within the study organization. Supported by the draft PACE toolkit, graded amoxicillin challenge may be offered to patients with low-risk histories and may be carefully considered for those classified as having medium risk with remote history of a reaction (i.e., more than 10 years before). This method would apply to 25 (86%) of the 29 participants in this study who were identified as candidates for delabelling. Compared with an oral challenge protocol, penicillin skin testing requires more resources, both material and human.21 If routinely adopted, graded oral amoxicillin challenge provides the potential for delabelling countless penicillin allergies. During data collection by means of the standardized interview, it was common for participants to request further information about oral challenge, as many wished to know their current allergy status; as such, this is a service for which there might be high demand.
Sigona and others7 found that 25 (75%) of inpatients receiving antimicrobial therapy who were interviewed by pharmacists were candidates for β-lactam therapy, and 65.6% were successfully switched from a non-penicillin antibiotic to a cephalosporin, carbapenem, or penicillin. In our study, 22 (46%) of interviewed patients who were receiving antimicrobial therapy were receiving non-β-lactam antibiotics; however, we did not assess whether these drugs were being administered as alternatives to first-line therapy, and no clinical intervention was undertaken, as doing so would have been outside the scope of this study. Given the high proportion of patients identified as having an inappropriate allergy label, it is likely that many of these patients would have been candidates for β-lactam therapy, depending on the infection.
Our results provide some evidence that the time required to conduct a standardized allergy history questionnaire is minimal, and that it may be feasible for other health care providers to administer the questionnaire. However, this would need to be validated through future research.
The results of this study have informed the revision of an allergy/sensitivity history form that is intended to be deployed across all of the institution’s sites in the future.
We acknowledge that this study had a number of limitations. Although obtaining allergy histories is a standard of care performed by pharmacists daily, consent was required from each patient before the interviews, with a minimum 24-hour waiting period before the allergy assessment was conducted. This waiting period, which was requested by the Clinical Research Ethics Board, significantly affected our sample size, because many patients were discharged during the waiting period, before the interview could be conducted. Participants’ reports of subsequent exposure (Figure 1, Question 7) were not verified with secondary sources (e.g., primary care clinics, community pharmacies, or dispensing databases), which limits the level of confidence in information about re-exposure. Verification of the history using secondary sources would have been valuable for those who did not recall their allergic reaction; however, this was beyond the scope of the current study. We also acknowledge that the time required for our assessor to complete the standardized allergy history questionnaire may not have been representative of all users, given that the time reported here was based on one person’s experience with the tool. However, the team believes that the tool itself is simple and intuitive for health care professionals to use, and should not pose an undue burden on those using it to obtain an accurate allergy history. We believe that implementation of a standardized allergy history questionnaire by health care professionals in this practice setting would improve appropriate antibiotic use, with benefit for clinical and pharmacoeconomic outcomes; however, further research is required to confirm this hypothesis.
In this study, documentation of allergy histories was often incomplete, inconsistent, and unreliable across records in the study institution. A detailed allergy assessment is the first step in identifying true IgE-mediated hypersensitivity reactions and is essential for complete and accurate documentation. Implementation of a standardized allergy history questionnaire may improve documentation of penicillin allergies, antimicrobial stewardship, and ultimately patient care.
1 Macy E. Elective penicillin skin testing and amoxicillin challenge: effect on outpatient antibiotic use, cost, and clinical outcomes. J Allergy Clin Immunol. 1998;102(2):281–5.
Crossref PubMed
2 Torda A, Chan V. Antibiotic allergy labels—the impact of taking a clinical history. Int J Clin Pract. 2018;72(3):e13058.
Crossref PubMed
3 van Dijk SM, Gardarsdottir H, Wassenberg MWM, Oosterheert JJ, de Groot MCH, Rockmann H. The high impact of penicillin allergy registration in hospitalized patients. J Allergy Clin Immunol Pract. 2016;4(5):926–31.
Crossref PubMed
4 Blumenthal KG, Shenoy ES, Varughese CA, Hurwitz S, Hooper DC, Banerji A. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115(4):294–300.e2.
Crossref PubMed PMC
5 Soria A, Autegarden E, Amsler E, Gaouar H, Vial A, Francès C, et al. A clinical decision-making algorithm for penicillin allergy. Ann Med. 2017;49(8):710–7.
Crossref PubMed
6 Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285(19):2498–505.
Crossref PubMed
7 Sigona NS, Steele JM, Miller CD. Impact of a pharmacist-driven beta-lactam allergy interview on inpatient antimicrobial therapy: a pilot project. J Am Pharm Assoc. 2016;56(6):665–9.
Crossref
8 Patterson RA, Stankewicz HA. Penicillin allergy. In: StatPearls [book on internet]. StatPearls Publishing; 2020 [cited 2020 Jun 23]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK459320/
9 Chen JR, Tarver SA, Alvarez KS, Tran T, Khan DA. A proactive approach to penicillin allergy testing in hospitalized patients. J Allergy Clin Immunol Pract. 2017;5(3):686–93.
Crossref
10 Blanca M, Torres MJ, García JJ, Romano A, Mayorga C, de Ramon E, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5 Pt 1):918–24.
Crossref PubMed
11 Jeffres MN, Narayanan PP, Shuster JE, Schramm GE. Consequences of avoiding β-lactams in patients with β-lactam allergies. J Allergy Clin Immunol. 2016;137(4):1148–53.
Crossref
12 Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501–6.
Crossref PubMed
13 Gell P, Coombs R. Classification of allergic reactions responsible for clnical hypersensitivity and disease. In: Clinical aspects of immunology. Blackwell Scientific Publications; 1975. p. 761–81.
14 Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–77.
Crossref PubMed PMC
15 Hippern LD, Halapy, H. Assessing penicillin allergies with a structured assessment form. Can J Hosp Pharm. 2000;53(3):184–92.
16 Inglis JM, Caughey GE, Smith W, Shakib S. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47(11):1292–7.
Crossref PubMed
17 Shah NS, Ridgway JP, Pettit N, Fahrenbach J, Robicsek A. Documenting penicillin allergy: the impact of inconsistency. PloS One. 2016; 11(3):e0150514.
Crossref PubMed PMC
18 Harig A, Rybarczyk A, Benedetti A, Zimmerman J. Clarification of drug allergy information using a standardized drug allergy questionnaire and interview. Pharm Ther. 2018;43(8):480–504.
19 Clark KE, Briand ME, Kapoor O, Pirasteh A. Impact of a standardized beta-lactam allergy questionnaire on aztreonam use. J Pharm Pract. 2019;32(4):399–403.
Crossref
20 Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99.
Crossref PubMed
21 Blumenthal KG, Li Y, Banerji A, Yun BJ, Long AA, Walensky RP. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018; 6(3):1019–1027.e2.
Crossref PMC
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors would like to acknowledge Alicia Rahier for her assistance with writing the final draft and for project support in her role as Antimicrobial Stewardship Program Coordinator within Northern Health.
Canadian Journal of Hospital Pharmacy, VOLUME 74, NUMBER 2, Spring 2021