Kevin Kwok, Caitlin Olatunbosun, Erin Ready, Osric Sin, Junine Toy, Alena Spears, Vickie Lau, Greg Bondy, Sarah StoneABSTRACT
Background
The population of people living with HIV is aging, and with aging come emergent comorbidities, including osteoporosis, for which screening and treatment are becoming increasingly important. Osteoporosis prevalence among those living with HIV is 3 times greater than among HIV-uninfected controls.
Objective
To assess and describe osteoporosis risk factors, screening, diagnosis, and treatment for people 50 years of age or older living with HIV and receiving care at a multidisciplinary HIV primary care clinic.
Methods
A retrospective chart review of people 50 years of age or older living with HIV was conducted at the John Ruedy Clinic in Vancouver, British Columbia, between June 1, 2016, and June 1, 2019. Patients who had had fewer than 2 yearly follow-up appointments were excluded.
Results
A total of 146 patients were included in the analysis; most were male (n = 134, 92%), and the median age was 55 years. Patients had a median of 3 osteoporosis risk factors (in addition to age and HIV infection), and 145 patients had at least 1 risk factor. All screening for osteoporosis was conducted by dual-energy X-ray absorptiometry (DXA). Thirty-nine (27%) of the patients were screened with DXA, 92 (63%) were not screened, and 15 (10%) already had a diagnosis of osteoporosis. The DXA screening identified osteoporosis in an additional 10 patients and osteopenia in 22 patients. Treatments for patients with osteoporosis included bisphosphonates (n = 15, 60%) and vitamin D or calcium (or both), without any other medications (n = 4, 16%). In the overall study population, 32 (22%) of the patients were taking calcium and 46 (32%) were taking vitamin D.
Conclusions
Many patients aged 50 years or older and receiving HIV care at the John Ruedy Clinic had or were at risk for osteoporosis. An opportunity exists to increase screening and treatment of these individuals. A multidisciplinary team may be crucial in achieving this goal.
KEYWORDS: HIV, osteoporosis, risk factors, screening, treatment
RÉSUMÉ
Contexte
La population des personnes vivant avec le VIH vieillit et, avec le vieillissement, des comorbidités émergent, dont l’ostéoporose, pour laquelle le dépistage et le traitement sont de plus en plus importants. La prévalence de l’ostéoporose chez les personnes vivant avec le VIH est 3 fois plus élevée que chez les témoins non infectés.
Objectif
Évaluer et décrire les facteurs de risque, le dépistage, le diagnostic et le traitement de l’ostéoporose chez les personnes d’au moins 50 ans vivant avec le VIH et qui reçoivent des soins dans une clinique pluridisciplinaire de soins primaires pour le VIH.
Méthodes
Un examen rétrospectif des dossiers des personnes d’au moins 50 ans vivant avec le VIH a été effectué à la clinique John Ruedy à Vancouver (Colombie-Britannique) entre le 1er juin 2016 et le 1er juin 2019. Les patients qui avaient eu moins de 2 rendez-vous de suivi annuels ont été exclus de l’étude.
Résultats
Au total, 146 patients ont été inclus dans l’analyse; la plupart étaient des hommes (n = 134, 92 %) et l’âge médian était de 55 ans. Les patients avaient une médiane de 3 facteurs de risque d’ostéoporose (en plus de l’âge et de l’infection par le VIH), et 145 patients avaient au moins 1 facteur de risque. Tous les dépistages de l’ostéoporose ont été réalisés par absorption biphotonique à rayons X (DXA). Trente-neuf patients (27 %) ont été dépistés par DXA, 92 (63 %) ne l’ont pas été et 15 (10 %) avaient déjà un diagnostic d’ostéoporose. Le dépistage par DXA a permis d’identifier l’ostéoporose chez 10 patients supplémentaires et l’ostéopénie chez 22 patients. Le traitement des patients atteints d’ostéoporose comprenait des bisphosphonates (n = 15, 60 %) et de la vitamine D ou du calcium (ou les deux) sans autre médicament (n = 4, 16 %). Dans la population générale de l’étude, 32 patients (22 %) prenaient du calcium et 46 (32 %) prenaient de la vitamine D.
Conclusions
De nombreux patients d’au moins 50 ans recevant des soins pour le VIH à la clinique John Ruedy présentaient un risque d’ostéoporose ou l’avaient déjà développée. Il est possible d’accroître leur dépistage et leur traitement, et une équipe multidisciplinaire peut être cruciale pour atteindre cet objectif.
MOTS CLÉS: VIH, ostéoporose, facteurs de risque, dépistage, traitement
The population of people living with HIV is aging, and the management of comorbidities is becoming an increasingly important part of their care. One significant consideration is bone mineral density (BMD) and osteoporosis. In the general population, osteoporosis accounts for 80% of fragility fractures in menopausal women over age 50 years. Among men over the age of 60 years, there is a 25% chance of osteoporotic fracture, and such fractures can lead to significant mortality, morbidity, and health care costs.1 People living with HIV have a higher risk of low BMD and fragility fractures than those without HIV infection. HIV is included as a risk factor for osteoporosis in the guidelines of the US National Osteoporosis Foundation (now known as the Bone Health and Osteoporosis Foundation).2 A meta-analysis of pooled prevalence data from the HIV population showed decreased BMD in 67% of patients and osteoporosis prevalence of 15% (3 times greater than that of HIV-uninfected controls).3
Lower BMD among people living with HIV can be explained by the prevalence of conventional risk factors, pathophysiological changes in HIV, and treatment with antiretroviral therapy (ART). Traditional risk factors for osteoporosis, such as low body weight and cigarette smoking, are more common among people living with HIV.4 In addition, the pro-inflammatory state of HIV affects bone formation and resorption.5 ART is associated with a 2% to 6% decrease in BMD during the first 2 years of treatment.6 Tenofovir disoproxil fumarate (TDF) and boosted protease inhibitors have been associated with decreased BMD, with TDF having the strongest association.7 Tenofovir alafenamide is a prodrug of tenofovir associated with significantly lower decrease in BMD than occurs with TDF8,9; it may be an attractive alternative for those with osteoporosis.10
Many factors can affect bone health, and a thorough assessment is warranted to screen for risk factors and to detect early decreases in BMD. Within the general population, individuals at high risk of osteoporotic fractures can be screened with a fracture risk assessment tool (FRAX) or dual-energy x-ray absorptiometry (DXA). Osteoporosis can be diagnosed on the basis of a history of fragility fracture or by measuring BMD with DXA. According to McComsey and others,7 the World Health Organization (WHO) classifies BMD as normal, osteopenia, or osteoporosis according to the number of standard deviations below the mean BMD for a healthy, young, sex- and ethnicity-matched reference population. Canadian guidelines recommend DXA for adults under age 50 if they have particular risk factors, including fragility fracture, prolonged use of glucocorticoids, or use of other high-risk medications.11 For adults over age 50, screening with DXA is indicated if any of the aforementioned risk factors are present, or the risk factors of smoking, high alcohol intake, or low body weight. All adults over age 65 should undergo BMD testing.11
The recommendations in published guidelines for osteoporosis screening in people living with HIV are comparable to those for the general population. The primary care guidelines of the BC Centre for Excellence in HIV/AIDS10 suggest screening for individuals 50 years of age or older, which is consistent with the Canadian osteoporosis guidelines.11 This differs from the approach offered by Brown and others,4 who recommended screening with FRAX for people living with HIV who are 40 to 49 years old. Unfortunately, there is no validated screening tool for use in patients living with HIV, and FRAX is known to underestimate the risk of fracture for this population.12–14 Brown and others4 recommended DXA for those with a FRAX score above 10%, as well as those with additional risk factors (men ≥ 50 years old, postmenopausal women, and those with a history of fragility fracture, long-term steroid use, or high risk of falls). Diagnosis of osteoporosis in people living with HIV follows the same criteria as those outlined by the WHO.
Treatment of osteoporosis in people living with HIV is the same as outlined in national guidelines for persons not infected with HIV. The foundation of bone health relies on adequate intake of calcium (1200 mg daily) and vitamin D (800–2000 IU daily), as well as lifestyle measures, including weight-bearing exercises and smoking cessation, both of which should be assessed for all patients. For patients with a history of fragility fracture or high fracture risk as indicated by FRAX score (> 20% risk of fracture in 10 years) or DXA (T-score ≤ −2.5), antiresorptive therapy should be considered.3,11,15 Bisphosphonates are considered first-line therapy because of their long-term efficacy and good safety profile. Denosumab is commonly used to treat osteoporosis in the general population, but there are few efficacy data, and this drug is associated with potential for increased risk of infection; as such, its use by people living with HIV has been limited.4,16 For patients with low BMD, a history of fragility fracture, or osteoporosis, an ART regimen that avoids TDF and protease inhibitors should be considered.13
The John Ruedy Clinic at St Paul’s Hospital in Vancouver, British Columbia, is a low-barrier multidisciplinary HIV primary care clinic. It hosts approximately 1300 active patients and includes primary care physicians, pharmacists, nurses, dieticians, and access to specialists such as endocrinologists, psychiatrists, and nephrologists. Although many of these patients have immediate urgent needs, screening and treatment for chronic diseases such as osteoporosis is becoming a significant part of their care, given the aging of the population. The purpose of this study was to assess risk factors for osteoporosis, as well as pertinent screening, diagnosis, and treatment, among HIV clinic patients at least 50 years of age.
This single-centre retrospective chart review was conducted at the John Ruedy Clinic in Vancouver. Electronic medical records were used to identify eligible patients. Participants were considered eligible if they were HIV-positive, 50 years of age or older as of June 1, 2016, and an active patient at the clinic during the study period of June 1, 2016, to June 1, 2019. Patients were excluded if they had had fewer than 2 yearly follow-up appointments with a clinic physician, as this low frequency would provide insufficient opportunity for thorough assessment. This study was approved by the local research ethics board, with a waiver of informed consent.
The planned sample size was 170 patients (147 patients plus 15% to account for attrition), calculated from the population of 582 clinic patients who were 50 years of age or older. The confidence interval was 95% with a margin of error of 7%. A random sample of clinic patients was drawn using Excel spreadsheet software (Microsoft Corporation).
All data were collected from the clinic’s electronic medical records by a single reviewer (K.K.), and data collection for 15% of the charts was audited by a second reviewer (C.O.). The collected data included patient history, medications, medical conditions, results of medical testing, specialist consults, and chart notes prepared by the multidisciplinary team. Data were also collected for osteoporosis risk factors, screening, diagnosis, treatment recommendations, and care team members involved. The risk factors were adapted, before the chart review began, from “A Tool for Preventing and Managing Bone Disease in HIV-Infected Adults”13 (factors are listed in Table 1). Any of the following qualified as screening: annual measurement of height and weight, FRAX scores, DXA scans, fall risk assessments, and laboratory assessments for differential diagnosis (e.g. thyroid-stimulating hormone, complete blood count). CAROC scores (Osteoporosis Canada’s 10-year fracture risk scores) were recorded if they appeared in the DXA scan report. The investigators calculated FRAX scores for all patients using the University of Sheffield calculator, with HIV included as a secondary cause.17
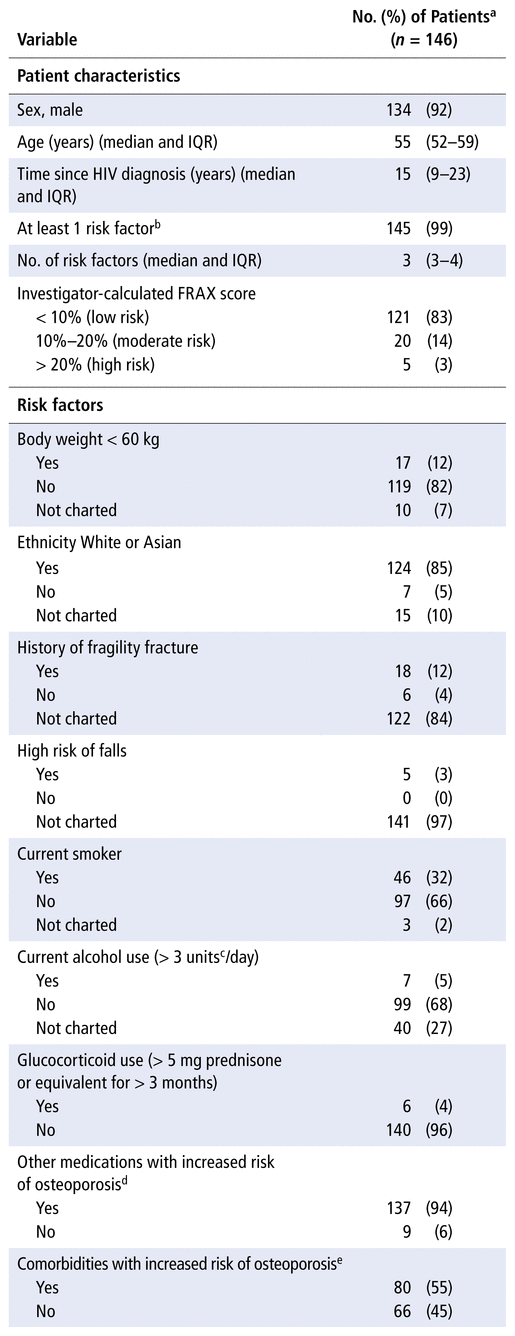
TABLE 1 Patient Characteristics and Risk Factors


The primary outcomes of this study were the numbers of patients screened for osteoporosis, given a diagnosis of osteoporosis, treated for osteoporosis with bisphosphonates, and given a recommendation for supplementation with calcium and vitamin D. The secondary outcomes were the number of patients with osteoporosis risk factors in addition to HIV and age, the specific types of risk factors, the types of screening done, the number of recommendations provided for bone health other than bisphosphonates and calcium/vitamin D, and the team members involved in the assessment of bone health.
Data were collected using Access database software (Microsoft Corporation) and analyzed using descriptive statistics in Excel spreadsheet software.
Of 170 charts screened, 146 charts met the inclusion criteria. Of those excluded, 21 patients joined the clinic after the beginning of the study period, 2 patients had fewer than 2 yearly visits with a clinic physician, and 1 patient was not seeing a clinic physician. The included patients were mostly male (92%), their median age was 55 years, and HIV had been diagnosed a median of 15 years previously.
Patients had a median of 3 osteoporosis risk factors (in addition to age and HIV), and 145 patients (99%) had at least 1 risk factor. Risk factors that would be accounted for in a FRAX score calculation (previous fracture, parental hip fracture, current smoker, glucocorticoid use, rheumatoid arthritis, alcohol use 3 or more units daily [where 1 unit = 8–10 g alcohol]) were present for 21 patients. Risk factors and their frequencies are listed in Table 1.
A total of 39 patients (27%) underwent screening for osteoporosis during the study period. All screening at the clinic involved DXA ordered by a general practitioner or specialist physician. There was no documentation of patients screened through assessment without DXA or by use of a FRAX score. Among those who were screened, median age was 56 years, the median number of risk factors was 4, and 29 (74%) had TDF in their regimen (defined here and subsequently as TDF use at any time during the study period). Among the 107 patients who did not undergo screening, the median age was 54 years, the median number of risk factors was 3, and 61 (57%) had TDF in their regimen. The full breakdown of screening results is shown in Figure 1.
|
| ||
|
FIGURE 1 Screening and diagnosis of osteoporosis. | ||
Overall, 25 patients in this study had osteoporosis (10 with prior diagnosis and 15 cases diagnosed during this study period). Among these patients with osteoporosis, the median age was 59 years and the median duration since HIV diagnosis was 18 years. Seventeen of these patients (68%) were receiving TDF. The investigator-calculated FRAX scores for patients with a diagnosis of osteoporosis were categorized as low risk (n = 13, 52%), moderate risk (n = 7, 28%), and high risk (n = 5, 20%). There were some slight differences in these characteristics for those who were screened and had normal BMD results. Among the 7 patients screened for osteoporosis who had normal BMD and the 13 patients with prior diagnosis of normal BMD, the median age was 54 years and the median duration since HIV diagnosis was 12 years. Nine (45%) of these 20 patients were receiving TDF. For the 7 patients screened for osteoporosis who had normal BMD, the investigator-calculated FRAX scores were categorized as low risk.
Among the 146 patients in our analysis, a total of 25 patients (17%) had osteoporosis at the end of the study period. Among patients with a diagnosis of osteoporosis (either previously or during this study period), 15 (60%) were treated with a bisphosphonate, 4 were treated with vitamin D or calcium supplementation only, and 6 were not given any treatment. Among patients not receiving treatment, there was no documentation of bisphosphonate contraindication. Denosumab treatment was not documented for any patients in this study. Twenty-two of the patients with osteoporosis were receiving an ART regimen associated with risk of decreased BMD: 5 were receiving a protease inhibitor without TDF, 5 were receiving TDF without a protease inhibitor, and 12 were receiving both TDF and a protease inhibitor concurrently. For 10 of the 17 patients (59%) who were receiving TDF, the TDF was changed to another ART for reasons of bone health.
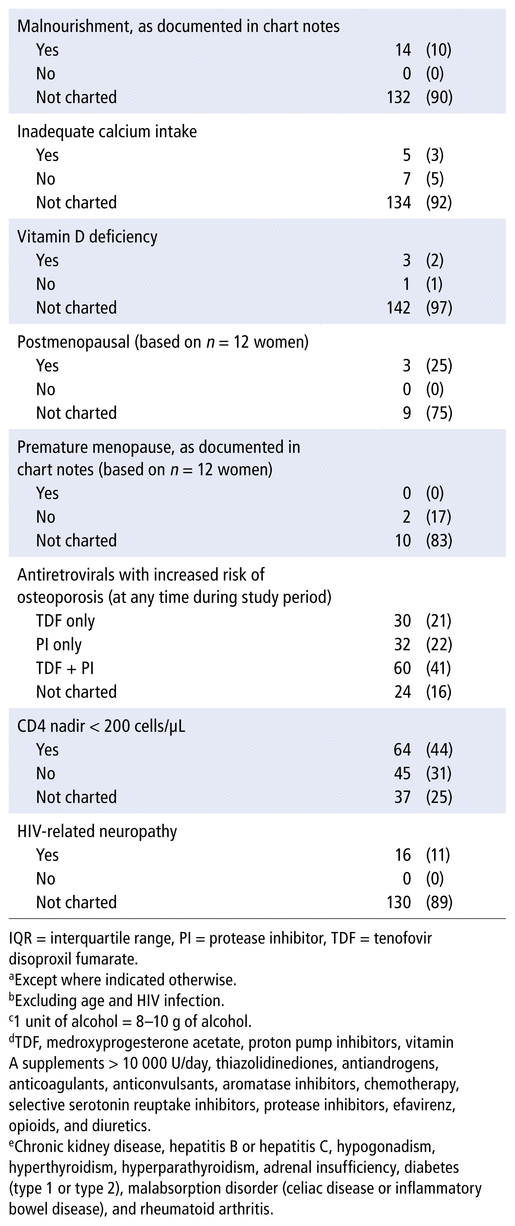
Various nutritional interventions were recommended for patients with and without osteoporosis: calcium supplementation for 32 patients (22%) and vitamin D supplementation for 46 patients (32%). TDF alone was discontinued or substituted for another ART in 13 patients (9% of the total sample) for reasons of bone health. Also for the purpose of bone health, 1 patient was switched off an ART regimen consisting of TDF and a protease inhibitor. None of the patients who were receiving an ART regimen that included a protease inhibitor without TDF had the protease inhibitor discontinued for reasons of bone health. The multidisciplinary team was involved in management of bone health for 57 patients (39%) with or without osteoporosis: pharmacists (n = 38), nurses (n = 21), and dieticians (n = 14). Additional results for interventions made are shown in Table 2.
TABLE 2 Treatments and Recommendations for Osteoporosis for All Patients
This study has showcased the high frequency of risk factors for osteoporosis among people living with HIV, has highlighted that screening is not routinely documented in many cases, and has shown that suitable treatment modalities may be underutilized. These problems may be common in primary care clinics that serve people living with HIV, as similar findings were reported in a study conducted in the United Kingdom.18 The investigators in that study found that within their sample of 20 people living with HIV who were at least 50 years of age, only 3 (15%) were assessed by DXA, and none had had a FRAX score calculated in the previous 3 years. Our study can serve as a prompt for local improvement in the management of osteoporosis and as a call for other clinics to evaluate their practices.
Osteoporosis is prevalent in the HIV population, and identifying and addressing risk factors for this condition are important, given data showing a higher prevalence of traditional risk factors among those with HIV.19 We looked at a comprehensive list of risk factors and found a median of 3 osteoporosis risk factors (in addition to age and HIV). These included risk factors, such as use of selective serotonin reuptake inhibitors, beyond those captured in risk calculators such as FRAX, which may partly explain why fracture risk is often underestimated by these risk calculators. In addition, there is a chance that risk factors were underdocumented, especially for women. For example, there was no documentation concerning menopause for 9 of the 12 women included in this study, despite evidence showing that early menopause can double the risk of fractures.20
Despite the presence of multiple risk factors, only 27% of the patients were screened for osteoporosis. DXA was the preferred screening tool for all of these patients. The FRAX scores calculated by investigators during the course of this study showed that 17% of patients were at moderate or high risk of fractures, which is on par with the literature,3 but there was high discordance among the scores. For example, for 52% of those in whom osteoporosis was diagnosed (previously or during this study) by DXA, the investigator-calculated FRAX score was “low risk”. Studies have shown that relying on FRAX as an independent risk assessment tool may lead to underdetection of patients who may be at risk for osteoporosis and who may be candidates for treatment,21,22 Applying the results of our study, it may be appropriate to recommend DXA for patients who, despite having a low FRAX score, may have numerous other risk factors for osteoporosis. We suspect that osteoporosis rates might have been higher than we observed, given the large number of patients in this study who were not screened.
For people living with HIV, treatment with bisphosphonates has been shown to significantly improve BMD while being well tolerated.23 However, bisphosphonates were being taken by only 11% of our population (15 patients with osteoporosis and 1 patient with osteopenia), despite 17% having a diagnosis of osteoporosis. There is a possibility that patients in our study were not offered bisphosphonates because of contraindications (e.g., renal insufficiency, cost concerns), but if so, there was no supporting documentation. Current guidelines recommend bisphosphonate for people living with HIV according to the same criteria as used for the general population, but this recommendation does not take into account the potential underestimation of fracture risk by FRAX and BMD in this population.24
In our study, only 22% of patients were receiving calcium supplementation, and only 32% were receiving vitamin D supplementation, despite the presence of numerous risk factors for osteoporosis, including 84% of patients receiving an ART regimen that included TDF or a protease inhibitor (or both). Calcium and vitamin D, known as crucial nutrients for optimal bone health, have been explored for their preventive role in bone health for patients who are taking ART regimens containing TDF, a medication that affects BMD. One study compared ART-naive HIV-infected patients who were taking vitamin D 4000 units daily and calcium carbonate 500 mg twice daily with patients who were taking placebo; the intervention group had a lesser decline in total hip BMD and lower markers for bone turnover over 48 weeks.25 There were no clear differences with regard to adverse events in the treatment and placebo groups. These results, albeit weak, suggest another possible intervention for preventing osteoporosis. These 2 supplements are relatively inexpensive, easy to take, and generally well tolerated. It may be appropriate to recommend vitamin D and calcium supplementation for all patients with HIV who have risk factors for osteoporosis.
Few patients in our study population were switched from TDF to another ART for reasons of bone health. Given the association of TDF and protease inhibitors with decreased BMD and fractures, people with osteopenia or osteoporosis who are taking these medications should have a discussion with their health care provider about alternative ART.4 Although it is common to modify regimens containing TDF or protease inhibitors and to consider alternatives,26,27 there are clinical scenarios in which a switch may not be possible. Data from ART switch studies are limited to surrogate markers such as DXA and bone turnover markers; there are currently no clinical data to suggest that discontinuing TDF (or other ART with negative bone effects) will reduce fracture risk over time.28 Additionally, there are many third-agent alternatives that can be used to replace protease inhibitors but fewer options for TDF. However, despite the conflicting evidence, this is still a potential avenue worthy of exploration. Alternatives to TDF include abacavir and tenofovir alafenamide, but both of these options have limitations. Abacavir may not be suitable for some patients because of resistance or the presence of the HLA-B*5701 allele marker for hypersensitivity.29 Tenofovir alafenamide is attractive because of its improved safety profile for kidney function and BMD with equivalent efficacy; however, its use may be limited by a variety of issues, including drug coverage, increases in serum lipids, weight gain, and limited data on long-term efficacy and safety.28,30–32 An additional strategy to optimize ART for bone health is to use a 2-drug regimen such as dolutegravir with lamivudine or dolutegravir with rilpivirine, both of which are available in Canada as coformulated, fixed-dose combination tablets. This option is limited to patients with virologic suppression, no history of virologic failure, no resistance to either agent in the 2-drug regimen, and no hepatitis B co-infection.33,34
Maximizing use of the multidisciplinary team can help to comprehensively address issues associated with osteoporosis. Studies have shown that involving allied health professionals, such as dieticians and nurses, in screening for chronic disease in primary care clinics can improve the identification of patients with modifiable comorbidities.18 Pharmacists can be instrumental in screening people who have or are at risk of major diseases, such as cardiovascular disease, certain cancers, and osteoporosis.35 This type of practice, specifically for osteoporosis, has been successfully implemented in community pharmacies.36 Although HIV teams traditionally focus on HIV and its manifestations, it is important for all team members to address the chronic diseases associated with HIV, given this population’s increasing age.
This study had several limitations. First, patients younger than 50 years of age were not included. These patients may have been underscreened or undertreated and might benefit from earlier intervention for bone health. Second, given the retrospective design, there is the possibility of underdocumentation of interventions such as screening or treatment, leading to inaccurate conclusions. Third, given the single-centre design, there may be limited generalizability to populations outside our clinic.
This study has highlighted an area for practice improvement in HIV care. Patients over age 50 at the John Ruedy Clinic had risk factors for osteoporosis that warranted screening, yet rates of screening were low. At the same time, rates of osteoporosis were high, and interventions were underutilized. An opportunity exists to improve care for these patients, by increasing screening with DXA, making proactive recommendations for lifestyle measures and intake of calcium and vitamin D, and selecting bone-friendly ART. Further study on the use of the multidisciplinary team to achieve this improvement in care is needed.
1 Nguyen T, Eisman J, Kelly P, Sambrook P. Risk factors for osteoporotic fractures in elderly men. Am J Epidemiol. 1996;144(3):255–63.
Crossref PubMed
2 Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–81.
Crossref PubMed PMC
3 Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006; 20(17):2165–74.
Crossref PubMed
4 Brown TT, Hoy J, Borderi M, Guaraldi G, Renjifo B, Vescini F, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis. 2015;60(8):1242–51.
Crossref PubMed PMC
5 Walker Harris V, Brown TT. Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. J Infect Dis. 2012;205(Suppl 3):S391–8.
Crossref PubMed PMC
6 Rey D, Treger M, Sibilia J, Priester M, Bernard-Henry C, Cheneau C, et al. Bone mineral density changes after 2 years of ARV treatment, compared to naive HIV-1-infected patients not on HAART. Infect Dis. 2015;47(2):88–95.
Crossref
7 McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51(8):937–46.
Crossref PubMed PMC
8 Mills A, Crofoot G, McDonald C, Shalit P, Flamm JA, Gathe J, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2015; 69(4):439–45.
Crossref PubMed
9 Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–15.
Crossref PubMed
10 Primary Care Guidelines Panel. Primary care guidelines for the management of HIV/AIDS in British Columbia. BC Centre for Excellence in HIV/AIDS; 2015 [cited 2019 Jul 4]. p. 1–80. Available from: http://www.cfenet.ubc.ca/sites/default/files/uploads/primary-care-guidelines/primary-care-guidelines_015-09-15.pdf
11 Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182(17):1864–73.
Crossref PubMed PMC
12 Saccomanno MF, Ammassari A. Bone disease in HIV infection. Clin Cases Miner Bone Metab. 2011;8(1):33–6.
PubMed PMC
13 Foisy M, Hughes C, Yuksel N. A tool for preventing and managing bone disease in HIV-infected adults (version 2). Development Team [self-published]; 2017 [cited 2019 Jun 17]. Available from: https://hivclinic.ca/downloads/BoneDiseasePCforWeb2017.pdf
14 Yin MT, Falutz J. How to predict the risk of fracture in HIV? Curr Opin HIV AIDS. 2016;11:261–7.
Crossref PubMed PMC
15 Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014; 58(1):e1–34.
Crossref
16 Chisati EM, Constantinou D, Lampiao F. Management of reduced bone mineral density in HIV: pharmacological challenges and the role of exercise. Front Physiol. 2018;9:1074.
Crossref PubMed PMC
17 FRAX fracture risk assessment tool: Calculation tool. Sheffield University; [cited 2019 Jul 4]. Available from: https://www.sheffield.ac.uk/FRAX/tool.aspx?country=9
18 Bradshaw H, Yoganathan K, Kneath S. Routine monitoring of adults with HIV-1 over 40. HIV Med. 2019;20(Suppl 5):46.
19 Thomsen MT, Wiegandt YL, Gelpi M, Knudsen AD, Fuchs A, Sigvardsen PE, et al. Prevalence of and risk factors for low bone mineral density assessed by quantitative computed tomography in people living with HIV and uninfected controls. J Acquir Immune Defic Syndr. 2020;83(2):165–72.
Crossref PubMed
20 Van der Voort DJM, Van der Weijer PHM, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int. 2003; 14(6):525–30.
Crossref PubMed
21 Tsai MS, Zhang JY, Sun HY, Liu WC, Wu PY, Yang CJ, et al. Performance of fracture risk assessment tool in HIV-positive male individuals aged ≥45 years on suppressive antiretroviral therapy. J Int AIDS Soc. 2019;22(8):e25383.
Crossref PubMed PMC
22 Vizcarra P, Gallego J, Vivancos MJ, Sifuentes WA, Llop M, Casado JL. Evaluation of the fracture risk assessment tool for determining bone disease and the impact of secondary causes of osteoporosis in people living with HIV. HIV Res Clin Pract. 2020;21(2–3):63–71.
Crossref PubMed
23 McComsey G, Kendall M, Tebas P, Swindells S, Hogg E, Alston-Smith B, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS. 2007;21(18):2473–82.
Crossref PubMed
24 Starup-Linde J, Rosendahl SB, Storgaard M, Langdahl B. Management of osteoporosis in patients living with HIV - a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2020;83(1):1–8.
Crossref
25 Overton ET, Chan ES, Brown TT, Tebas P, McComsey GA, Melbourne KM, et al. Vitamin D and calcium attenuate bone loss with antiretroviral therapy initiation: a randomized trial. Ann Intern Med. 2015; 162(12):815–24.
Crossref PubMed PMC
26 Pozniak A, Arribas JR, Gathe J, Gupta SK, Post FA, Bloch M, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr. 2016;71(5):530–7.
Crossref PMC
27 Mills A, Arribas JR, Andrade-Villanueva J, DiPerri G, Van Lunzen J, Koenig E, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52.
Crossref
28 Hill A, Hughes SL, Gotham D, Pozniak AL. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety [editorial]. J Virus Erad. 2018;4(2):72–9.
Crossref PubMed PMC
29 Ma JD, Lee KC, Kuo GM. HLA-B*5701 testing to predict abacavir hypersensitivity. PLoS Curr. 2010;2:RRN1203.
Crossref PubMed PMC
30 BC-CfE eligibility criteria for emtricitabine/tenofovir alafenamide (FTC/TAF; Descovy®). British Columbia Centre for Excellence in HIV/AIDS; 2019 [cited 2020 Jan 10]. Available from: http://bccfe.ca/sites/default/files/uploads/dear-doctor-letters/2019-11-26_ftc_taf_eligibility_criteria_bc-cfe.pdf
31 Gomez M, Seybold U, Roider J, Härter G, Bogner JR. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015–2017. Infection. 2019;47(1):95–102.
Crossref
32 Hill A, McCann K, Qavi A. Risks of metabolic syndrome, diabetes, and cardiovascular disease in ADVANCE trial [abstract]. Conference on Retroviruses and Opportunistic Infections; 202 Mar 8–11; Boston (MA).
33 Therapeutic guidelines: antiretroviral treatment of adult HIV infection. British Columbia Centre for Excellence in HIV/AIDS, Committee for Drug Evaluation and Therapy; 2019 [cited 2019 Jun 20]. Available from: http://bccfe.ca/sites/default/files/uploads/Guidelines/2020_06_30-BC-CfE_Adult_ARV_Therapeutic_Guidelines.pdf
34 Panel on Antiretroviral Guidelines for Adults and Adolescents. Management of the treatment-experienced patient: optimizing antiretroviral therapy in the setting of virologic suppression. In: Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. p. I-22–I-32. Department of Health and Human Services (US); 2019 [cited 2019 Jul 4]. Available from: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/optimizing-antiretroviral-therapy-setting-virologic-suppression?view=full
35 George J, Zairina E. The potential role of pharmacists in chronic disease screening. Int J Pharm Pract. 2016;24(1):3–5.
Crossref PubMed
36 Ayorinde AA, Porteous T, Sharma P. Screening for major diseases in community pharmacies: a systematic review. Int J Pharm Pract. 2013; 21(6):349–61.
Crossref PubMed
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 75, NUMBER 3, Summer 2022