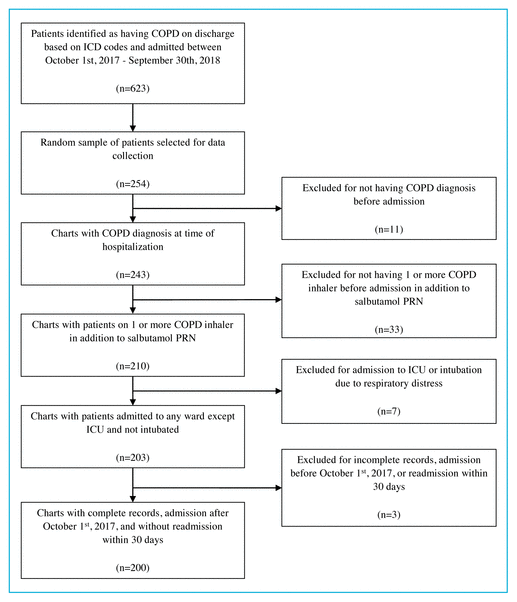
FIGURE 1 Sample selection. COPD = chronic obstructive pulmonary disease, ICD = International Classification of Diseases, ICU = intensive care unit, PRN = as needed.
Brittany Gage*, Julia Lamb*, Karen DahriABSTRACT
Background
In the past decade, the number of inhaled devices approved for management of chronic obstructive pulmonary disease (COPD) has tripled. Management of at-home inhaled COPD therapy can present a problem when patients are admitted to hospital, because only a limited number of these therapies are currently included in hospital formularies and there is a lack of established interchanges.
Objectives
To characterize and evaluate the appropriateness of management of patients’ before-admission inhaled therapy upon hospital admission.
Methods
This retrospective chart review involved patients with COPD admitted to a tertiary care centre over a 1-year period (October 2017 to September 2018). Before-admission inhaled therapy was compared with inhalers ordered in hospital and at discharge. Inhaler device type, regimen, therapeutic class, and disease severity were used to assess the appropriateness of inpatient management.
Results
The charts of 200 patients were reviewed. Of these patients, 124 (62%) were kept on the same inhaler, 43 (22%) had one or more of their inhalers discontinued, 35 (18%) had to provide their own medication, and 24 (12%) had their medication changed to a formulary equivalent. An average delay of 2.6 (standard deviation 3.2) days occurred when patients provided their own medication. Formulary substitution resulted in most patients receiving a medication from the same class (75% [18/24]); however, other aspects of therapy, such as device type (17% [4/24]), regimen (29% [7/24]) and drug combination (47% [9/19]), were not maintained. Only 55% (6/11) received an equivalent dose of inhaled corticosteroids when the medication was interchanged to a formulary inhaler.
Conclusions
The majority of patients’ inhaled therapies continued unchanged upon admission to hospital, which suggests that despite the proliferation of new inhalers on the market, their use is still limited. For patients who did require interchange to formulary inhalers, maintenance of the same regimen, device, and combination product was rare. Provision of the medication supply by patients themselves often resulted in a delay in therapy.
KEYWORDS: chronic obstructive pulmonary disease, formulary, inhaled therapy
RÉSUMÉ
Contexte
Au cours des dix dernières années, le nombre de dispositifs d’inhalation approuvés pour gérer la maladie pulmonaire obstructive chronique (MPOC) a été multiplié par trois. La gestion de la thérapie à domicile de la MPOC peut présenter un problème lors de l’admission à l’hôpital, car seul un nombre limité de ces thérapies est actuellement inclus dans la pharmacopée des hôpitaux et les tableaux d’équivalence des médicaments font défaut.
Objectifs
Au moment de l’admission à l’hôpital, définir et évaluer l’adéquation dentre l’inhalothérapie des patients avant leur admission et celle offert à l’hôpital.
Méthodes
Cet examen rétrospectif des dossiers concernait des patients atteints d’une MPOC ayant été admis dans un centre de soins tertiaires sur une période d’un an (d’octobre 2017 à septembre 2018). Il portait sur la comparaison entre l’inhalothérapie avant l’admission et les inhalateurs commandés à l’hôpital et au moment du congé. Le type de dispositif d’inhalation, le régime, la classe thérapeutique et la gravité de la maladie ont servi à évaluer la pertinence de la gestion de l’inhalothérapie des patients hospitalisés.
Résultats
L’examen portait sur les dossiers de 200 patients. De ceux-ci, 124 (62 %) ont gardé le même inhalateur; 43 (22 %) ont vu la suppression d’au moins un inhalateur; 35 (18 %) ont dû fournir leurs propres médicaments; et les médicaments de 24 (12 %) d’entre eux ont été remplacés par un équivalent de la pharmacopée. Les investigateurs ont observé un retard moyen de 2,6 jours (écart type 3,2) lorsque les patients fournissaient leurs propres médicaments. La substitution par des médicaments de la pharmacopée a conduit la plupart des patients à en recevoir un de la même classe (75 % [18/24]); cependant, d’autres aspects de la thérapie n’ont pas été maintenus, comme le type de dispositif (17 % [4/24]), le régime (29 % [7/24]) et la combinaison de médicaments (47 % [9/19]). Seuls 55 % (6/11) ont reçu une dose équivalente de corticostéroïdes en inhalation, lors du remplacement du médicament par un inhalateur de la pharmacopée.
Conclusions
La majorité des inhalothérapies des patients sont restées inchangées au moment de l’admission à l’hôpital, ce qui laisse entendre que, malgré la prolifération de nouveaux inhalateurs sur le marché, leur utilisation est encore limitée. Pour les patients qui nécessitaient le remplacement par un inhalateur de la pharmacopée, le maintien du même régime, du même dispositif et du même produit de combinaison était rare. L’approvisionnement en médicaments par les patients eux-mêmes entraînait souvent un retard dans la thérapie.
MOTS CLÉS: maladie pulmonaire obstructive chronique, pharmacopée, inhalothérapie
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease characterized by fixed airflow obstruction and chronic inflammation.1 It is the fourth leading cause of death worldwide and a major source of financial and medical burden.2,3 An estimated 17% of Canadians aged 35 to 79 years meet the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) for a diagnosis of COPD, based on spirometry, and this proportion is expected to increase.4
There is no cure for the irreversible lung damage that occurs in COPD, but pharmacologic therapy is employed to reduce symptoms, prevent exacerbations, and improve patients’ quality of life.2 Three main drug classes—bronchodilators, muscarinic antagonists, and corticosteroids—are used in various combinations and strengths, depending on disease progression and other patient factors. Specific drugs within each class are further classified according to their duration of action, termed “short-acting” or “long-acting”. As the prevalence of COPD has increased, so too have the number of inhaler devices and the therapeutic options within each drug class. Since 2010, the number of inhaled devices approved in Canada for the treatment of COPD has almost tripled.5,6 Inhalers are primarily classified by their mechanism of drug delivery. For COPD, these devices are either pressurized metered dose inhalers, dry powder inhalers, or soft mist inhalers. Although all devices have been shown to be effective and clinical guidelines do not recommend one over another,2,7 patients’ satisfaction with their inhaler is positively correlated with adherence and health status.8 Consequently, when prescribing these medications, it is clinically important to consider patient preference along with drug coverage and provincial formularies.
Drug classes with a large variety of therapeutic options often present a problem at the time of hospital admission, particularly when no standard equivalence table for the class has been established. Such is the case for the majority of inhaled drugs for COPD. Hospital formularies are commonly utilized to control costs and standardize care by enabling clinicians to become familiar with a smaller subset of medications.9 Conversely, the risk of medication errors upon admission and discharge may be increased when a patient’s home medications are not available on the hospital formulary. In response to this problem, hospitals commonly implement therapeutic interchange, which is defined as the automatic replacement of a prescribed medication with a pre-approved medication from the same drug class that is chemically different, but therapeutically equivalent.10 An estimated 88% of major Canadian hospitals (> 100 beds) use a therapeutic interchange program,11 including Vancouver General Hospital (VGH). Relative to the number of devices that are commercially available in Canada, VGH has a limited number of inhaler devices on formulary (complete list available from the corresponding author upon request), in accordance with the provincially mandated hospital formulary for all health authorities in British Columbia.12 Within VGH, there are no direct therapeutic interchange guidelines for COPD drugs other than inhaled corticosteroids (ICS).13
There is a lack of literature characterizing how patients’ inhaled medications are dealt with upon admission to hospital and reporting the prevalence of therapeutic interchange for these drug classes. When patients are admitted, the following outcomes are possible for each of their before-admission medications:
The same medication is provided in hospital (i.e., the medication may be on formulary or the hospital may provide it as a nonformulary item).
The medication is interchanged with a therapeutic equivalent on the formulary.
The medication is discontinued until discharge.
Patients provide their own medication during the hospital stay.
No studies have evaluated inpatient management of medications specifically for COPD, but studies of therapeutic interchange for other indications have elicited concerns about the safety and efficacy of this practice. For example, therapeutic interchange of proton pump inhibitors has been shown to increase the rate of treatment failure (inadequate clinical response, need for dose titration, or discontinuation due to adverse effects)14 and is associated with more medication discrepancies at discharge than when the same drug is continued in hospital.15 The overall medication reconciliation error rate for all drug classes has also been shown to be much higher for interchanged medications than for unchanged medications.9 Even when therapeutic interchange is done correctly, switching devices can lead to decreased patient adherence or improper inhalation technique.16 Patients may be confused about new treatment regimens or device-specific handling instructions, particularly after a hospital stay during which pharmacotherapy for their other conditions has been adjusted; this situation may lead to duplicate therapy or accidental discontinuation if the errors are not resolved. Furthermore, patients may face financial difficulties and be intentionally nonadherent if their discharge prescriptions include cost-prohibitive drugs.17 It is therefore important to characterize the prevalence of therapeutic interchange and critically evaluate its consequences in this patient population to ensure safe and effective care.
The purpose of this study was to determine how the inhaled respiratory maintenance medications of patients with COPD were managed when they were admitted to hospital and, in cases where therapeutic interchange occurred, to assess the appropriateness of the medication and regimen prescribed. Appropriateness was evaluated in terms of dose equivalence, similarity of device, maintenance of combination product devices, and drug class, according to COPD severity and clinical guidelines.
The study was a retrospective chart review of patients with COPD who were admitted to a tertiary care centre.
International Classification of Diseases (9th Revision) codes were used to identify patients with COPD at discharge who had been admitted to the hospital between October 1, 2017, and September 30, 2018. The inclusion criteria were as follows: patient was taking at least 1 COPD medication by inhalation and was using salbutamol on an as-needed basis. The exclusion criteria were as follows: incomplete records in the patient’s chart, admission to the intensive care unit or another critical care area, intubation for respiratory distress, and readmission within less than 30 days. Incomplete records were defined as those missing any portion of data that prevented assessment of the primary outcome. From all admissions that met the initial criteria, a convenience sample was identified by random selection; for this purpose, each of the charts was given a random number, and the charts were selected in ascending numeric order.
Two investigators (B.G. and J.L.) independently abstracted the data. The following baseline demographic and clinical data were collected: age, comorbidities, spirometry results, and the reason and date of admission. In addition, all before-admission inhalers that were being used and all inhalers prescribed upon discharge were recorded.
The primary objective of the study was to determine if patients with COPD who were admitted to hospital had their before-admission inhaler medications continued, discontinued, or therapeutically interchanged, or if the patient supplied their own medication for use in the hospital. For each patient with multiple inhalers that were all managed using the same technique, the inhalers were grouped and counted as one for that method of management. For patients with multiple inhalers that were managed differently, each inhaler was counted individually for the particular technique. After initial determination of how the inhalers were managed, secondary outcomes were also captured. For patients who received at least 1 inhaler from their own supply, the investigators determined whether the inhaler was actually received during the hospital stay and if so, the number of days’ delay from the date ordered to the first dose being administered (if any such delay occurred). For patients with at least 1 inhaler interchanged for a formulary inhaler, the secondary outcomes were whether the therapeutic interchange resulted in the same device being ordered, whether the same class of medication was maintained (i.e., short-acting muscarinic antagonist, long-acting muscarinic antagonist, long-acting β-agonist, or ICS), whether the before-admission regimen was maintained, and whether, in the case of ICS therapy, the equivalent dose (as per the VGH therapeutic interchange dosing equivalency chart) was given after the therapeutic interchange. Additionally, if there was an indication for therapeutic optimization (which includes altering the dose of existing medications or adding or removing an inhaled therapy, as outlined in the 2020 GOLD guidelines2), the investigators determined whether that was done. If patients had been admitted for a COPD exacerbation and treated per the hospital-based protocol, this was counted as optimization; where applicable, inhalers ordered post-exacerbation were assessed in terms of the primary and secondary outcomes outlined above. At VGH, the administration of all inhaled therapies is either performed or witnessed by nurses and then documented.
For this retrospective study, ethics approval was obtained from the University of British Columbia Clinical Research Ethics Board, and operational approval to conduct the study was obtained from Vancouver Coastal Health.
Descriptive statistics were used to analyze all outcomes. Data were entered into Excel 365 Pro Plus v. 1902 software (Microsoft Corporation), which was used to carry out all statistical analyses.
A total of 623 admissions occurred during the defined time frame; random numbers were assigned to the 623 charts, and a convenience sample of 254 charts was selected for analysis. Of the 254 charts reviewed, 200 met the inclusion criteria. The reasons for exclusion are detailed in Figure 1.
|
|
||
|
FIGURE 1 Sample selection. COPD = chronic obstructive pulmonary disease, ICD = International Classification of Diseases, ICU = intensive care unit, PRN = as needed. |
||
The mean age of patients included in the study was 74.0 (standard deviation [SD] 11.8) years (Table 1). According to the most recent spirometry data available, the mean forced expiratory volume in 1 second (FEV1) was 44.7% (SD 17.7%) of predicted (n = 70 patients), whereas the mean ratio of FEV1 to forced vital capacity (FEV1/FVC) was 0.53 (SD 0.11) (n = 50 patients) (Table 1). For patients with both pre- and post-bronchodilator spirometry values recorded in the chart, the most recent post-bronchodilator values were used for this analysis.
TABLE 1 Baseline Characteristics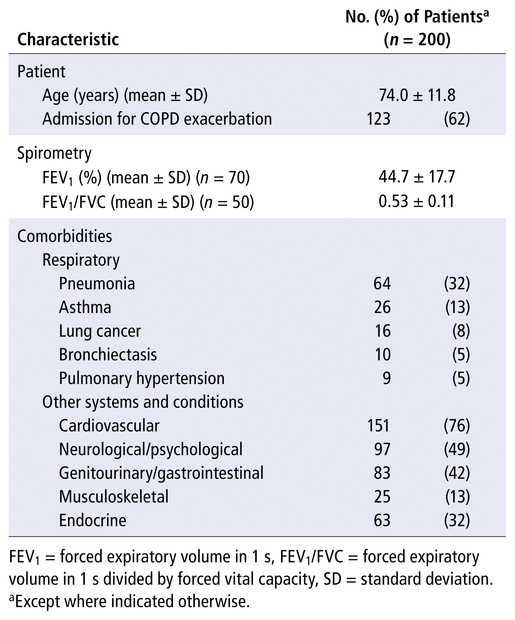
The list of inhalers that patients were using at the time of admission was generated from VGH records; at this hospital, patient interviews are typically used to confirm or update the list as soon as possible after admission. The 200 patients included in the analysis had a total of 326 inhalers for COPD medications for which management was assessed. For the majority of the 200 patients, before-admission inhalers were continued in hospital (62%), followed by discontinuation of the inhaler during hospitalization (22%) and use of the patient’s own inhalers (18%) (Table 2). The least common management course was therapeutic interchange (12%) (Table 2).
TABLE 2 Evaluation of Inpatient Management of Inhalers for Treatment of COPD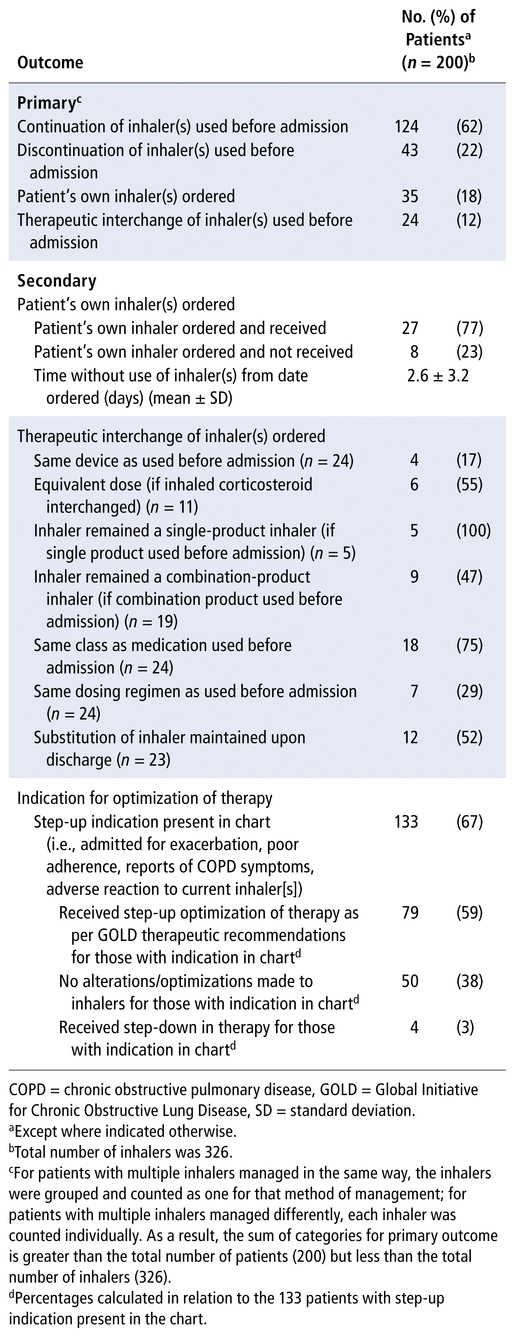
Among the 35 patients with self-provision of nonformulary inhalers, 27 (77%) received a dose during their hospital stay; among these patients, there was an average delay of 2.6 (SD 3.2) days from the order date to the date of the first dose (Table 2). The other 23% of patients with an order to use their own inhaler did not receive a dose during their hospital stay (Table 2).
For inhalers with therapeutic interchange, the majority were substituted for a different device, with only 17% (4/24) of the patients remaining on the same device as before admission (Table 2). Similarly, dosing regimens were maintained for only a minority of patients (29% [7/24]) (Table 2). Additionally, for those who were previously taking a combination product with 2 or more inhaled medications, substitution resulted in separation of the combination, with maintenance of the dosing regimen for the components occurring in less than half of cases (47% [9/19]); conversely, all single-product inhalers that were interchanged were maintained as single products (100% [5/5]) (Table 2). Of the patients with combination inhalers that were separated upon admission, 70% (7/10) were returned to their combination inhaler upon discharge. The medication class being administered before admission was preserved upon substitution for most patients (75% [18/24]), as was the dose equivalency for patients who were receiving an ICS (55% [6/11]) (Table 2). Of the 23 patients who had a formulary substitution and had discharge medications documented in the chart, 12 (52%) remained on the substituted inhaler upon discharge (Table 2).
For the majority of patients (67% [133/200]), one or more of the following indications for therapy optimization was present (Table 2): admission for a COPD exacerbation, poor before-admission adherence to their COPD medications, ongoing reports of COPD symptoms, or adverse reaction to 1 or more of their inhaler medications. Of those with indications for optimization, 79 (59%) received a step-up in therapy, whereas 50 (38%) received no alterations to their medications during the hospital stay or upon discharge (Table 2). A small proportion of patients (3% [4]) received step-down therapy, as per the GOLD guideline2 (Table 2).
This study adds to the literature by characterizing the current state of inhaler management when patients were admitted to one tertiary care hospital. Despite the presence of institutional formulary restrictions, the majority of patients with COPD were maintained on their home regimen after admission, which could indicate a lack of prescribing of the newer COPD inhaler medications within the study population. However, elucidating the prescribing practices for COPD medications was beyond the scope of this study and would be an area for future research.
Patients providing their inhalers from home for in-hospital use was the third most common management strategy employed and resulted in a notable delay of, on average, 2.6 days before the first dose of medication was administered. The delay in receipt of a long-term medication could result in worsening of patients’ symptoms. In addition, if a patient does not have home medications with them at the time of admission, the onus for procuring the patient’s own supply shifts to an external caregiver, which may be impossible for some patients. To decrease potential delays in therapy, a predetermined period during which the patient can bring in their own supply should be defined. Once this period has passed, the pharmacy should re-evaluate the situation and determine if there is a need to obtain a nonformulary supply or if there are possible formulary alternatives that could be used.
Formulary substitution for COPD inhalers was the least common management technique employed at VGH, with only 12% of patients receiving an alternative formulary product. At this hospital, only ICS medications have a defined therapeutic interchange with respect to within-class dosing equivalence, which is likely the reason this approach was less well utilized. Developing therapeutic interchanges for other classes of inhaled therapies may be needed in the future, as the use of newer agents increases.
The appropriateness of any substitutions that did occur was evaluated with respect to maintenance of several factors: device, class of medication, dosing regimen, and single-product or combination-product inhaler. Often it was the delivery device that was not maintained, which resulted in patients being exposed to an unfamiliar inhalation device. If an unfamiliar device is used and proper device technique is not practised, the likelihood of receiving the correct dose decreases, which can alter the control of COPD symptoms.15 Inhaler technique for patients’ regular before-admission medication has been evaluated previously at VGH; in that study, critical errors occurred in 59% of participants.18 If hospital inpatients are using devices different from what they use at home, an opportunity to reinforce proper technique for home inhalers is lost. For many patients with formulary substitutions, the dosing regimen was also altered, with just under one-third of patients remaining on the same regimen. Also of concern is the possibility that in-hospital substitutions will be maintained upon discharge, which occurred for 52% of our study population. For other medication classes in which therapeutic interchange occurs, such as proton pump inhibitors and angiotensin-converting enzyme inhibitors, previous studies have shown that therapy changes during hospital admission can result in medication errors upon discharge, nonadherence, and associated increased costs to the health care system.9,19,20
Another potential barrier to adherence that could be introduced in the inpatient setting is the separation of a combination product into multiple inhalers, which occurred for 47% of the patients in our study who had therapeutic interchange of COPD medications; notably, however, most patients (70%) were returned to their combination product at discharge. The current formulary does not include a combination inhaler for a long-acting muscarinic antagonist and a β-agonist, but it does have single-product inhalers of these drug classes available; as such, unless patients provide their own inhalers, there will likely continue to be separation of combination products for those receiving this specific combination therapy before admission. Seventy-five percent of the patients received a different medication within the same drug class for long-acting β-agonists, long-acting muscarinic antagonists, and ICS. For substitutions involving long-acting muscarinic antagonist and β-agonist, there are no established equivalent doses between different agents within the same class. In addition, there are variations between specific agents within the same class, such as onset of action, duration, and need for renal adjustment.21 The clinical impact of such differences is unknown.
With respect to ICS inhalers, for which therapeutic interchange exists, this study showed that just over half of patients (55%) with a therapeutic interchange received an equivalent ICS dose. All but one of the patients who did not receive an equivalent ICS dose had an indication for optimizing therapy. Overall, 67% of the patients had 1 or more indications for alteration of their current therapy, based on GOLD recommendations.2 The most common indication for therapy adjustment was admission for a COPD exacerbation, which occurred in 62% of the study population. Among the patients with an indication for optimization, therapy was stepped up for the majority (59%), unaltered for a large proportion (38%), and decreased for only a few (3%). The decision to have patients remain on their before-admission therapy may be due to factors such as concerns about adherence, complexity of the regimen, and the prohibitive cost of adding another medication.22 Future studies could investigate the underlying factors that prevent stepping-up of therapy with respect to COPD inhalers, despite presence of an indication.
The study was a retrospective chart review, and as such was limited by the comprehensiveness and accuracy of the information presented in the charts. Charts without complete information were excluded, as a way of minimizing the impact of incomplete documentation. Of the 4 criteria used to determine indications for optimization, 3 relied on chart notes, and limitations related to the comprehensiveness of chart notes likely prevented us from recognizing some patients who had an indication for optimization. Related to this limitation is the fact that adherence before admission was not always documented. Additionally, the investigators could not guarantee that patients who were to use their own medication supply did not take doses without the knowledge of care providers, despite procedures within VGH requiring the identification of patients’ own medications by the pharmacy before administration and documentation on the medication administration record of all medications given. Other factors, such as length of stay, severity of presentation, or the risks versus benefits of a given treatment could affect decisions regarding management of a patient’s inhaler therapy during the hospital admission and were beyond the scope of this retrospective chart review. Also beyond the scope of this type of study was any ability to determine whether changes in therapy were intentional, unless such information is clearly stated in the chart. Complete spirometry results were available for only 25% of the patient population; therefore, the diagnosis of COPD could not be confirmed using objective criteria. The secondary outcome of ICS dosing equivalency was based on evidence for asthma-based ICS equivalency, which represents another limitation, given that COPD-specific dosing equivalencies could not be evaluated. Finally, there was no postdischarge follow-up with patients in the community, so the ramifications for those who were discharged with different inhalers could not be investigated.
The increasing prevalence of COPD is adding to the burden for patients and health care teams alike. The admission to hospital of rising numbers of patients with COPD highlights the need to develop a safe and efficacious management strategy for COPD inhalers, especially given the limited options currently available on formulary relative to the large number of devices that have entered the market. In this study, most patients were continued on their before-admission inhalers, an approach that for other drug classes has been shown to result in fewer medication errors and fewer patient adherence issues than occur with therapeutic interchange.9,16 Discontinuation of an inhaler was the second most common management technique, followed by patients supplying their own inhaler. Despite the large number of different devices and medication combinations for COPD currently available, this study showed that it is still possible, with current formulary options, to keep patients on the same inhaler therapy, although this is ultimately dependent on community prescribing practices.
1 MacNee W. Pathology, pathogenesis, and pathophysiology. BMJ. 2006;332(7551):1202–4.
Crossref PMC
2 Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Global Initiative for Chronic Obstructive Lung Disease; [updated 2020; cited 2020 Jun 19]. Available from: www.goldcopd.org
3 May SM, Li JT. Burden of chronic obstructive pulmonary disease: healthcare costs and beyond. Allergy Asthma Proc. 2015;36(1):4–10.
Crossref PubMed PMC
4 Evans J, Chen Y, Camp PG, Bowie DM, McRae L. Estimating the prevalence of COPD in Canada: reported diagnosis versus measured airflow obstruction. Health Rep. 2014;25(3):3–11.
PubMed
5 Respiratory training and educator courses: COPD medications brochure. Lung Association of Saskatchewan; 2019 [cited 2018 Oct 5]. Available from: https://sk.lung.ca/health-professionals/resources/resptrec-resources
6 Gaebel K, Blackhouse G, Robertson D, Xie F, Assasi N, McIvor A, et al. Triple therapy for moderate-to-severe chronic obstructive pulmonary disease. Report 127. Canadian Agency for Drugs and Technologies in Health; 2010 [cited 2018 Oct 5]. Available from: http://www.cadth.ca/index.php/en/hta/reports-publications/search/publication/1690
7 Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airway disease: a systematic review of the literature. Health Technol Assess. 2001;5(26):1–149.
Crossref PubMed
8 Chrystyn H, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of patient’s satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med. 2014;108(2):358–65.
Crossref
9 Wang JS, Fogerty RL, Horwitz LI. Effect of therapeutic interchange on medication reconciliation during hospitalization and upon discharge in a geriatric population. PLoS One. 2017;12(10):e0186075.
Crossref PubMed PMC
10 Schachtner J, Guharoy R, Medicis J, Newman N, Speizer R. Prevalence and cost savings of therapeutic interchange among U.S. hospitals. Am J Health Syst Pharm. 2002;59(6):529–33.
Crossref PubMed
11 Eurich D, Poulin S, Semchuk W, Taylor J. Therapeutic interchange in Canadian hospitals: a national survey. Can J Hosp Pharm. 2001;54(1): 28–34.
12 BC Health Authorities formulary initiative - update. Lower Mainland Pharmacy Services; 2012 Sep 27 [cited 2019 Dec 19]. Available from: https://pediatrics.med.ubc.ca/files/2012/10/BCHA-Formulary-Initiative-Update-Memo.27Sep2012.pdf
13 Shalansky K, editor. Therapeutic interchange program and prescription interpretations at Vancouver community of care. Drugs and Therapeutics Committee; 2008 [updated 2018 Sep 19; cited 2018 Sep 28]. Available from: http://vhpharmsci.com/PageFormulary/index.html
14 Amidon PB, Jankovich R, Stoukides CA, Kaul AF. Proton pump inhibitor therapy: preliminary results of a therapeutic interchange program. Am J Manag Care. 2000;6(5):593–601.
PubMed
15 Chua D, Chu E, Lo A, Lo M, Pataky F, Tang L, et al. Effect of misalignment between hospital and provincial formularies on medication discrepancies at discharge: PPITS (proton pump inhibitor therapeutic substitution) study. Can J Hosp Pharm. 2012;65(2):98–102.
PubMed PMC
16 Braido F, Lavorini F, Blasi F, Baiardini I, Canonica GW. Switching treatments in COPD: implications for costs and treatment adherence. Int J Chron Obstruct Pulmon Dis. 2015;10:2601–8.
Crossref PubMed PMC
17 Glaholt S, Hayes GL, Wisniewski CS. Evaluation of discharge medication orders following automatic therapeutic substitution of commonly exchanged drug classes. P T. 2014;39(4):267–77.
PubMed PMC
18 Batternik J, Dahri K, Aulakh A, Rempel C. Evaluation of the use of inhaled medications by hospital inpatients with chronic obstructive pulmonary disease. Can J Hosp Pharm. 2012;65(2):111–8.
19 Harris C, Sridharan A, Landis R, Howell E, Wright S. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9(3):150–3.
Crossref PubMed
20 D’Amore M, Masters P, Maroun C. Impact of an automatic therapeutic interchange program on discharge medication selection. Hosp Pharm. 2003;38(10):942–6.
Crossref
21 Ejifor S, Turner AM. Pharmacotherapies for COPD. Clin Med Insights Circ Respir Pulm Med. 2013;7:17–34.
22 Jin J, Sklar GE, Min V, Oh S. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008; 4(1): 269–86.
Crossref PubMed PMC
Competing interests: None declared. ( Return to Text )
Brittany Gage and Julia Lamb made equal contributions to the study and this article and should be considered joint first authors on the paper. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 74, NUMBER 2, Spring 2021