Scott E Walker, Hanif Sachedina, Katia BicharABSTRACT
Background
Clozapine oral suspension is not commercially available in Canada but is required for administration to patients who cannot swallow intact tablets.
Objective
To evaluate the stability of 25 mg/mL and 50 mg/mL clozapine suspensions prepared in a 50:50 mixture of methylcellulose gel 1% and Oral Syrup (flavoured syrup vehicle, Medisca Pharmaceutique Inc) and stored in amber glycol-modified polyethylene terephthalate (PET-G) bottles over 120 days at 4°C and 25°C.
Methods
This study used a validated reverse-phase stability-indicating liquid chromatographic method capable of quantifying clozapine, 3 known degradation compounds, a known impurity, and an unknown compound. Three separate batches of 25 mg/mL and 50 mg/mL clozapine suspensions were prepared, divided into 100-mL aliquots, and stored in 120-mL PET-G bottles. Half of the bottles from each concentration were stored at room temperature (20°C to 25°C) and the other half were stored in the refrigerator (2°C to 8°C). On study days 0, 28, 60, 90, and 120, concentrations of clozapine, each of the 3 known clozapine degradation products, a known impurity, and an unknown compound were determined.
Results
When suspensions were stored in PET-G containers at room temperature or under refrigeration for 120 days, the concentration of clozapine remained above 95% of initial concentration, and the measured concentration of degradation products and impurities did not exceed the 0.5% limits set by regulatory authorities worldwide. The proportion of the initial concentration of clozapine remaining on day 120, based on fastest degradation rate with 95% confidence (1-sided), exceeded 92%, and the only degradation product found (clozapine lactam, 0.2%) and an unknown impurity (0.2%) also did not exceed allowable limits.
Conclusions
Compounded clozapine suspensions of 25 mg/mL and 50 mg/mL can be stored in amber PET-G containers for up to 120 days after preparation with storage at room temperature or under refrigeration.
KEYWORDS: clozapine, stability, suspension
RÉSUMÉ
Contexte
La clozapine en suspension orale n’est pas disponible sur le marché canadien, mais elle est nécessaire pour les patients qui ne peuvent l’avaler sous forme de comprimé intact.
Objectif
Évaluer la stabilité des suspensions de clozapine de 25 mg/mL et de 50 mg/mL, préparées dans un mélange 50:50 de gel méthylcellulose à 1 % et de Sirop Oral (véhicule de sirop aromatisé, MEDISCA) et conservées dans des flacons ambrés en polytéréphtalate d’éthylène modifié au glycol (PET-G) pendant 120 jours à des températures de 4°C et 25°C.
Méthode
Cette étude a utilisé une méthode validée par chromatographie liquide indicatrice de stabilité en phase inverse pouvant quantifier la clozapine, trois composés de dégradation connus, une impureté connue et un composé inconnu. Trois lots séparés de suspensions de clozapine de 25 mg/mL et de 50 mg/mL ont été préparés, divisés dans des aliquotes de 100-mL et stockés dans des flacons en PET-G de 120-mL. La moitié des flacons de chaque concentration a été conservée à température ambiante (de 20°C à 25°C), et l’autre moitié au réfrigérateur (de 2°C à 8°C). Aux jours 0, 28, 60, 90 et 120 de l’étude, on a déterminé les concentrations de clozapine, celles de chacun des trois produits de dégradation de la clozapine, celles d’une impureté connue et d’un complexe inconnu.
Résultats
Lorsque les suspensions étaient stockées dans des contenants en PET-G à température ambiante et réfrigérées pendant 120 jours, la concentration de clozapine demeurait au-dessus de 95 % de la concentration initiale; la concentration mesurée des produits de dégradation et des impuretés ne dépassait pas la limite de 0,5 % fixée par les autorités de règlementation mondiales. La proportion de concentration initiale de clozapine restante au 120e jour, sur la base du taux de dégradation le plus rapide avec un intervalle de confiance de 95 % (unilatéral), dépassait 92 %, et le seul produit de dégradation trouvé (clozapine lactam, 0,2 %) ainsi qu’une impureté inconnue (0,2 %) ne dépassaient pas non plus les limites autorisées.
Conclusions
Les suspensions de clozapine composées de 25 mg/mL et de 50 mg/mL peuvent être conservées dans des contenants ambrés PET-G jusqu’à 120 jours après leur préparation, soit à température ambiante, soit dans un réfrigérateur.
MOTS CLÉS: clozapine, stabilité, suspension
Clozapine suspension is not commercially available in Canada, yet there is a need for this suspension to be available for patients who may have dysphagia or require dose administration under observation. To date, only 1 study concerning the stability of clozapine suspensions has been published.1 In that study, 20 mg/mL suspensions of clozapine were prepared in 6 different suspending vehicles (Ora-Sweet and Ora-Plus suspending vehicles, a 1:1 mixture of Ora-Sweet and Ora-Plus, the suspending vehicle used by the Hospital for Sick Children [Toronto, Ontario], simple syrup, and a noncommercial vehicle known as Guy’s pediatric mixture), and their stability was evaluated. Each suspension was stored in amber plastic containers at 23°C and retained more than 95% of the initial concentration during 63 days of storage, regardless of the suspending vehicle.1 However, with commonly used maintenance doses of 300 to 600 but not exceeding 900 mg/day,2,3 and an average dosage of 200 mg bid, suspensions with concentration of 25 mg/mL or 50 mg/mL have been judged as more convenient. Furthermore, since prescriptions can be written for up to a 3-month supply, we wished to test the stability of such suspensions over a period of at least 3 months. However, no data exist at either concentration or for this storage period, and the formulations of suspending agents may have changed since the original study was published in 2005.
In pharmacy practice, the end points used to judge stability of a drug have changed over the past 50 years. In the 1970s, the last study day on which the observed concentration was greater than 90% of the initial concentration was deemed to be the expiry date. This criterion works where analytical error is exceptionally low or zero. However, because analytical variability always exists, the trend for the concentration to decline to a concentration of 90%, established by linear regression, came to be considered a more robust end point than the last day on which more than 90% remained.4 Although this end point is statistically stronger, on the day that linear regression indicates 90% will remain, there is a 50% chance that the proportion remaining is actually less than 90% of the initial concentration. Calculation of confidence intervals can reduce this uncertainty and may have been first proposed in 1991 by Chow and Shao,5 was supported by Carstensen and others6 in 1992, and was restated by Shao and Chow7 in 1994. Confidence intervals have been used in pharmacy practice stability studies only in the past 20 years. The lower 95% confidence limit constructed about the slope of the drug product of interest represents the fastest degradation rate. The intersection of this lower limit of the 95% confidence interval and 90% remaining is generally accepted as the beyond-use date (BUD). A 1-sided (lower-bound) 95% confidence interval increases the confidence that a pharmacist can have in compounded products, reducing to just 5% the chance that less than 90% will remain.
Degradation products of various medications have been identified and noted to be sensitive indicators of stability8; however, such degradation products are generally not commercially available, so pharmacy practitioners have not developed or used criteria based on degradation products to determine the BUD. In contrast, regulatory authorities, which do not regulate pharmacist-compounded products, define shelf life on the basis of degradation product limits and approve drugs on the basis of safety and efficacy. As part of their evaluation of safety, regulatory authorities worldwide, through the International Council on Harmonisation (ICH), have agreed to limit the amount or proportion of degradation products and impurities in pharmaceutical dosage forms.9,10 Lower thresholds may be set if the degradation product is unusually toxic. Manufacturers determine the shelf life and release each lot of product according to analysis demonstrating that degradation products and impurities do not exceed these limits. For clozapine, the measured amount of any 1 of 3 known degradation compounds (clozapine lactam, a bis-compound, and a desmethyl compound) or of a known impurity (N-methyl piperazine, also known as aminopiperazide) cannot exceed 0.5%. Furthermore, if the amount of an individual unknown impurity exceeds 0.2%, or the total amount of all degradation products, the known impurity, and the unknown compound exceeds 1%, the shelf life is deemed to have been reached.9,10 This approach can be followed only if practitioners have access to reference standards of the degradation products and an analytical method capable of separating and measuring each degradation product specifically. Since the identity of and reference standards for degradation products are generally not available to pharmacists early in a product’s life cycle, pharmacy practitioners have not routinely measured, reported, or evaluated BUDs using degradation products. Nevertheless, the concentrations of degradation products have been included in the current evaluation of clozapine stability as an additional measure of the potency, purity, and quality of the formulation.
To our knowledge, this is the first study in which a product compounded by pharmacy has been evaluated with the degradation testing methodology defined by regulatory bodies such as Health Canada and the US Food and Drug Administration, in addition to confidence interval analysis of the active pharmaceutical ingredient (clozapine). Although regulatory agencies have limited authority over compounding, concentrations of degradation products have been included as a measure of product purity, to provide compounding pharmacists with confidence in the stability of the formulation and the recommended BUD.
The objective of this study was to evaluate the stability of 25 mg/mL and 50 mg/mL oral suspensions of clozapine stored for up to 120 days in amber glycol-modified polyethylene terephthalate (PET-G) bottles at 4°C (2°C to 8°C) and room temperature (20°C to 25°C; described hereafter as 25°C) through measurement of concentrations of clozapine, known degradation products, and impurities. During the 120-day study period, suspensions were visually inspected for appearance, colour, the ability to be resuspended or caking, odour, and measured pH.
In the evaluation of stability, the intersection of the lower limit of the 95% confidence interval constructed about the clozapine concentration was used to estimate a BUD. As an assurance of safety, the 0.5% limit on each of 3 known clozapine degradation products and a known impurity was also used in establishing a safe storage period.
Before the stability study, physical studies were undertaken to determine the suitability of the suspension in various suspending vehicles, including mixtures of vehicles at various ratios. These studies evaluated commercially available methylcellulose gel 1% (product no. 3060, Medisca Pharmaceutique Inc), Oral Syrup flavoured syrup vehicle (product no. 2511, Medisca Pharmaceutique Inc), and a 50:50 mixture of these 2 products. These suspending vehicles were screened for their ability to form a well-dispersed suspension and maintain the initial pH (between 5.5 and 6.2), with limited foaming, particle aggregation, and caking. Samples were examined for changes in colour, any clumping, ease of resuspension, and pH (digital pH meter, Beckman) over 120 days storage at 25°C and 4°C.
The most acceptable suspending agent was the 50:50 mixture of methylcellulose gel 1% and the flavoured syrup vehicle, Oral Syrup. This mixture of suspending agents was used to compound the suspensions used in our evaluation of clozapine stability.
Reverse-phase stability-indicating liquid chromatographic methods with ultraviolet (UV) detection, either at 230 nm using a 31.5% acetonitrile and 68.5% phosphate buffer mobile phase1 or at 254 nm using a 40% acetonitrile and 60% water mobile phase,11 have been described previously. The previous investigators observed but did not identify degradation products.1,11 Skibiński and others12 forced the degradation of clozapine, identified 6 degradation products, determined their respective masses, and obtained fragmentation spectra by tandem mass spectrometry, which allowed structural identification. Two of the degradation products were observed following forced UV photodegradation, although they were not observed under typical photolysis conditions or during storage. The remaining 4 degradation products are now commercially available as individual standards or as a mixture,13 which can be useful for assessing the stability-indicating nature of a particular method.
The analytical method used for the current study was similar to the previously published methods, although here the degradation products were identified, separated, and quantified, which did not occur in the previous studies.1,11 This method is used by the manufacturer for product release in Canada and is stability-indicating. The reverse-phase liquid chromatographic method used a phosphate buffer and acetonitrile (80:20 v/v) solvent system to elute clozapine and its degradation products from the formulation. Clozapine eluted at 5.7 minutes, well separated from the 4 degradation products and an impurity, when the mobile phase was pumped at 1.0 mL/min through a 4.6 cm × 50 mm reverse-phase 3-μm LC-18 column (ACME Canadian Life Science; product no. ACMC 18-3-05046). Samples of 5 μL were introduced into the liquid chromatographic system using an auto injector (automatic liquid sampler, Hewlett-Packard). The column effluent was monitored with a photodiode array (diode array detector, Agilent) at 226 nm. A signal from the detector was integrated and recorded with a chromatography data system (OpenLAB, version A.01.05, Agilent).
The acceptance criteria of the validated analytical method require that the within-day residual standard deviation (RSD%) for triplicate injections of each standard be not more than 2%, the between-days RSD% not exceed 5%, and recovery (accuracy) not exceed an absolute deviation of 2%. This method was capable of separating clozapine from the 3 known clozapine degradation products (clozapine lactam, a bis-compound, desmethyl clozapine), the known impurity (aminopiperazide), and an unknown compound.
The separation and quantification of these compounds indicates that the method is stability-indicating and that it meets or exceeds published and accepted standards.14–16
Samples of the suspending vehicle mixture, with and without clozapine, were assayed to ensure that the vehicle did not interfere with the assay.
Once assurance of the specificity of the analytical method had been completed, the validation phase followed, during which accuracy and reproducibility of the standard curves were evaluated over a 5-day period, and system suitability criteria (theoretical plates, tailing, and retention time) were developed to ensure consistent chromatographic performance on each study day.17 On each validation day, 10 mg of clozapine (USP reference standard) was accurately weighed and dissolved in water to prepare standards. Then, 5-μL samples of each standard and a blank were chromatographed in duplicate to create the standard curve. The range of the calibration curve encompassed the diluted test concentration of clozapine samples.
Within-day and between-day errors were assessed by the coefficients of variation of the peak areas of standards.
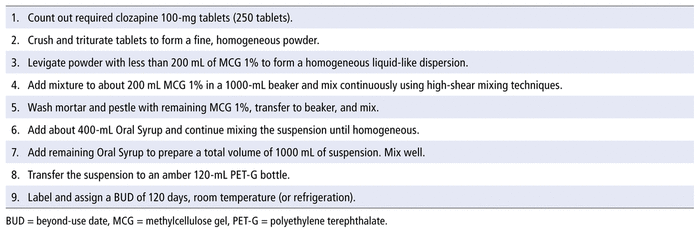
Clozapine suspensions (25 mg/mL and 50 mg/mL) were prepared with 100-mg clozapine tablets (Clozaril, HLS Therapeutics Inc, Etobicoke, Ontario) in a 50:50 mixture of methylcellulose gel 1% and Oral Syrup to prepare 3 separate 1-L batches of each concentration of suspension. The suspensions were prepared with 2 different lots of 100-mg clozapine tablets (HLS Therapeutics Inc; lot 18027, expiry August 2021, and lot 19050, expiry April 2022). The suspending vehicle was prepared with 2 different lots of methylcellulose gel 1% (Medisca Pharmaceutique Inc; lot 627663, expiry May 2020, and lot 628337, expiry July 2020) and 2 lots of flavoured Oral Syrup (Medisca Pharmaceutique Inc; lot 622919, expiry September 2021, and lot 622919A, expiry September 2021). The procedure for making the 25 mg/mL suspension is presented in Appendix 1. The suspensions were all well suspended, not thick or viscous, and were easy to pour.
APPENDIX 1 Compounding instructions to prepare 1 L of clozapine suspension 25 mg/mL
Two 100-mL aliquots of each batch were poured into 120-mL amber coloured PET-G plastic graduated bottles (product no. 7293, Medisca Pharmaceutique Inc), for a total of 6 bottles for each concentration. One bottle from each of the 3 batches of each concentration was placed in a refrigerator at 4°C (2°C to 8°C). The other bottle from each of the 3 batches of each concentration was stored at 25°C (i.e., 20°C to 25°C).
Each test container was manually shaken, and 5 mL was withdrawn from each separate bottle (using a pipette) following initial compounding on day 0 and then subsequently on days 28, 60, 90, and 120; the concentration of clozapine and degradation products was determined in these samples. From each of the well-mixed 25 mg/mL suspensions, a 5-mL sample (taken individually from each of the 3 separate containers at each temperature) was diluted to 100 mL with a 50:50 mixture of methanol and water diluent. The mixture was then vortexed and a 10-mL aliquot was further diluted to 100 mL with a 50:50 mixture of methanol and water. The solution was then filtered through a 0.7-μm glass fibre filter, and 5 μL of the supernatant was injected into the high-performance liquid chromatography system. A similar method of preparation was followed for the 50 mg/mL suspension, although the first dilution in equal parts of methanol and water was completed with 200 mL of solvent. Chromatographic analysis was completed using the validated liquid chromatographic system described above, with UV detection at 226 nm. The area under the clozapine peak at 226 nm was subjected to least-squares linear regression, and the actual clozapine concentration in each sample was determined by interpolation from the standard curve and correction by the dilution factor. The percent of declared content (25 mg/mL or 50 mg/mL) was reported in summary tables.
Within-day and between-day analytical error was assessed by replicate analysis of standards. After determining the coefficient of variation of the analytical method, a power calculation indicated that duplicate injection could distinguish between concentrations that differed by at least 10% within each individual container.18,19 During the study, analytical error was assessed by replicate analysis of study samples. The mean and coefficient of variation were calculated for duplicate analyses from each of the 3 different bottles on each study day. These results are reported in summary tables.
The percent remaining was analyzed by linear regression, and a 95% confidence interval (1-sided, lower-bound) was constructed around the slope of percent remaining versus study day. The lower limit of this confidence interval represents the fastest degradation rate with 95% confidence (1-sided), and the time to achieve 90% remaining using this fastest degradation rate was calculated. Concentrations were considered within acceptable limits if the following 2 conditions were met: first, the measured clozapine concentration on that study day was greater than 90% of the initial (day zero) concentration, and second, the concentration on that day, estimated using the fastest degradation rate with 95% confidence, also exceeded 90% of the initial (day zero) concentration.
As an additional evaluation of suspension stability and purity, the measured amount of any 1 of 3 known degradation compounds (clozapine lactam, a bis-compound, and a desmethyl compound) or of a known impurity (N-methyl piperazine, known as aminopiperazide) cannot exceed 0.5%. Furthermore, if the amount of an unknown compound exceeds 0.2%, or the total amount of all degradation products, the known impurity, and the unknown compound exceeds 1%, the shelf life would be judged to have been reached.9,10 A 2003 Health Canada guidance document10 recommended application of a 1-sided 95% confidence limit; however, given that clozapine was commercially available more than 20 years before adoption of this guidance, the stability and lot acceptance criteria used by the pharmaceutical industry have not changed and do not use the confidence interval method. To replicate current pharmaceutical standards, the current study applied the “not-more-than” limits for each degradation product (0.5%), impurities (0.2%), and total degradation and impurities (1%), rather than limits based on a confidence interval. Although regulatory agencies have no authority in compounding, the inclusion of degradation compounds when evaluating the stability of these formulations provides additional confidence in product purity, as well as confidence in the recommended BUD.
The physical study of clozapine suspensions demonstrated that both concentrations of the suspension in a 50:50 mixture of methylcellulose gel 1% and flavoured Oral Syrup remained an opaque, yellow, milky suspension for the 120-day storage period at both temperatures. During the 120-day physical study period, some separation did occur, but no caking or clumping was visually evident, and all suspensions were easily redispersed with shaking. The pH of the suspensions stored at both 4°C and 25°C in suspending vehicle ranged between 5.60 and 5.97 for the 25 mg/mL preparations and between 5.79 and 6.05 for the 50 mg/mL suspensions for the duration of the 120-day study period.
The separation and detection of clozapine in the presence of degradation compounds and impurities must be demonstrated before the method can be considered stability-indicating. Clozapine eluted at 5.7 minutes, and the degradation products did not interfere with clozapine quantification (Figure 1). Furthermore, each of the degradation products was well separated from clozapine and the other degradation products, and each could be measured specifically. The suspending vehicle did not interfere with measurement of clozapine or any of the degradation products.
|
|
||
|
FIGURE 1 Chromatograms of clozapine. Panel A represents desmethyl clozapine at concentration 12.5 μg/mL (labelled “D” and eluting at 2.1 minutes), clozapine lactam (labelled “L” and eluting at 2.7 minutes), the aminopiperazide (labelled “A” and eluting at 3.2 minutes), and an unknown compound (labelled “C” and eluting at 9.2 minutes), with 50 mg/mL clozapine (eluting at 5.7 minutes) in the suspending vehicle. Panel B represents the 50 mg/mL clozapine suspension on day 0, when no degradation products are present. Panel C represents the same 50 mg/mL suspension after 120 days storage at room temperature. Small amounts of clozapine lactam (labelled “L”) were detected at 2.7 minutes, representing concentrations of about 0.1%. |
||
As a result of the chromatographic separation of the degradation products from clozapine and the lack of interference of the suspending agent with clozapine and the degradation products, it was concluded that this analytical method was stability-indicating.
During the study period, within-day analytical variability of the study samples averaged 2.13%, and between-day analytical reproducibility (as measured by the standard deviation of regression, Sy.x) averaged 2.05%.
The percent remaining of the initial clozapine concentration as observed on each day during the study period is presented in Table 1. The concentration of clozapine in the suspending vehicle in all study samples (both concentrations) remained at or above 95.1% of the initial concentration when stored in amber PET-G bottles at both storage temperatures for 120 days. The 1-sided 95% confidence limits showed that the lowest percent remaining of clozapine exceeded 92% on study day 120 for all combinations of concentration and storage temperature. Analysis of variance detected no differences in percent remaining in the clozapine suspensions due to study day (p = 0.26), temperature (p = 0.16), or concentration (p = 0.08). Multiple linear regression also detected no differences in percent remaining due to study day (p = 0.34), temperature (p = 0.17), or concentration (p = 0.09).
TABLE 1 Percent of Clozapine Remaining on Each Study Day and Calculation of Time to Achieve 90% Remaining (T90) with 95% Confidence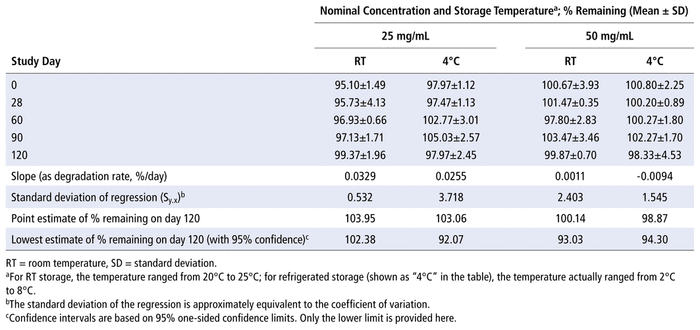
Two of the known clozapine degradation products (a bis-compound and the desmethyl clozapine), as well as the known impurity (aminopiperazide), were not detected in any sample during the 120-day study period. The other known degradation product, clozapine lactam, was detected in some samples (Table 2); however, the limit of 0.5% was not reached after 120 days of storage at either temperature or concentration. The unknown compound was also detected in some samples (Table 3); the observed maximum of 0.2% was reached on days 90 and 120 in the 25 mg/mL suspension stored at room temperature. Table 4 presents combined data for impurities and degradation products. None of the degradation products or impurities were detected at concentrations exceeding their specific limits during the 120-day study period.
TABLE 2 Clozapine Lactama Observed, by Study Day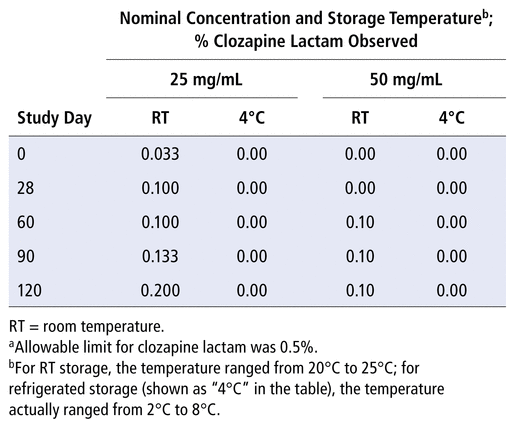
TABLE 3 Unknown Clozapine Impuritya Observed, by Study Day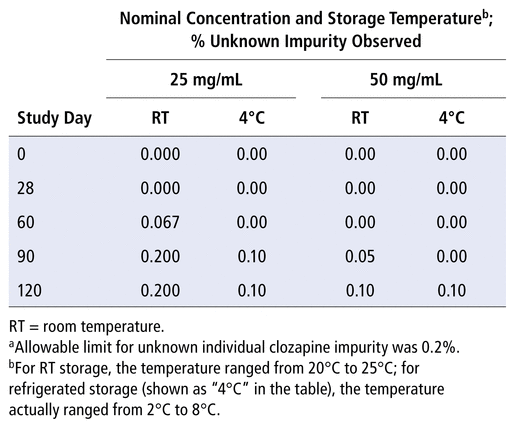
TABLE 4 Total Degradation Products and Impuritiesa Observed, by Study Day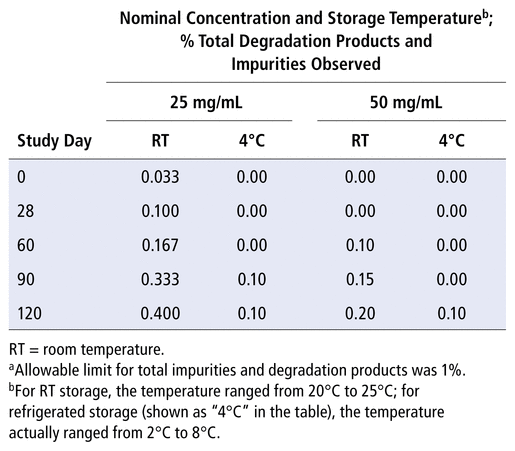
This study has demonstrated the physical and chemical stability of 25 mg/mL and 50 mg/mL clozapine suspensions stored in amber PET-G bottles for 120 days. The measured concentration of clozapine remained greater than 95% for the entire 120-day study period, and no degradation product or impurity was measured above its allowable limit. Regression analysis also demonstrated that the concentration of clozapine was likely to remain above 92.1% (with 95% confidence) for the 120-day study period. This analysis supports a BUD of 120 days for both concentrations at both temperatures. While it is recognized that regulatory agencies have limited authority over compounding, the inclusion of pharmaceutical regulatory standards for the concentration of degradation products provide an assurance of product purity and should increase compounding pharmacists’ confidence in the recommended BUD.
The clozapine results obtained in this study are very similar to the results reported by Walker and others,1 although the previous publication did not measure or report concentrations of degradation products, and the longer duration of this study allowed a longer BUD to be reported. Re-analysis of the data of Walker and others1 by a method similar to the one used in the current study estimated that the amount remaining on day 61 ranged from 91.9% to 97.9%, for concentrations of 20 mg/mL in a variety of suspending agents stored in amber polyethylene bottles. Comparable values in the current study on day 61 ranged from 95.97% to 101.21%. Both studies used the Clozaril brand of clozapine tablets.
In the evaluation of methods for determining stability, assessment based solely on the observed concentration or percent remaining at the end of the study period does not take into account analytical variability and error, whereas the confidence interval approach5–7,20 does account for this variability and presents a conclusion that more conservatively estimates the time to reach 90% remaining. For example, although the measured concentration of clozapine remained greater than 95% for the entire 120-day study period of the current study, confidence interval analysis predicts a less than 5% chance that the concentration of clozapine will be below 92% on the 120th day of storage. Stability studies are conducted in completely controlled environments, yet in real life, suspensions will be removed from the fridge on a daily basis and may be exposed to temperatures above 25°C. The use of confidence intervals yields a more conservative conclusion, reducing the possibility that a product with lower-than-desired potency is administered to patients.
A power calculation using the mean square error from the analysis of variance indicated that this study had the ability to detect a difference in concentration of more than 6%. Given that all of the observed differences due to temperature or container were less than 2%, none of these differences were statistically significant.
Assurance of the specificity of the analytical method is also very important. The separation and detection of intact drug in the presence of degradation compounds must be demonstrated before the method can be considered stability-indicating. Since all known degradation products and impurities were measured specifically and their concentrations estimated, the method was judged to be stability-indicating. Furthermore, the accuracy and reproducibility of the analytical method on each study day during the stability study provides the required confidence in the assay methodology.
The stability observed with these suspensions cannot be extrapolated to other suspending vehicles or formulations compounded with other clozapine tablet formulations. This report and the previous publication1 both used the same brand of clozapine product, Clozaril. Therefore, although we have no evidence that any differences in excipients that might exist between formulations would or would not affect the stability of suspensions, we cannot extrapolate these data to other clozapine tablet formulations.
Clozapine, as 25 mg/mL and 50 mg/mL suspensions, stored in amber PET-G bottles at 4°C or 25°C for 120 days retained more than 95% of the measured initial concentration, and no degradation product or impurity exceeded the allowable limit during the entire storage period. This study also estimated a less than 5% chance that the clozapine concentration would be below 92% of the initial concentration after 120 days of storage at either temperature. Clozapine suspensions of 25 mg/mL and 50 mg/mL can therefore be stored for up to 120 days after preparation, maintaining the desired potency and purity.
1 Walker SE, Baker D, Law S. Stability of clozapine stored in oral suspension vehicles at room temperature. Can J Hosp Pharm. 2005;58(5):279–84.
2 Clozaril [product monograph]. HLS Therapeutics Inc; 2019 Jan 4.
3 Clozaril [product monograph]. Novartis Pharmaceuticals Canada Inc; 2015 Aug 26.
4 Walker SE, Paton TW, Fabian TM, Liu CC, Coates PE. Stability and sterility of cimetidine admixtures frozen in minibags. Am J Hosp Pharm. 1981;38(6):881–3.
PubMed
5 Chow SC, Shao J. Estimating drug shelf-life with random batches. Biometrics. 1991;47(3):1071–9.
Crossref PubMed
6 Carstensen JT, Franchini M, Ertel K. Statistical approaches to stability protocol. J Pharm Sci. 1992;81(3):303–8.
Crossref PubMed
7 Shao J, Chow SC. Statistical inference in stability analysis. Biometrics. 1994;50(3):753–63.
Crossref
8 Walker SE, Lau DWC, DeAngelis C, Iazzetta J, Coons C. Epirubicin stability in syringes and glass vials and evaluation of chemical contamination. Can J Hosp Pharm. 1990;43(6):265–72.
9 Guidance for industry. Impurities in new drug products: ICH Topic Q3B(R) [guidance document]. Health Canada, Health Products and Food Branch; 2003 Sep 25 [cited 2020 May 21]. Accessed from: http://www.hc-sc.gc.ca/hpfb-dgpsa/tpd-dpt/; now available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/international-conference-harmonisation/quality/industry-impurities-new-drug-products-topic.html
10 Guidance for industry. Stability testing of new drug substances and products: ICH Topic Q1A(R2) [guidance document]. Health Canada, Health Products and Food Branch; 2003 Sep 25 [cited 2020 Jun 1]. Accessed from: http://www.hc-sc.gc.ca/hpfb-dgpsa/tpd-dpt/; now available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/international-conference-harmonisation/quality/stability-testing-new-drug-substances-products-topic.html
11 Hasan NY, Elkawy MA, Elzeany BE, Wagieh NE. Stability indicating methods for the determination of clozapine. J Pharm Biomed Anal. 2002;30(1):35–47.
Crossref PubMed
12 Skibiński R, Trawiński J, Komsta Ł, Bajda K. Characterization of forced degradation products of clozapine by LC-DAD/ESI-Q-TOF. J Pharm Biomed Anal. 2016;131:272–80.
Crossref
13 USP reference standards: Clozapine resolution mixture (30 mg). USP; [cited 2020 May 26]. Available for purchase from: http://store.usp.org/OA_HTML/ibeCCtpItmDspRte.jsp?item=33562
14 Trissel LA. Avoiding common flaws in stability and compatibility studies of injectable drugs. Am J Hosp Pharm. 1983;40(1):159–60.
15 Trissel LA, Flora KP. Stability studies: five years later. Am J Hosp Pharm. 1988;45(7):1569–71.
PubMed
16 Policy for publication of chemical stability study manuscripts. Can J Hosp Pharm. 1990;43(1):3–4.
17 Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, et al. Analytical methods validation: bioavailability, bioequivalence, and pharmacokinetic studies. J Pharm Sci. 1992;81(3):309–12.
Crossref
18 Frieman JA, Chalmers TC, Smith H Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 “negative” trials. N Engl J Med. 1978;299(13):690–4.
Crossref
19 Stolley PD, Strom BL. Sample size calculations for clinical pharmacology studies. Clin Pharmacol Ther. 1986;39(5):489–90.
Crossref PubMed
20 Magari RT. Uncertainty of measurement and error in stability studies. J Pharm Biomed Anal. 2007;45(1):171–5.
Crossref PubMed
Competing interests: None declared (other than employment as indicated above and in the Funding paragraph below). ( Return to Text )
Funding: This study was funded by HLS Therapeutics Inc (manufacturer and distributor of Clozaril) through a grant to Scott Walker. Hanif Sachedina is an employee of HLS Therapeutics. Katia Bichar was, at the time of the study, an employee of Medisca Pharmaceutique Inc, which manufactures the suspending vehicles used in the study. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 74, NUMBER 3, Summer 2021