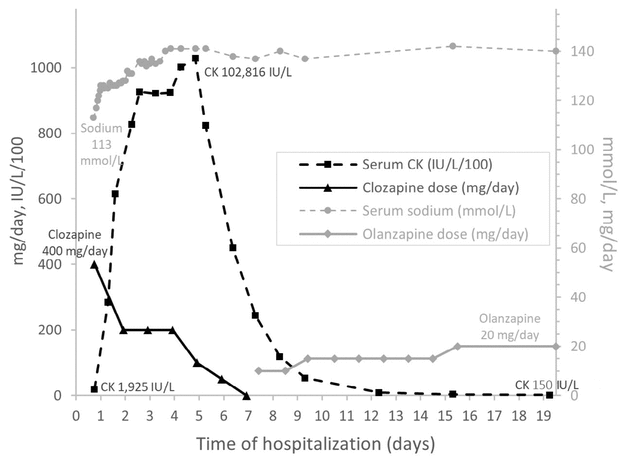
FIGURE 1 Time course of changes in the patient’s doses of clozapine and olanzapine, as well as serum sodium and creatine kinase (CK) levels during the hospital stay.
Myriam Lemelin , Nicolas Gagnon , Emmanuelle Jacques , Philippe Sirois , Alexandrine Coulombe
This case report describes a patient with refractory schizophrenia admitted to the intensive care unit for symptomatic hyponatremia. This electrolyte imbalance was attributable to psychogenic polydipsia (PPD), a medical condition associated with psychiatric disorders and characterized by abnormal thirst and excessive fluid intake.1 Rhabdomyolysis was also diagnosed and worsened during the correction of serum sodium, with the potential contribution of clozapine, paliperidone palmitate, and psychotic agitation.
Rhabdomyolysis is a clinical and biochemical syndrome resulting from the breakdown of muscle cells. This syndrome ultimately leads to leakage of intracellular content—specifically creatinine kinase (CK), myoglobin, and electrolytes—from the muscle cells into the circulation. The occurrence of rhabdomyolysis is rare, but the complications can be severe (e.g., acute kidney injury, electrolyte disturbance leading to cardiac arrhythmias).2 Biological markers such as CK can help to confirm the diagnosis.3 Common etiological risk factors for rhabdomyolysis include trauma, illicit drugs (e.g., cocaine, heroin, amphetamines), alcohol abuse, immobilization (e.g., coma, sedation, prolonged surgery), and medications (e.g., statins, antipsychotics, lithium, valproic acid, quinolones, colchicine).4,5 Case reports in the literature have also suggested hyponatremia secondary to PPD as a precipitating factor for rhabdomyolysis.6–21 Therefore, psychiatric patients can be considered at higher risk of rhabdomyolysis because of their prescribed psychiatric medications (e.g., neuroleptics and antipsychotics, such as haloperidol and atypical antipsychotics; selective serotonin reuptake inhibitors; lithium; valproic acid) and comorbidities (e.g., substance use disorder, involving illicit drugs and alcohol).5
Of particular interest in this case was the rapid increase in CK level to over 100 000 IU/L (normal range 0–150 IU/L), in a period of 4 days, in the presence of multiple contributing factors. This article highlights the numerous potential factors contributing to rhabdomyolysis and aims to increase health care providers’ awareness of the importance of closely monitoring serum CK in patients with such a clinical presentation.
A 35-year-old white man with a history of schizophrenia, borderline personality disorder, attention deficit hyperactivity disorder, PPD, and substance use disorder was brought to the emergency department for confusion, vomiting, and umbilical pain.* A social worker had been making daily home visits to verify treatment adherence and had reported no issues of concern before this episode. His current medications included clozapine 400 mg at bedtime, sublingual atropine 1% drops at bedtime, and olanzapine 5 mg as needed. The clozapine had been gradually introduced to his therapy a month before his presentation to the emergency department. His smoking status had remained stable since then. Before clozapine, the patient had been receiving injections of paliperidone palmitate 350 mg every 3 months. His last dose was 3 months before presentation to the emergency department.
In the emergency department, physical examination revealed tachycardia, diaphoresis, tremor, and apyrexia. Initial laboratory investigations revealed severe hyponatremia (defined as serum sodium less than 120 mmol/L), with serum sodium of 113 mmol/L, urine osmolarity of 133 mmol/kg of water (reference range 50–1200 mmol/kg of water), and elevated serum CK level, at 1925 IU/L (reference range 0–185 IU/L). In addition, the serum clozapine level was at the upper limit of the normal range, at 1533 nmol/L (reference range 306–1836 nmol/L). The toxicology screen was negative for alcohol or other drugs of abuse (e.g., cocaine, cannabis, amphetamines, barbiturates, phencyclidine).
The patient was rapidly transferred to the intensive care unit. Initial treatment consisted of 150 mL of 3% sodium chloride administered over 20 minutes, followed by an infusion of 0.9% sodium chloride at a rate of 100 mL/h for 3 hours to compensate for the sodium deficit. Five hours after administration of the bolus, the patient experienced a serum sodium increase of 13 mmol/L, reaching a serum sodium level of 126 mmol/L (reference range 135–145 mmol/L). Administration of IV fluid was stopped, and he received 2 μg of IV desmopressin acetate to decelerate the sodium correction rate. Four hours later, an infusion of 0.45% sodium chloride was started, at a rate varying between 80 and 250 mL/h, and was continued for approximately 36 hours to maintain the sodium repletion and prevent rhabdomyolysis-induced renal failure. Natremia normalization was achieved between 35 and 42 hours after admission, with the serum sodium level reaching 136 mmol/L.
At the same time, progressive worsening of the rhabdomyolysis was observed. The serum CK level abruptly increased, reaching a maximum value of 102 816 IU/L on the fourth day of the hospital stay (116 hours after admission). It started to decrease on the fifth day after admission (see Figure 1). CK normalization was achieved 19 days after admission, with a level of 150 IU/L (reference range 0–185 IU/L). To prevent acute renal failure, a high volume of fluids (i.e., 200–300 mL/h for about 120 hours) was administered until the serum CK level fell below 10 000 IU/L at day 8, with close monitoring of natremia. The patient’s renal function remained stable during the admission, with an estimated glomerular filtration rate above 120 mL/min.
|
|
||
|
FIGURE 1 Time course of changes in the patient’s doses of clozapine and olanzapine, as well as serum sodium and creatine kinase (CK) levels during the hospital stay. |
||
With regard to his medication, clozapine was gradually reduced starting on day 1 of the admission and was stopped on day 6. Olanzapine 10 mg daily was introduced 36 hours after clozapine discontinuation (on day 7) and was titrated over a week up to 20 mg daily. During this therapeutic substitution, the patient had psychotic agitation. He fully recovered after the introduction of olanzapine. He was discharged on day 20 with olanzapine 5 mg in the morning and 15 mg at bedtime. The serum CK level was maintained in the normal range for at least a month after discharge.
According to the Naranjo probability scale, there was a probable causal relationship (score 5) between the use of clozapine and the clinical event.22
Usually, practitioners consider the possibility of rhabdomyolysis when the serum CK level is 5 times the upper limit of normal.23 This patient had a mild case of rhabdomyolysis on admission (CK 1925 IU/L), which worsened during the hospital stay. It is essential to point out the pharmacokinetics of CK to establish the timeline of possible causes of the rhabdomyolysis. This biological marker usually increases approximately 2 to 12 hours following the breakdown of muscle cells. The peak occurs within 3 to 5 days after the injury. CK level diminishes over the subsequent 6 to 10 days, when multifactorial causes are corrected.24 The potential factors contributing to the pathological state of rhabdomyolysis in this patient, discussed below, were medications, hyponatremia, and rapid correction of the hyponatremia.
Certain medications (e.g., statins, antipsychotics, lithium, valproic acid, quinolones, colchicine), alcohol, and convulsions can precipitate rhabdomyolysis.4,5 This patient did not manifest any sign of seizures and had negative results on toxicology screening for alcohol and drugs. Therefore, we excluded these as potential contributing factors to the rhabdomyolysis, keeping in mind that drug testing has its limitations.
On the basis of the literature, we found a plausible relationship between the patient’s medication regimen and his rhabdomyolysis. In a descriptive retrospective study, Packard and others25 tried to establish a relationship between antipsychotics and rhabdomyolysis in patients admitted to a medical centre in Nebraska. Of the 673 cases of rhabdomyolysis, 71 (10.5%) involved patients who were taking at least 1 antipsychotic agent.25 The exact mechanism of antipsychotic-induced rhabdomyolysis is still unclear. Two hypotheses have been proposed: serotonin antagonism increasing permeability to CK and dopaminergic blockade resulting in excessive movements and rigidity.25 In the case reported here, recent exposure to olanzapine, paliperidone palmitate, and clozapine may have contributed to the patient’s rhabdomyolysis. Because the olanzapine had been prescribed on an as-needed basis and the patient was not actually taking it, this antipsychotic will not be considered in our analysis. His last deltoid injection of 3-month paliperidone palmitate had been administered 3 months before the admission. Considering its long half-life (84 to 95 days) and the fact that only 1 half-life had passed, the significant amount of residual systemic paliperidone palmitate could have contributed to the rhabdomyolysis, as reported in the literature.25–27 The recent introduction of clozapine might also have caused CK elevation. In the study by Packard and others,25 clozapine was involved in 4% of the reported cases. To our knowledge, at least 7 cases specifically involving clozapine-associated rhabdomyolysis have been published.7,16,19,28–31 In these cases, the following concomitant risk factors for rhabdomyolysis were identified: PPD, hyponatremia, seizure, lithium use, and electroconvulsive therapy.25 Rhabdomyolysis occurred at various times after the introduction of clozapine, ranging from weeks to years. The serum CK level fluctuated between approximately 10 000 IU/L and 98 000 IU/L.7,16,19,28–31 Discontinuation of clozapine and initiation of fluid therapy appear to have been the treatment adopted in the majority of cases.7,16,19,28–31 All episodes of rhabdomyolysis resolved after the treatment. In 2 cases, a second episode of rhabdomyolysis occurred after a rechallenge.29,30 Thus, it is plausible that clozapine could have contributed to rhabdomyolysis in the case reported here, even though the clozapine serum level was within the therapeutic range. There was no clozapine rechallenge in this case. Only 3 other case reports in the literature describe the combination of the same 3 contributing factors as we observed (clozapine, PPD, rapid serum sodium correction).7,16,19 Of these, the case reported by Wicki and others19 is the only one in which clozapine was rechallenged, and no recurrence of rhabdomyolysis was reported.
Moreover, the patient’s psychiatric disorder was unstable during the gradual dose reduction of clozapine and the introduction of olanzapine, leading to psychotic agitation. Substantial psychomotor activation is also known as a potential contributor to rhabdomyolysis.4,5
Hyponatremia and hypo-osmolarity can cause rhabdomyolysis, which is attributable to a change in the transmembrane potential, leading to myolysis.18
A week before presentation to the emergency department, the patient’s serum sodium was 140 mmol/L, subsequently decreasing to 113 mmol/L. PPD can lead to severe and symptomatic hyponatremia (< 120 mmol/L) in the following situations: excessive and rapid hydration (> 100 mL/kg daily), maximal urine dilution (< 100 mmol/kg of water), or additional contributing factors (e.g., syndrome of inappropriate antidiuretic hormone secretion, certain medications, malnutrition, alcohol, intrinsic renal disease).1,32 According to Ali and Bazzano,32 second-generation antipsychotics may also cause hyponatremia. The current medical history (i.e., development of symptoms consistent with severe hyponatremia in less than 24 hours in combination with PPD) and the laboratory results (i.e., low urine sodium, 21.0 mmol/L; dilute serum potassium, 3.1 mmol/L, and serum chlorine, 73 mmol/L; low urine osmolality, 133 mmol/kg of water; low serum osmolality, 235 mmol/kg of water) were consistent with excessive fluid intake over a short period secondary to PPD as the cause of severe hyponatremia.
The incidence of rhabdomyolysis in patients with PPD and severe hyponatremia is approximately 30% to 60%.33,34 The first report of rhabdomyolysis associated with hyponatremia secondary to PPD was published in 1979.6 We searched PubMed, MEDLINE, Embase (up to February 25, 2021), and the grey literature for case reports describing rhabdomyolysis with severe hyponatremia secondary to PPD. More than 20 cases, including ours, have now been reported (Table 1).6–21,35,36 However, the underlying mechanism remains controversial. All patients in the reported cases had severe hyponatremia and serum sodium level on admission between 104 and 120 mmol/L. Among these cases, most patients were male and had a diagnosis of schizophrenia. Other contributing factors included seizures and medications (e.g., antipsychotics, lithium, valproic acid). Clozapine was used to treat schizophrenia in 4 cases, including the current case. The peak level of CK ranged from 10 642 to 102 816 IU/L between 24 and 144 hours after the diagnosis of hyponatremia. Hyponatremia was quickly managed in less than 48 hours. In rhabdomyolysis due to hyponatremia, the serum CK peak is often reached at 48 to 96 hours with a level less than 100 000 IU/L. Therefore, the high and delayed serum CK peak in the case reported here (102 816 IU/L at 116 hours) could be explained by another contributing factor: the serum sodium correction rate.7,37
TABLE 1
Reported Cases of PPD-Related Rhabdomyolysis and Their Contributing Factorsa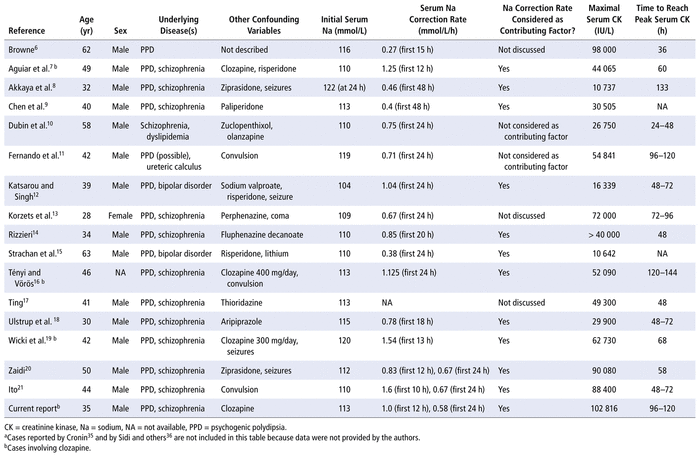
A rapid rate of serum sodium correction can cause rhabdomyolysis.16,33,34 More specifically, it can lead to the failure of cell volume regulation, which in turn results in membrane fragility and enzyme leakage.16,33,34 Correction of hyponatremia, along with the rate of sodium correction, was considered a contributing factor to rhabdomyolysis in 70% of the cases reported in Table 1. Between 2012 and 2016, in a single-centre retrospective cohort study, Kashiura and others34 showed that rapid correction of serum sodium was independently associated with rhabdomyolysis (defined as CK ≥ 1500 IU/L) in 56 cases of water intoxication. The median serum sodium correction was 1.15 mmol/L/h (interquartile range [IQR] 0.74–1.31 mmol/L/h) and 1.02 mmol/L/h (IQR 0.63–1.20 mmol/L/h) in the first 12 and 24 hours following admission, respectively. Among the 56 patients, 35 patients (62.5%) had rhabdomyolysis. The serum sodium level on admission was similar between patients with and without rhabdomyolysis (about 110 mmol/L). Patients with rhabdomyolysis had a higher median serum sodium correction rate in the first 12 and 24 hours than those without rhabdomyolysis (1.22 versus 0.71 mmol/L/h at 12 hours and 1.11 versus 0.60 mmol/L/h at 24 hours; p < 0.001). The serum CK level on admission was higher in patients with rhabdomyolysis (661 versus 215 IU/L, p < 0.001), which suggests that rhabdomyolysis might have occurred before admission. However, as an independent risk factor for rhabdomyolysis, the serum sodium correction rate may have contributed to the median serum CK peak of 10 323 IU/L (IQR 5775 to 35 695 IU/L) in patients with rhabdomyolysis.34
According to the literature, to prevent rhabdomyolysis, a serum sodium correction rate of less than 0.50 to 0.80 mmol/L/h and an increase in serum sodium of 10 to 12 mmol/L/24 h are recommended, and this approach to sodium correction should be undertaken within the first 24 hours of hyponatremia.33,34 In the case reported here, the serum sodium increased from 113 to 126 mmol/L (a difference of 13 mmol/L) over 5 hours, and the serum sodium correction rate was 1.0 mmol/L/h for the first 12 hours after admission to hospital and 0.58 mmol/L/h for the first 24 hours after admission. These correction rates are faster than what is recommended. Consequently, rapid correction of hyponatremia could have enhanced rhabdomyolysis in this patient. Furthermore, rhabdomyolysis due to the correction of hyponatremia is often associated with a CK peak delayed over 96 hours, which was the case for this patient (CK peak 102 816 IU/L after 116 hours).
In the case reported here, the underlying cause of rhabdomyolysis could not be determined with certainty, given the multifactorial etiology of this condition. We considered several contributing factors, such as clozapine, paliperidone palmitate, psychotic agitation, severe hyponatremia secondary to PPD, and rapid correction of serum sodium. In assessing the risk of rhabdomyolysis, clinicians should be aware of the interplay of multiple factors, and serum CK level should be closely monitored when one or more of these contributing factors are identified. In addition, clinicians should be vigilant in setting the rate of correction of hyponatremia and should extend close monitoring of serum CK levels, given that the CK peak may be delayed.
1 Dundas B, Harris M, Narasimhan M. Psychogenic polydipsia review: etiology, differential, and treatment. Curr Psychiatry Rep. 2007;9(3): 236–41.
Crossref PubMed
2 Chatzizisis YS, Misirli G, Hatzitolios AI, Giannoglou GD. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19(8):568–74.
Crossref PubMed
3 Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58–69.
PubMed PMC
4 Cabral BMI, Edding SN, Portocarrero JP, Lerma EV. Rhabdomyolysis. Dis Mon. 2020;66(8):101015.
Crossref PubMed
5 Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3): 1058–65.
Crossref PubMed
6 Browne PM. Rhabdomyolysis and myoglobinuria associated with acute water intoxication. West J Med. 1979;130(5):459–61.
PubMed PMC
7 Aguiar DT, Monteiro C, Coutinho P. Recurrent rhabdomyolysis secondary to hyponatremia in a patient with primary psychogenic polydipsia. Rev Bras Ter Intensiva. 2015;27(1):77–81.
Crossref PubMed PMC
8 Akkaya C, Sarandol A, Sivrioglu EY, Kotan Z, Kirli S. A patient using ziprasidone with polydipsia, seizure, hyponatremia and rhabdomyolysis. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1535–8.
Crossref PubMed
9 Chen LC, Bai YM, Chang MH. Polydipsia, hyponatremia and rhabdomyolysis in schizophrenia: a case report. World J Psychiatry. 2014; 4(4):150–2.
Crossref PubMed PMC
10 Dubin I, Gelber M, Schattner A. Rare times rare: the hyponatremia, rhabdomyolysis, anterior compartment syndrome sequence. JRSM Open. 2016;7(5):2054270416629326.
Crossref PubMed PMC
11 Fernando S, Sivagnanam F, Rathish D. A compulsive act of excess water intake leading to hyponatraemia and rhabdomyolysis: a case report. Int J Emerg Med. 2019;12(1):34.
Crossref PubMed PMC
12 Katsarou A, Singh S. Hyponatraemia associated rhabdomyolysis following water intoxication. BMJ Case Rep. 2010;2010:bcr0220102720.
Crossref PubMed PMC
13 Korzets A, Ori Y, Floro S, Ish-Tov E, Chagnac A, Weinstein T, et al. Case report: severe hyponatremia after water intoxication: a potential cause of rhabdomyolysis. Am J Med Sci. 1996;312(2):92–4.
Crossref PubMed
14 Rizzieri DA. Rhabdomyolysis after correction of hyponatremia due to psychogenic polydipsia. Mayo Clin Proc. 1995;70(5):473–6.
Crossref PubMed
15 Strachan P, Prisco D, Multz AS. Recurrent rhabdomyolysis associated with polydipsia-induced hyponatremia - a case report and review of the literature. Gen Hosp Psychiatry. 2007;29(2):172–4.
Crossref PubMed
16 Tényi T, Vörös V. Successful switch to olanzapine after rhabdomyolysis caused by water intoxication and clozapine use. Pharmacopsychiatry. 2006;39(4):157–8.
Crossref PubMed
17 Ting JY. Rhabdomyolysis and polydipsic hyponatraemia. Emerg Med J. 2001;18(6):520.
Crossref PubMed PMC
18 Ulstrup A, Ugleholdt R, Rasmussen JV. Fulminant crural compartment syndrome preceded by psychogenic polydipsia. BMJ Case Rep. 2015;2015:bcr2014208603.
Crossref PubMed PMC
19 Wicki J, Rutschmann OT, Burri H, Vecchietti G, Desmeules J. Rhabdomyolysis after correction of hyponatremia due to psychogenic polydipsia possibly complicated by clozapine. Ann Pharmacother. 1998;32(9): 892–5.
Crossref PubMed
20 Zaidi AN. Rhabdomyolysis after correction of hyponatremia in psychogenic polydipsia possibly complicated by ziprasidone. Ann Pharmacother. 2005;39(10):1726–31.
Crossref PubMed
21 Ito H. A Sudden fluctuation in creatinine kinase: water intoxication and rhabdomyolysis. Cureus. 2020;12(9):e10698.
PubMed PMC
22 Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Crossref PubMed
23 Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20(1):135.
Crossref PubMed PMC
24 Nance JR, Mammen AL. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve. 2015;51(6):793–810.
Crossref PubMed PMC
25 Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract. 2014;27(5):501–12.
Crossref PubMed
26 Paliperidone. In: Lexi-drugs online [database on Internet]. Lexicomp, Inc.; ©1978–2021 [updated 2021 Jun 13; cited 2021 Jun 16]. Available from: http://online.lexi.com. Subscription required to access content.
27 Fernández-Macho JG, Espárrago-Llorca G, Morales-Gómez GR, Guisado-Macías JA. Paliperidone-induced rhabdomyolysis: a case report. Actas Esp Psiquiatr. 2015;43(2):66–8.
PubMed
28 Jansman FG, Crommelin HA, van Hout FJ, Meulenbelt J. Rhabdomyolysis in clozapine overdose. Drug Saf Case Rep. 2015;2(1):9.
Crossref
29 Koren W, Koren E, Nacasch N, Ehrenfeld M, Gur H. Rhabdomyolysis associated with clozapine treatment in a patient with decreased calcium-dependent potassium permeability of cell membranes. Clin Neuropharmacol. 1998;21(4):262–4.
PubMed
30 Lam YWF. Rhabdomyolysis associated with clozapine and haloperidol. Brown Univ Psychopharmacol Update. 2020;31(12):2–3.
31 Tseng KC, Hwang TJ. Rhabdomyolysis following dose increase of clozapine and combination therapy with lithium. J Clin Psychopharmacol. 2009;29(4):398–9.
Crossref PubMed
32 Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230–5.
Crossref PubMed PMC
33 Morita S, Inokuchi S, Yamamoto R, Inoue S, Tamura K, Ohama S, et al. Risk factors for rhabdomyolysis in self-induced water intoxication (SIWI) patients. J Emerg Med. 2010;38(3):293–6.
Crossref
34 Kashiura M, Sugiyama K, Hamabe Y. Association between rapid serum sodium correction and rhabdomyolysis in water intoxication: a retrospective cohort study. J Intensive Care. 2017;5:37.
Crossref PubMed PMC
35 Cronin RE. Psychogenic polydipsia with hyponatremia: report of eleven cases. Am J Kidney Dis. 1987;9(5):410–6.
Crossref PubMed
36 Sidi Y, Gassner S, Sandbank U, Keren G, Pinkhas J. Water intoxication, hyperpyrexia and rhabdomyolysis in a patient with psychogenic polydipsia. N Y State J Med. 1984;84(9):462–4.
PubMed
37 Menashe G, Borer A, Gilad J, Horowitz J. Rhabdomyolysis after correction of severe hyponatremia. Am J Emerg Med. 2000;18(2):229–30.
Crossref PubMed
The authors thank Louise Mallet for presubmission comments on the manuscript.
*The curateur public du Québec provided written consent for publication of this case report. ( Return to Text )
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy , VOLUME 75 , NUMBER 2 , Spring 2022