Hana Sakly, Ines Chakroun, Khouloud Ben JeddouABSTRACT
Background
In the hospital setting, the medication-use system for temperature-sensitive drugs is a high-risk process.
Objectives
To analyze the risks associated with the hospital-based medication-use process and to propose corrective and preventive actions for the most critical failure modes.
Methods
A multidisciplinary team was trained to analyze the medication-use process for temperature-sensitive drugs and to identify potential failures using a risk analysis method known as failure mode, effects, and criticality analysis (FMECA). The medication-use process, from initial supply to administration to patients, was investigated using “the 5 Ws and How” method (Who? What? Where? When? Why? How?), and the causes of the failure modes were analyzed using Ishikawa diagrams. The most critical failure modes were selected using the Pareto law, and relevant improvement actions were proposed.
Results
This analysis identified 41 failure modes for the 9 stages of the medication-use process, of which only 36 were deemed assessable by the participants. Eighteen (50%) of these failure modes were critical, according to the Pareto law, with criticality indices between 12 and 60. The stage of tidying up and storage in patient care units had the highest number of critical failures (n = 5). A total of 48 corrective actions were proposed.
Conclusion
The proposed action plan prioritized 3 areas for improvement: the documentation system, staff training, and equipment acquisition. A second FMECA should be carried out to reassess the medication-use process after implementation of these improvement actions. The second FMECA, allowing detection of residual risks and identification of new risks, will be part of a continuous improvement process.
KEYWORDS: cold chain, temperature-sensitive drug, risk management, patient safety
RÉSUMÉ
Contexte
Le circuit des médicaments thermosensibles en milieu hospitalier fait partie des processus à risque.
Objectifs
Analyser a priori les risques liés à ce circuit et proposer des actions correctives et préventives contre les modes de défaillances les plus critiques.
Méthodes
Une équipe pluridisciplinaire a été formée pour analyser le circuit des médicaments thermosensibles et identifier les défaillances par la méthode de l’analyse des modes de défaillances, de leurs effets et de leurs criticités (AMDEC). Le processus, qui va de l’approvisionnement jusqu’à l’administration des médicaments aux patients, a été décortiqué en utilisant la méthode « Qui? Quoi? Où? Quand? Comment? Pourquoi? » (QQOQCP) et les causes des défaillances ont été analysées à l’aide de diagrammes d’Ishikawa. Les défaillances les plus critiques ont été sélectionnées par la loi de Pareto, et des mesures d’amélioration pertinentes ont été proposées.
Résultats
Cette analyse a mis en évidence 41 modes de défaillances pour les 9 étapes du circuit, dont uniquement 36 sont jugés évaluables par les membres de l’équipe pluridisciplinaire. Dix-huit modes sur 36 (soit 50 %) sont critiques selon la loi de Pareto, avec des indices de criticité répartis entre 12 et 60. L’étape comprenant le nombre le plus élevé de défaillances critiques est celle du rangement et du stockage dans les services de soins, avec 5 défaillances. Au total, 48 mesures correctives ont été proposées.
Conclusions
Le plan d’action proposé priorisait 3 domaines d’amélioration : le système de documentation, la formation du personnel et l’acquisition d’équipements. Une deuxième AMDEC devrait être réalisée pour réévaluer le circuit après la mise en œuvre des mesures d’amélioration. La deuxième AMDEC permettra la détection des risques résiduels et l’identification de nouveaux risques et s’inscrira dans une démarche d’amélioration continue.
MOTS CLÉS: chaîne du froid, médicament thermosensible, gestion des risques, sécurité des patients
The cold chain is the set of logistic links that guarantee maintenance of a temperature between 2°C and 8°C during the stages of storage, handling, transport, and distribution of drugs. Any improper operation in the process of cold chain logistics may have a significant effect on the quality of temperature-sensitive drugs and vaccines, endangering the safety of patients receiving them. For example, power outages, inadequate conditions during transport, or unsuitable storage conditions can lead to a break in the cold chain, causing the degradation of vaccines and other pharmaceuticals.1,2 In fact, a drug’s properties may change with any temperature variation, depending on the temperature reached and the duration of storage at that temperature.1,3 Certain drugs may lose some clinical effectiveness, whereas others may have a complete loss of activity or may even become toxic.1,4,5 Thus, the cold chain is a major concern for hospitals, both financially and in terms of patient safety. Managing the operation of the cold chain, defining its players, ensuring its performance, and securing the medication-use process for temperature-sensitive drugs must therefore be included among the hospital’s priorities, especially for the pharmacy department. Indeed, the hospital pharmacist represents an essential link in the cold chain, intervening in almost all stages of the system, from purchase and supply to administration to patients. Errors may occur at any stage of the process, with the pharmacist being responsible not only for identifying the failures but also for evaluating them and implementing the measures necessary to correct or prevent them.6,7
The objectives of this study were to analyze a priori the potential failure modes of the medication-use process for temperature-sensitive drugs and to propose corrective and preventive actions, by setting up a concrete action plan to minimize the risks and ensure the safety of the system and thus the safety of patients.
This study was a failure mode, effects, and criticality analysis (FMECA), carried out in the pharmacy department of a university hospital over a 3-month period (October to December 2019). The FMECA involved 6 steps: training, functional analysis, qualitative study, quantitative study, determination of the hierarchy of criticality, and proposals for improvement.8–10
A multidisciplinary working group was trained to ensure consistency in the discussion and rating of failure modes. The group was made up of 8 members: 2 pharmacists (H.S., K.B.J.), 1 doctor, 3 pharmacy interns (including I.C.), 1 nurse, and 1 pharmacy assistant. For implementation of the study, 4 bimonthly meetings were scheduled.
The working group described the medication-use process for temperature-sensitive drugs, from supply to administration, using the “5 Ws and How” method (Who? What? Where? When? Why? How?). This method allowed us to analyze the various stages, to define the main actors at each stage, and to describe the medication-use process clearly and simply.
The working group identified potential failure modes by brainstorming. For each failure mode, the possible effects on or consequences for both the patient and the temperature-sensitive drugs were defined, the causes of failure were identified, and various means of detecting the risk were found. The causes were analyzed using Ishikawa (“fishbone”) diagrams showing the following 5 aspects: personnel (man/labour), machinery (equipment, technology), material (raw materials, consumables, and information), method (process), and measurement or environment.
For each stage of the medication-use process, a summary matrix of failure modes, as well as their causes and effects, was developed.
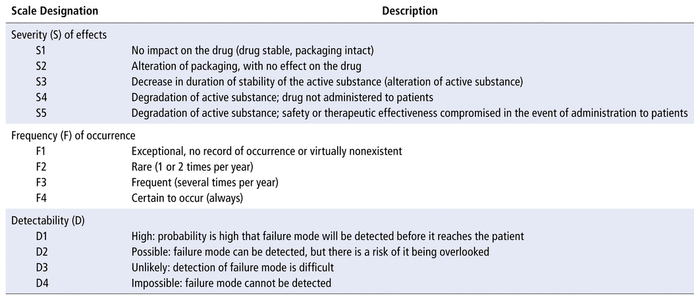
The same matrix was used for rating the frequencies, detectability, and severity of failure modes according to proposed scales (Table 1). Decisions were based on voting by the working group, with divergent ratings subject to discussion to ensure consensus.
TABLE 1 Scales Considered for Scoring Failure Modes

The criticality index of each failure mode was calculated as the product of frequency, detectability, and severity. This calculation made it possible to prioritize the various failure modes identified and to appropriately target actions to be taken.
For this purpose, the working group used a Pareto diagram. This type of diagram is based on the empirical law of 80/20, whereby about 20% of causes explain up to 80% of a problem. In this case, 20% of the failure modes were assumed to account for 80% of the criticality index.
After ranking the failure modes in descending order according to their respective criticality indexes, the cumulative criticality indexes and the percentages of cumulative criticality indexes were calculated for application of the Pareto law.
Once the most critical failure modes were identified, corrective action plans were put into place.
Figure 1 shows an excerpt of the medication-use process for temperature-sensitive drugs, as described by the “5 Ws and How” method.
|
| ||
|
FIGURE 1 Excerpt from description of the medication-use process for temperature-sensitive drugs (TSDs), according to the “5 Ws and How” method. | ||
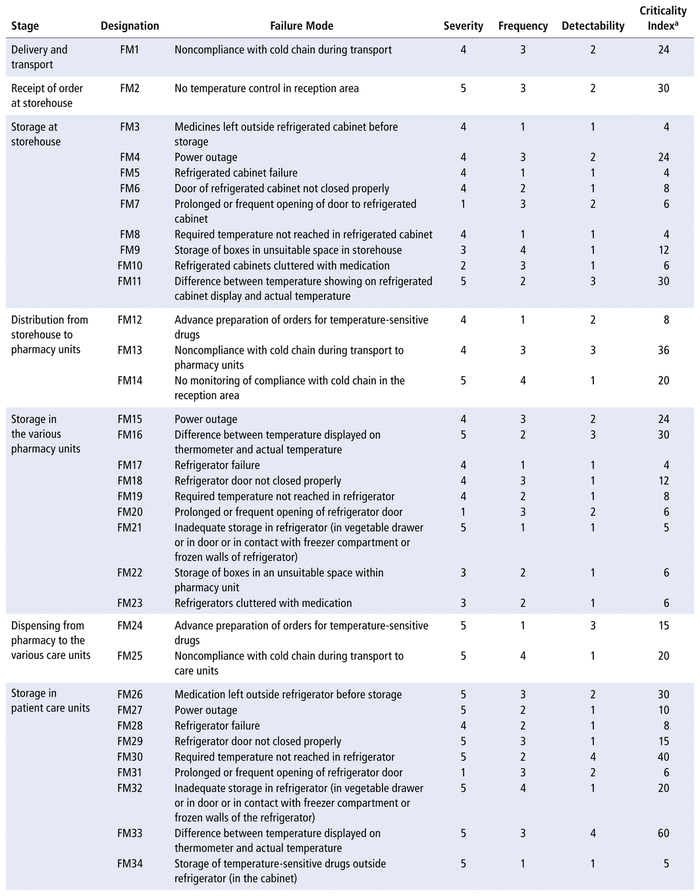
A total of 41 failure modes were identified and subjected to analysis. The highest number of failure modes was recorded for storage stages at the storehouse, in pharmacy units, and in patient care units (n = 9 failure modes for each location). The lowest number of failures (n = 1) was found for the stage of order reception at the storehouse.
The working group deemed 5 failures to be non-evaluable, because they were not specific to the medication-use process for temperature-sensitive drugs. Three of these failure modes belonged to the first stage, relating to purchase and supply (error in supply of the product, delay in supply of the product, insufficient amount ordered relative to need); one occurred in the second stage, relating to delivery and transport (delivery error); and one occurred in the seventh stage, relating to dispensing to care units (error in dispensing). In the end, 36 failure modes were selected for evaluation (Table 2).
TABLE 2 Rating of Failure Modes and Calculation of Criticality Indices

As an example, Figure 2 shows an Ishikawa (“fishbone”) diagram for the failure mode of a power outage.
|
| ||
|
FIGURE 2 Example of an Ishikawa (“fishbone”) diagram for the failure mode of a power outage. | ||
Criticality indexes were calculated for the failure modes identified in the qualitative study. The calculated values were between 4 and 60, as shown in Table 2.
According to the Pareto law, 18 of the 36 failure modes were critical, having criticality indexes between 12 and 60. Figure 3 illustrates the Pareto chart.
|
| ||
|
FIGURE 3 Pareto chart. The failure modes (FMs) are presented along the horizontal axis in descending order of their criticality index. The solid black curve depicts the cumulative percentage of the criticality index. The point along the horizontal axis intersecting the 80% value on the right-hand vertical axis (as shown by dashed grey lines) defines the failure modes that were deemed to be critical (spanned by the horizontal grey arrow). | ||
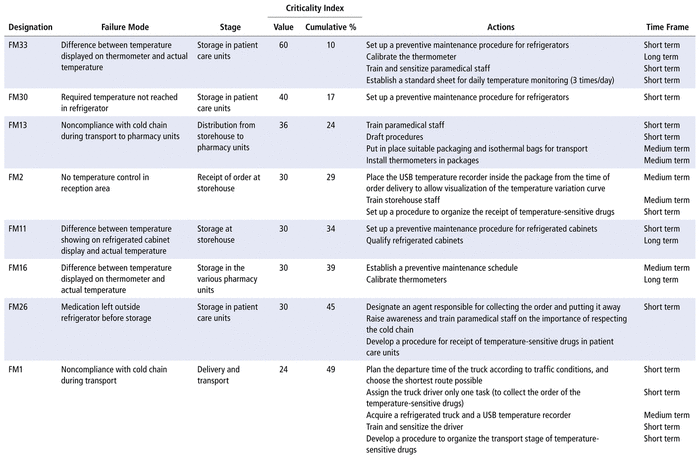
Most of the critical failures (n = 11) were related to the storage stages: in patient care units (n = 5), in the storehouse (n = 3), and in the pharmacy units (n = 3).
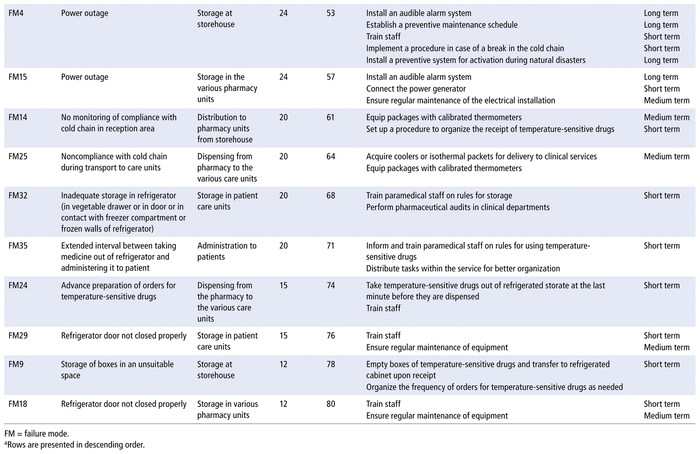
According to the most critical failure modes selected by the Pareto law, the working group proposed several ameliorative, corrective, and preventive actions. These actions will be implemented gradually over time, with deadlines for action in the long, medium, and short terms, according to the hospital’s financial resources (Table 3).
TABLE 3 Critical Failure Modes Selected by Pareto Law and Proposed Improvement Actionsa
Monitoring indicators were put in place to measure the impact of these improvement actions. The first indicator chosen by the working group was the number of temperature alerts, an indirect measure of the efficacy and safety of temperature-sensitive drugs. The second monitoring indicator was the state of operation of cold chain equipment, to identify maintenance needs, with the goal of preserving the quality of temperature-sensitive drugs. This indicator is used for operational purposes (i.e., updating the maintenance plan).
Controlling the cold chain remains a topical issue for hospitals. It is linked to a triple risk: a financial or economic risk, a regulatory risk, and above all a risk to patients if the quality of a drug is altered.11 As intervenors in the cold chain, pharmacists play an important role in preserving the integrity and safety of each drug.12
In this study, the a priori analysis of risks associated with the medication-use process for temperature-sensitive drugs was carried out by the FMECA method because it is the most suitable method for the hospital setting, according to several previous studies.9,13–15 It has been recommended by the French Haute Autorité de Santé (National Authority for Health)16 as a reference method for risk analysis and has been recommended by several other health organizations, in particular the Institute for Healthcare Improvement,17 the Institute for Safe Medication Practices Canada,18 and the American Association of Physicists in Medicine.19
The FMECA method is based on the concept of brainstorming. This method of combining ideas is 1 of the 7 basic tools of quality.20 It is a creative technique that allows the emergence of new ideas in groups.21 The CHU Sainte-Justine in Montréal, Quebec, applied this technique in the search for failure modes for an evaluation of the various stages of drug administration.22 The major strengths of FMECA are its simplicity and the quantitative evaluation it allows through a combination of 3 parameters: frequency, severity, and detectability. Moreover, the FMECA helps in identifying the most important critical events, which is helpful for deciding upon and prioritizing improvement actions.22 However, the FMECA methodology does not specify the procedure to be followed for prioritization. In the AMELIORE study, the team opted for a criticality threshold beyond which recommendations were made23; however, they did not explain their choice of threshold value. Arenas Villafranca and others24 classified failure modes according to their relative importance. They subsequently developed a checklist to determine the “most critical aspects of the process”. Again, however, the method of selecting priority risks was not specified. In the current study, the Pareto law was used to define critical failure modes. This choice was approved by all participants, given the method’s advantages, including time savings. The criticality index can be rated according to a scale that allows visual assessment using a colour code: green for acceptable, yellow for tolerable, and red for unacceptable.25 In other studies,9,14 the criticality index was positioned in a matrix developed by the working group. It can be divided into 3 or more levels, for example, acceptable, tolerable, and unacceptable risk or, alternatively, low, high, and major criticality. Improvement actions are then prioritized according to these categories.
The Pareto law allowed us to select 18 critical failure modes. The stage with the most failure modes was storage in patient care units. Conversely, the stages of delivery and transport and of receipt of the order at the storehouse had the least critical failure modes.
These results cannot be directly compared with any study in the literature because we found no prior studies using FMECA to analyze the medication-use process for temperature-sensitive drugs. Instead, we compared our results with those of studies using the FMECA method and studies on improvement of the cold chain. One study of the latter type, conducted in a health care establishment, highlighted 14 critical points along the entire medication-use process for temperature-sensitive drugs, beginning with delivery by suppliers and ending with delivery to patients11; by comparison, we found 18 points of critical failure. Some failure modes were common to the earlier cold chain study11 and our analysis, namely, noncompliance with the cold chain during transport from the supplier, absence of temperature indicators in the medication packages, noncompliance of pharmacy refrigerators and cold rooms, noncompliance with the cold chain during transport to clinical departments, and storage conditions for products requiring temperature control in compliant refrigerators in clinical departments. Indeed, the delivery and transport stage in our study showed a single critical failure with a criticality index of 24, suggesting noncompliance with the cold chain during transport. This crucial stage must be appropriately controlled, given it is the first link in the entire cold chain. The study by Saint-Lorant and others11 similarly showed that the transport stage is critical. The only efficient way to transport temperature-sensitive drugs is to use a refrigerated truck, to equip the packages with a USB temperature recorder, and to train logistics staff. The stage of order reception at the pharmacy storehouse also had a single failure (lack of temperature control in the reception area), which was deemed to be critical, according to the 80/20 law. To secure this stage, it is important to check the USB temperature recorder of the order upon receipt, to prioritize documentation of receipt of temperature-sensitive drugs, and to put them away promptly. Saint-Lorant and others11 suggested limiting the number of people involved in accepting medication deliveries and to make the receptionist aware of the importance of this task.
Eleven of the 18 critical failure modes identified in the current study involved storage: at the storehouse, in the various pharmacy units, and in patient care units. Three failure modes related to storage in the storehouse were deemed critical. The first was a difference between the temperature shown on the display and the actual temperature. To remedy this potential failure mode, the working group proposed qualifying the refrigerated cabinets, to verify that the device continued to operate well under actual conditions of use over time (monitoring of continuous temperature recording, regular verification of proper functioning of the alarms, renewal of the characterizations of the refrigerated cabinet), calibrating the thermometers, and putting in place preventive maintenance procedures. The second critical failure mode was a power outage, for which the main action proposed was installation of an audible alarm system. A remedial procedure in case of a break in the cold chain and the establishment of a preventive maintenance schedule for electrical installations were also proposed. Saint-Lorant and others11 planned to group temperature-sensitive drugs into categories according to their stability, to allow their retrieval in case of a break in the cold chain. The third critical failure mode was the storage of boxes containing temperature-sensitive drugs in the refrigerated cabinet, a situation in which the temperature could exceed 8°C. To address this potential failure mode, staff were asked to unpack the drugs quickly before storing them.
The stage of storage in the various pharmacy units had 3 critical failure modes according to the Pareto law: a difference between the temperature displayed by the thermometer and the actual temperature, power outage, and refrigerator door not closed properly. The proposed improvement actions were to train staff and ensure preventive maintenance of equipment.
Storage in the patient care units represents the penultimate stage of the medication-use process; it is crucial because it is the last step before drug administration. This stage also had the greatest number of failure modes of any stage: there were 9 failures, 5 of which were critical according to the Pareto law: difference between the temperature displayed by the thermometer and actual temperature, required temperature not reached in refrigerator, medication left outside the refrigerator before storage, inadequate storage in the refrigerator (e.g., in the door or the vegetable drawer), and refrigerator door not closed properly. The corrective actions were as follows: training of paramedical staff and sensitization to the challenges of the cold chain, monitoring the temperature of refrigerators 3 times daily, and returning to the pharmacy any temperature-sensitive drugs not administered to patients (to avoid cluttering refrigerators on the care units). Regular pharmaceutical audits within the various clinical services have also been proposed. Saint-Lorant and others11 have also provided for pharmaceutical audits in clinical services to remedy fault points and to put in place any corrective actions needed to improve the cold chain.
Similar to other work using FMECA,26,27 subjectivity in the rating of each risk was the main limitation of this study. In fact, the identification of failure modes and their rating are mainly based on the risk perceptions of various health care professionals, given that everyone participates and reacts according to their own experience.
In this study, storage in patient care units was the stage of the medication-use process with the most critical failure modes and required the most interventions to ensure patient safety. Securing this high-risk process is a means of ensuring the efficiency of patient care within the framework of continuous quality improvement. Admittedly, implementation of quality improvement measures appears to be difficult, time-consuming, and expensive; however, such an approach makes it possible to prevent the deterioration of temperature-sensitive drugs and thus to reduce the financial losses due to breakdown of the cold chain. A second FMECA should be carried out to reassess the medication-use process after implementation of improvement actions.
1 Periáñez Parraga L, Gómez-Lobón A, Gamón Runnenberg I, Seco Melantuche R, Delgado Sánchez O, Puigventós Latorre F. Thermolabile drugs. Operating procedure in the event of cold chain failure. Farm Hosp (Engl Ed). 2011;35(4):190.e1–190.e28.
2 Kosari S, Walker EJ, Anderson C, Peterson GM, Naunton M, Castillo Martinez E, et al. Power outages and refrigerated medicines: the need for better guidelines, awareness and planning. J Clin Pharm Ther. 2018; 43(5):737–9.
Crossref PubMed
3 Wen Z, Liao H, Ren R, Bai C, Zavadskas EK, Antucheviciene J, et al. Cold chain logistics management of medicine with an integrated multi-criteria decision-making method. Int J Environ Res Public Health. 2019; 16(23):4843.
Crossref PMC
4 Bogale HA, Amhare AF, Bogale AA. Assessment of factors affecting vaccine cold chain management practice in public health institutions in east Gojam zone of Amhara region. BMC Public Health. 2019;19(1): Article 1433.
Crossref PubMed PMC
5 Ogboghodo EO, Omuemu VO, Odijie O, Odaman OJ. Cold chain management practices of health care workers in primary health care facilities in Southern Nigeria. Pan Afr Med J. 2017 [cited 2021 Jan 20];27:Article 34. Available from: http://www.panafrican-med-journal.com/content/article/27/34/full/
Crossref
6 Bedouch P, Baudrant M, Detavernier M, Rey C, Brudieu É, Foroni L, et al. La sécurisation du circuit du médicament dans les établissements de santé : données actuelles et expérience du centre hospitalier universitaire de Grenoble. Ann Pharm Fr. 2009;67(1):3–15.
Crossref PubMed
7 Ricote-Lobera I. Medicamentos termolábiles: intervención farmacéutica como garantía del mantenimiento de la cadena del frío. Farm Hosp. 2014;38(3):211–5. Article in Spanish.
PubMed
8 Bonnabry P, Despont-Gros C, Grauser D, Casez P, Despond M, Pugin D, et al. A risk analysis method to evaluate the impact of a computerized provider order entry system on patient safety. J Am Med Inform Assoc. 2008;15(4):453–60.
Crossref PubMed PMC
9 Meyrieux C, Garcia R, Pourel N, Mège A, Bodez V. Analyse des risques a priori du processus de prise en charge des patients en radiothérapie : exemple d’utilisation de la méthode Amdec. Cancer/Radiother. 2012; 16(7):613–8.
Crossref
10 Boulé M, Lachapelle S, Collin-Lévesque L, Demers É, Nguyen C, Lebel D, et al. Approche commentée par étape pour réaliser une AMDEC dans le cadre du circuit du médicament. Pharm Hosp Clin. 2018; 53(4):315–24.
11 Saint-Lorant G, Souchon J, Guillard P, Barbier-Courteille F, Hecquard C. Amélioration continue de la chaîne du froid des médicaments dans un établissement de santé. Pharm Hosp Clin. 2014;49(3):162–75.
12 Noblot-Rossignol M, Brunet L, Fleck C, Marechal B, Jost V. Délivrance de médicaments réfrigérés depuis une plateforme hospitalière d’approvisionnement : quelles configurations optimales? Pharm Hosp Clin. 2017;52(3):276–84.
13 Demers É, Collin-Lévesque L, Boulé M, Lachapelle S, Nguyen C, Lebel D, et al. Analyse des modes de défaillance, de leurs effets et de leur criticité dans le circuit du médicament: revue de littérature. Can J Hosp Pharm. 2018;71(6):376–84.
14 Darcissac C, Duvert L, Hoegy D, Chappuy M, Pivot C, Janoly-Dumenil A. Analyse des risques a priori en unité de rétrocession hospitalière : focus sur le processus de dispensation. Ann Pharm Fr. 2020;78(1):12–20.
Crossref
15 Hurtrel F, Beretz L, Renard V, Hutt A. Analyse des risques liés au circuit de gestion et de dispensation des produits en expérimentation clinique par «AMDEC». Risques Qual. 2012;1:22–30.
16 Gestion des risques et protocoles de coopération. Saint-Denis (France): Haute Autorité de Santé; 2013.
17 Failure modes and effects analysis (FMEA) tool. Institute for Healthcare Improvement; 2022 [cited 2022 May 4]. Available from: http://www.ihi.org/resources/Pages/Tools/FailureModesandEffectsAnalysisTool.aspx
18 Failure mode and effects analysis (FMEA). Institute for Safe Medication Practices Canada; 2022 [cited 2022 May 4]. Available from: https://www.ismp-canada.org/fmea.htm
19 Thomadsen B, Dunscombe P, Ford E, Huq S, Pawlicki T, Sutlief S, editors. Quality and safety in radiotherapy: learning the new approaches in Task Group 100 and beyond. AAPM Med Phys Monogr 36. Medical Physics Publishing; 2013.
20 The 7 basic quality tools for process improvement. American Society for Quality; 2022 [cited 2022 May 4]. Available from: https://asq.org/quality-resources/seven-basic-quality-tools
21 Méthodes et outils des démarches qualité pour les établissements de santé. Saint-Denis (France): Agence Nationale d’Accréditation et d’Evaluation en Santé; 2000.
22 Nguyen C, Côté J, Lebel D, Caron E, Genest C, Mallet M, et al. The AMÉLIE project: failure mode, effects and criticality analysis: a model to evaluate the nurse medication administration process on the floor. J Eval Clin Pract. 2013;19(1):192–9.
Crossref
23 Boulé M, Lachapelle S, Collin-Lévesque L, Demers É, Nguyen C, Fournier-Tondreau M, et al. Failure mode, effect, and criticality analysis of the parenteral nutrition process in a mother–child hospital: the AMELIORE study. Nutr Clin Pract. 2018;33(5):656–66.
Crossref PubMed
24 Arenas Villafranca JJ, Sánchez AG, Guindo MN, Felipe VF. Using failure mode and effects analysis to improve the safety of neonatal parenteral nutrition. Am J Health Syst Pharm. 2014;71(14):1210–8.
Crossref PubMed
25 Allou KRS, El Harti J. Cartographie de la gestion des risques de la prédésinfection des dispositifs médicaux en milieu hospitalier. Cas de la stérilisation centrale de l’hôpital Ibn Sina, Rabat. Pharm Hosp Clin. 2018;53(4):279–87.
26 Berruyer M, Atkinson S, Lebel D, Bussières JF. Utilisation de l’insuline en établissement de santé universitaire mère–enfant : analyse des modes de défaillance. Arch Pediatr. 2016;23(1):1–8.
Crossref
27 Bonnabry P, Cingria L, Ackermann M, Sadeghipour F, Bigler L, Mach N. Use of a prospective risk analysis method to improve the safety of the cancer chemotherapy process. Int J Qual Health Care. 2006;18(1):9–16.
Crossref
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 75, NUMBER 3, Summer 2022