Marie-Pier Roy, Frédéric Calon, David Simonyan, Luc BergeronABSTRACT
Background
Neutropenia is an adverse effect associated with the use of several antibiotics, including piperacillin–tazobactam (P/T). Previous findings have suggested that the risk of neutropenia in children is significantly higher with P/T than with ticarcillin–clavulanate.
Objectives
To compare the risk of neutropenia associated with P/T and with cefazolin in an adult population and to describe the characteristics of neutropenia episodes observed.
Methods
This descriptive retrospective study involved patients aged 18 years or older who received a minimum of 10 days of treatment with P/T or cefazolin between January 2009 and December 2013. Patients who experienced neutropenia (absolute neutrophil count < 1.5 × 109/L) were compared, using univariate and multivariate logistic regression models, between those who received P/T and those who received cefazolin.
Results
A total of 207 patients were included (104 who received P/T and 103 who received cefazolin). Ten episodes of neutropenia were observed, 5 with each antibiotic (4.8% and 4.9%, respectively; odds ratio 0.99, 95% confidence interval 0.278–3.527). The mean cumulative dose of piperacillin was 290.4 g among patients who experienced neutropenia and 247.0 g among all patients treated with P/T, and the mean treatment duration was 24.0 days and 21.0 days, respectively. The average time before the onset of neutropenia was slightly longer with P/T than with cefazolin (22.0 versus 17.2 days, p = 0.38).
Conclusions
Although these results require confirmation in a larger clinical trial (to lessen possible attribution bias), the risk of neutropenia appeared to be similar between P/T and cefazolin.
KEYWORDS: neutropenia, piperacillin, tazobactam, cefazolin
RÉSUMÉ
Contexte
La neutropénie est un effet indésirable associé à l’utilisation de plusieurs antibiotiques, dont la pipéracilline-tazobactam (P/T). Des données récentes indiquent que le risque de neutropénie chez les enfants est significativement plus élevé avec la P/T qu’avec l’association ticarcilline-clavulanate.
Objectifs
Comparer le risque de neutropénie associé à la P/T et à la céfazoline chez une population adulte et décrire les caractéristiques des épisodes de neutropénie observés.
Méthodes
Cette étude rétrospective descriptive impliquait des patients âgés d’au moins 18 ans ayant reçu un traitement d’au moins 10 jours par P/T ou céfazoline entre janvier 2009 et décembre 2013. Les patients ayant présenté une neutropénie (nombre absolu de neutrophiles < 1,5 × 109/L) ont été comparés, à l’aide de modèles de régression logistique univariée et multivariée, entre ceux qui ont reçu de la P/T et ceux qui ont reçu de la céfazoline.
Résultats
Au total, 207 patients ont été inclus (104 ayant reçu de la P/T et 103 ayant reçu de la céfazoline). Dix épisodes de neutropénie ont été observés, 5 avec chaque antibiotique (4,8 % et 4,9 %, respectivement; rapport des cotes 0,99; intervalle de confiance à 95 % 0,278–3,527). La dose cumulée moyenne de pipéracilline était de 290,4 g chez les patients ayant présenté une neutropénie et de 247,0 g chez tous les patients traités par P/T. La durée moyenne du traitement était de 24,0 jours et 21,0 jours, respectivement. Le délai moyen avant l’apparition de la neutropénie était légèrement plus long avec la P/T qu’avec la céfazoline (22,0 contre 17,2 jours, p = 0,38).
Conclusions
Bien que ces résultats nécessitent une confirmation dans un essai clinique de plus grande envergure (afin de réduire d’éventuels biais d’attribution), le risque de neutropénie semble être similaire chez les personnes ayant reçu de la P/T et ceux ayant reçu de la céfazoline.
MOTS CLÉS: neutropénie, pipéracilline-tazobactam, céfazoline
Hematologic toxic effects are well-known side effects of several drugs, including multiple classes of antimicrobials. β-Lactam antibiotics are particularly implicated and are among the most commonly used antimicrobials known to cause agranulocytosis.1,2 The pathophysiology of β-lactam–associated neutropenia remains poorly defined and only a few case reports and literature reviews have been published on the subject; notably, no randomized studies regarding the risk of neutropenia secondary to piperacillin–tazobactam (P/T) relative to other antibiotics have been published.3–6 Antibiotic-associated neutropenia is characterized by a decline, either sudden or gradual, in circulating neutrophils. There is no consensual definition for drug-induced neutropenia, but it has been previously defined as an absolute neutrophil count below 1.0 or 2.0 × 109/L for β-lactam antibiotics.6–9 Among other factors, increasing cumulative exposure to β-lactams has been linked to increases in the occurrence of neutropenia.3,6
An initial study conducted in our centre in 2012 established that there was a significantly higher risk of neutropenia among children who received P/T than among those who received ticarcillin–clavulanate.10 We wanted to confirm whether the higher risk of P/T-associated neutropenia was also present for adults. When we started the current study (with data collection beginning in 2009), ticarcillin–clavulanate was unavailable (on long-term back order). Since then, in 2015, the sole manufacturer of ticarcillin–clavulanate ceased production in North America. As such, for the adult study, we could not use the same comparator drug as was used in the pediatric study.10 In terms of a substitute comparator, few β-lactam antibiotics are used on a regular basis, for relatively long periods, at daily doses comparable to those of piperacillin. Cloxacillin would be one example, as it is typically used in amounts between 8 and 12 g daily; however, it was seldom used in our centre. We therefore selected cefazolin, a parenteral β-lactam that is often used on a long-term basis, as the comparator, although the usual daily doses were predictably lower with cefazolin (6 g/day) than with P/T (12 g/day).
The primary objective of this study was to compare the proportions of cases of neutropenia observed during treatment with P/T or cefazolin in adult patients. The secondary objectives were to compare the following variables in the 2 groups: duration of antibiotic therapy before occurrence of neutropenia, cumulative dose of each antibiotic, presence of confounding variables (age, sex, concurrent drug use), duration of neutropenia, and neutrophil recovery time.
This retrospective cohort study was conducted in a university teaching medical centre in Quebec City, Quebec. Cefazolin was chosen as the comparator drug for practical reasons, as outlined in the Introduction. Like P/T, this drug is widely used in practice for infections requiring prolonged courses of treatment in adults.
The medical records of patients 18 years of age or older who had received at least 10 days of treatment with P/T or cefazolin between January 2, 2009, and December 3, 2013, were reviewed. The minimum of 10 days of exposure was selected because the time to onset of neutropenia associated with β-lactam antibiotics was previously estimated at 10–15 days.3,9–11 Potential participants were identified through a list of patients who received P/T or cefazolin, generated by the hospital pharmacy software. Patients were excluded if other antimicrobials had been administered in the week preceding prescription of P/T or cefazolin. Also excluded were patients with immunosuppression, those undergoing treatment for active malignancy (according to the list of antineoplastic drugs shown in Appendix 1, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/209), HIV infection, neutropenia at the start of treatment with P/T or cefazolin, or congenital abnormalities associated with the development of neutropenia. Patients treated concomitantly with drugs known to cause neutropenia (see Appendix 2, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/209), such as carbamazepine or antithyroid drugs, were not excluded, but the concomitant use of these drugs was taken into account in the analyses.2,11,12
Episodes of neutropenia were defined by the first value of absolute neutrophil count less than 1.5 × 109/L observed after initiation of P/T or cefazolin until the end of the studied episode of care. The time to recovery was defined by the date when absolute neutrophil count greater than 1.5 × 109/L was first reported following the original decline.
Quantitative variables are reported as means with standard deviations (SDs) or medians with interquartile ranges and ranges, and qualitative variables are reported as frequencies with percentages. Bivariate analyses were performed using Wilcoxon Mann–Whitney tests, Student t tests after normality verification, and χ2 or Fisher exact tests as appropriate. Crude and adjusted odds ratios (ORs) were estimated by univariate and multivariate logistic regression models. The goodness of fit of the logistic models was checked by the Hosmer and Lemeshow test. All statistical analyses were performed using SPSS 24 statistics software (IBM Corporation) and SAS 9.4 statistics software (SAS Institute) with 2-sided significance level set at p < 0.05.
This study was approved by the clinical research ethics review board of the CHU de Québec – Université Laval (approval number GU14-166 AQUEM 2013-11).
A total of 583 medical records were identified in which P/T or cefazolin was documented as the first antibiotic administered. A total of 207 records met the inclusion criteria and were included in the analysis. The primary reasons for exclusion of the remaining 376 records were treatment duration less than 10 days and unavailability of the medical record at the time of data collection (n = 237, 63.0%), administration of another antibiotic within the 7 days preceding the prescription of P/T or cefazolin (n = 102, 27.1%), use of immunosuppressive drugs (n = 16, 4.3%; see Appendix 3, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/209, for a list of the immunosuppressive drugs used for these exclusions), and neoplasia or active chemotherapy (n = 21, 5.6%).
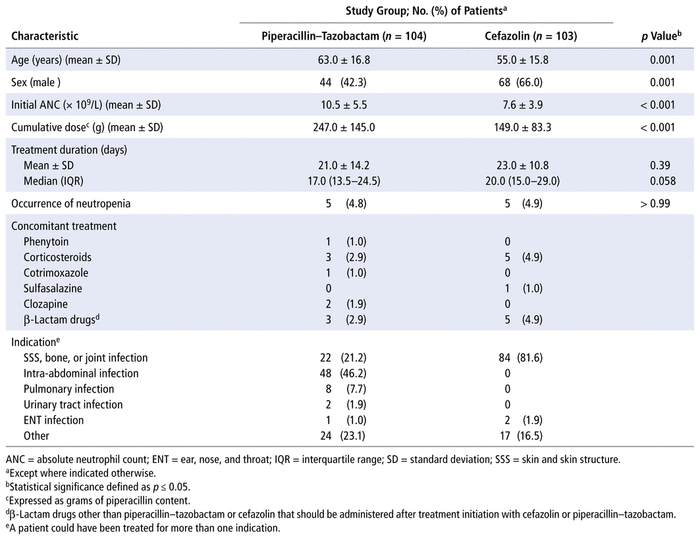
Among the 207 patients who met the inclusion criteria, 104 (50.2%) were in the P/T group and 103 (49.8%) in the cefazolin group. Table 1 summarizes patients’ characteristics according to the antibiotic received. Patients in the P/T group were older and there was a lower proportion of men relative to the cefazolin group (p = 0.001 for both). In addition, patients in the P/T group had, on average, a higher baseline neutrophil count (10.5 × 109/L versus 7.6 × 109/L, respectively; p < 0.001) and shorter duration of treatment (median 17 versus 20 days, respectively; p = 0.058). The mean cumulative dose in the P/T group was 247.0 (SD 145.0) g. Cefazolin was given primarily for skin and skin structure infections and for bone and joint infections (n = 84/103, 81.6%). P/T was given for a greater variety of indications (Table 1).
TABLE 1 Patient Characteristics

Ten episodes of neutropenia were observed, 5 in each group (4.8% in the P/T group, 4.9% in the cefazolin group). The unadjusted logistic regression model showed no difference in the risk of neutropenia, whether patients received P/T or cefazolin (OR 0.99, 95% confidence interval 0.278–3.527; p = 0.99). This difference remained statistically nonsignificant after adjustment for age, sex, baseline neutrophil count, and presence of concomitant treatments. However, the 10 patients who experienced neutropenia were younger than those without neutropenia (mean age 49 [SD 14] years, range 33–74 years, versus 60 [SD 17] years, range 21–94 years; p = 0.042).
Table 2 summarizes the characteristics of the patients who experienced neutropenia. The neutropenia occurred in the P/T group after a mean cumulative dose of 290.4 g, which was slightly higher than the average dose administered in this group as a whole (247.0 g), but the difference was not statistically significant. The mean cumulative dose recorded for cefazolin among patients who experienced neutropenia was 145.2 g, less than the mean cumulative dose of P/T for patients with neutropenia (290.4 g), but similar to the mean dose within the cefazolin group overall (149.0 g).
TABLE 2 Characteristics of Episodes of Neutropenia (n = 10 Patients)
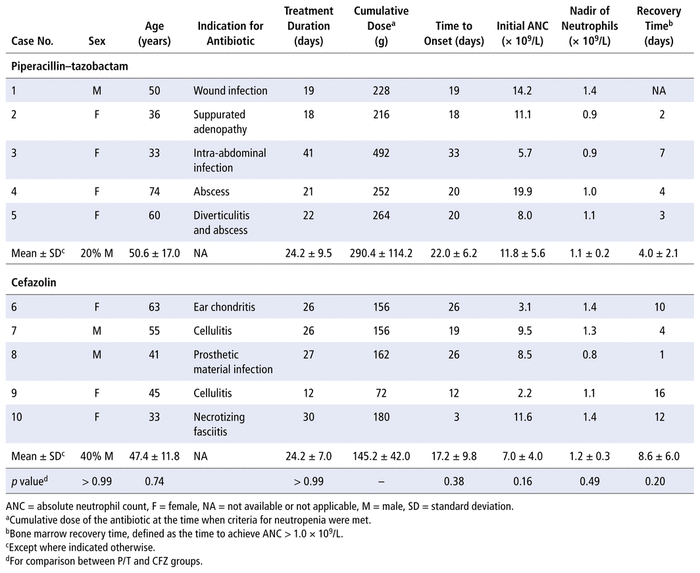
The mean time to onset of neutropenia and the neutrophil baseline value were slightly higher in the P/T group than in the cefazolin group, although these differences were not statistically significant (time to onset 22.0 days versus 17.2 days, respectively, p = 0.38; 11.8 × 109/L versus 7.0 × 109/L, respectively, p = 0.16). The neutrophil nadir was similar for the 2 antibiotics (1.1 × 109/L in the P/T group and 1.2 × 109/L in the cefazolin group; p = 0.49). Finally, the mean time for the bone marrow to recover after the antibiotics were stopped was twice as long in the cefazolin group as in the P/T group (8.6 days versus 4.0 days, respectively; p = 0.20); the absence of statistical significance may have been related to the small sample sizes.
In this study, the prevalence of neutropenia was similar between patients who received P/T and those who received cefazolin (4.8% and 4.9%, respectively), which suggests that the risk of this adverse effect was also similar in the 2 groups. In 2 small studies published in the 1990s, the prevalence of neutropenia induced by P/T was between 1% and 4%, similar to the prevalence observed in the present study.13,14 A systematic review of case reports, retrospective cohort studies, and clinical trials describing neutropenia associated with piperacillin and P/T was published in 2007.6 This review showed that the prevalence of neutropenia associated with piperacillin or P/T was less than 1%, but the authors stated that the true prevalence of piperacillin- or P/T-associated neutropenia was unknown.6 Similarly, the prevalence of cefazolin-associated neutropenia is not well defined and is mostly based on observational cohort studies. The prevalence of neutropenia in patients treated with cefazolin has been reported between 1.3% and 3.3%, which is slightly lower than what we observed.15–18Table 3 summarizes the results of previous studies describing neutropenia associated with P/T and cefazolin.
TABLE 3 Summary of Studies of Neutropenia Associated with Piperacillin–Tazobactam and Cefazolin in Adults
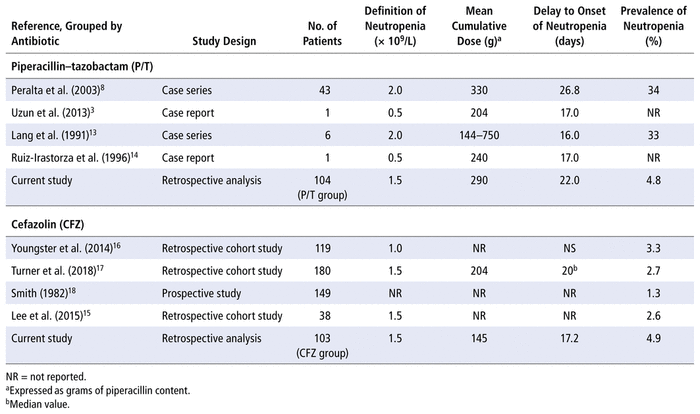
The criteria for defining neutropenia strongly influence the reported rates of β-lactam–associated neutropenia. For instance, Scheetz and others6 defined neutropenia as absolute neutrophil count less than 0.5 × 109/L, which is much more stringent than the definitions used by other authors (e.g., ≤ 2.0 × 109/L).8,13 The use of this criterion may have led Scheetz and others6 to underestimate the rate of neutropenia. We deliberately selected a cut-off of 1.5 × 109/L, halfway between the definition often used in clinical practice (1.0 × 109/L ) and the definition selected in most studies (2.0 × 109/L).8,13 When we performed the same statistical analyses with a different definition of neutropenia (absolute neutrophil count < 1.0 × 109/L), the prevalence of antibiotic-associated neutropenia with the 2 antibiotics was predictably lower, but still not significantly different (1.9% [2/104] for P/T versus 1.0% [1/103] for cefazolin). Similar results were obtained when neutropenia was defined as an absolute neutrophil count below 2.0 × 109/L (7.7% [8/104] for P/T versus 5.8% [6/103] for cefazolin).
Increased cumulative exposure to β-lactams has been linked to increases in the occurrence of neutropenia. A retrospective cohort study published in 2003 reported the occurrence of neutropenia at a threshold cumulative dose of P/T between 204 and 612 g.8 Patients treated with piperacillin who experienced neutropenia received an average cumulative dose of 330 g, whereas the average cumulative dose was lower, at 237 g, in the non-neutropenic group. In that previous study, neutropenia was defined by a neutrophil count of 2.0 × 109/L or less. In our study, the cumulative dose of P/T was only slightly higher among patients who experienced neutropenia than among those who did not experience neutropenia (290.4 g versus 244.8 g; difference not significant). This value is similar to the cumulative dose (284 g) described for an episode of neutropenia associated with the use of piperacillin in a young man after 21 days of treatment.19 Unsurprisingly, the cumulative dose of cefazolin was lower than that of P/T, which reflects the respective dosing schedules of these medications.
The defined daily dose (DDD) represents another way to appreciate the magnitude of antibiotic use. The DDD, a benchmarking tool developed by the World Health Organization (www.whocc.no), represents the assumed average daily maintenance dose for a drug when used for its main indication in adults. However, the DDD does not always reflect precisely the recommended or prescribed daily dose. For instance, the DDD for cefazolin is 3 g, whereas in our institution cefazolin is seldom prescribed at less than 6 g/day. Conversely, the DDD for piperacillin is 14 g, which reflects more accurately its usual dosing range, between 12 and 16 g/day. The mean amounts of antibiotic administered to patients who experienced neutropenia were 20.7 DDD for piperacillin and 48.3 DDD for cefazolin. However, if 6 g/day is used to represent the usual maintenance dose of cefazolin, rather than 3 g/day, patients in the cefazolin group received an average of 24.2 DDD, which would be comparable to the DDD for piperacillin. To date, no clear threshold cumulative dose leading to neutropenia has been identified for β-lactam antibiotics, and to our knowledge no study has examined this issue from the perspective of DDDs.
Ceftaroline, a fourth-generation cephalosporin active against methicillin-resistant Staphylococcus aureus, has recently been associated with an increased risk of neutropenia, relative to other commonly used antistaphylococcal antibiotics, such as cefazolin, nafcillin, and vancomycin.17 In that cohort study, cumulative dose and duration of exposure were not significant predictors of neutropenia, whereas age, baseline absolute neutrophil count, presence of a bone and joint infection, and use of ceftaroline were significantly associated with neutropenia.17
Neutropenia is an uncommon side effect of β-lactam therapy and usually requires more than 10 days of exposure to the antibiotic.1,6,16 In our study, the time to onset of neutropenia observed in the P/T group (mean 22.0 days, median 20 days, IQR 19–20 days) was slightly longer than values previously reported.3,15 For cefazolin, the observed time to onset was shorter (mean 17.2 days, median 19 days, IQR 12–26 days) and similar to the value reported in a retrospective cohort study.17 The bone marrow recovery time after β-lactam–induced neutropenia is reported to be about 7 days,3,4,6,8,9,19 and Peralta and others8 reported an average recovery time of 3.8 days. This is in line with our observations for the P/T group (bone marrow recovery after a mean of 4.0 [SD 2.1] days). Mean bone marrow recovery time was longer for cefazolin (8.6 [SD 6.0] days), although the difference was not statistically significant (p = 0.20).
Two mechanisms have been proposed to explain β-lactam–associated neutropenia. The first suggests an immunological phenomenon as the cause of the decrease in neutrophils. Circulating immunoglobulin G (IgG) directed against neutrophils was found in several patients who had been exposed to β-lactams and P/T.9 The IgG reacts with granulocytes and platelets.6,18,19 Such an immunological hypothesis could explain in part the fact that “cross-neutropenia” is possible between β-lactam antibiotics.19,20 The second hypothesis concerns a direct toxic effect of the antibiotic on the bone marrow, thereby hindering granulopoiesis.19,20
The diversity in the infectious diseases observed in our study reflects the clinical usefulness and spectrum of antibacterial activity of both P/T and cefazolin. As might be expected, cefazolin was used mainly for treatment of skin, skin structure, and osteo-articular infections, whereas P/T, which displays a broader antibacterial spectrum, was used for a greater variety of situations, particularly for polymicrobial infections. However, the types of infections for which the patients were treated were so varied that we were unable to establish any association with the outcome (Table 2). Some potential confounding factors such as age, sex, and initial absolute neutrophil count were significantly different between the 2 groups. Therefore, we fitted a multivariate model with adjustment for these factors. However, the results of adjusted and crude models evaluating neutropenia in both the P/T and cefazolin groups were very similar.
Our study had some limitations, mainly because of the retrospective nature of the study design. In particular, the data were dependent on the time when white blood cell count was determined, which varied greatly depending on the length of stay and whether the treatment was administered on an outpatient basis. This limitation has a potential influence on the estimation of time to recovery from neutropenia. Finally, the small sample size limited the possibility of detecting a meaningful difference in the risk of neutropenia between the 2 antibiotics.
The results of this study suggest a comparable risk of neutropenia after 10 days of treatment with P/T or cefazolin. The risk observed here was lower than that reported in the literature, at least for an adult population. Neutropenia associated with β-lactam antibiotics remains a poorly documented adverse effect. Further studies are needed to better establish the individual risk for each antibiotic, as well as other contributing factors, such as the cumulative dose.
1 Andrès E, Maloisel F. Antibiotic-induced agranulocytosis: a monocentric study of 21 cases. Arch Intern Med. 2001;161(21):2619.
Crossref PubMed
2 Andrès E, Maloisel F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol. 2008;15(1):15–21.
Crossref
3 Uzun G, Onem Y, Hatipoglu M, Turhan V, Mutluoglu M, Ay H. Piperacillin/tazobactam-induced neutropenia, thrombocytopenia, and fever during treatment of a diabetic foot infection. Scand J Infect Dis. 2013;45(1):73–6.
Crossref
4 Pérez-Vázquez A, Pastor JM, Riancho JA. Immune thrombocytopenia caused by piperacillin/tazobactam. Clin Infect Dis. 1998;27(3):650–1.
Crossref PubMed
5 Gerber L, Wing EJ. Life-threatening neutropenia secondary to piperacillin/tazobactam therapy. Clin Infect Dis. 1995;21(4):1047–8.
Crossref PubMed
6 Scheetz MH, McKoy JM, Parada JP, Djulbegovic B, Raisch DW, Yarnold PR, et al. Systematic review of piperacillin-induced neutropenia. Drug Saf. 2007;30(4):295–306.
Crossref PubMed
7 Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56–e93.
Crossref PubMed
8 Peralta FG, Sánchez MB, Roíz MP, Pena MA, Tejero MA, Arjona R. Incidence of neutropenia during treatment of bone-related infections with piperacillin-tazobactam. Clin Infect Dis. 2003;37(11):1568–72.
Crossref PubMed
9 Olaison L, Belin L, Hogevik H, Alestig K. Incidence of beta-lactam-induced delayed hypersensitivity and neutropenia during treatment of infective endocarditis. Arch Intern Med. 1999;159(6):607–15.
Crossref PubMed
10 Lemieux P, Grégoire JP, Thibeault R, Bergeron L. Higher risk of neutropenia associated with piperacillin-tazobactam compared with ticarcillin-clavulanate in children. Clin Infect Dis. 2015;60(2):203–7.
Crossref
11 Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146(9): 657–65.
Crossref PubMed
12 Van der Klauw MM, Goudsmit R, Halie MR, van’t Veer MB, Herings RM, Wilson JH, et al. A population-based case-cohort study of drug-associated agranulocytosis. Arch Intern Med. 1999;159(4):369–74.
Crossref PubMed
13 Lang R, Lishner M, Ravid M. Adverse reactions to prolonged treatment with high doses of carbenicillin and ureidopenicillins. Rev Infect Dis. 1991;13(1):68–72.
Crossref PubMed
14 Ruiz-Irastorza G, Barreiro G, Aguirre C. Reversible bone marrow depression by high-dose piperacillin/tazobactam. Br J Haematol. 1996; 95(4):611–2.
Crossref PubMed
15 Lee B, Tam I, Weigel B 4th, Breeze JL, Paulus JK, Nelson J, et al. Comparative outcomes of β-lactam antibiotics in outpatient parenteral antibiotic therapy: treatment success, readmissions and antibiotic switches. J Antimicrob Chemother. 2015;70:2389–96.
Crossref PubMed PMC
16 Youngster I, Shenoy ES, Hooper DC, Nelson SB. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin Infect Dis. 2014;59(3):369–75.
Crossref PubMed PMC
17 Turner RB, Wilson DE, Saedi-Kwon H, Chang E, Won R, Chan D, et al. Comparative analysis of neutropenia in patients receiving prolonged treatment with ceftaroline. J Antimicrob Chemother. 2018;73(3):772–8.
Crossref
18 Smith CR. Cefotaxime and cephalosporins: adverse reactions in perspective. Rev Infect Dis. 1982;4 (Suppl):S481–S488.
Crossref PubMed
19 Kumar A, Choudhuri G, Aggarwal R. Piperacillin induced bone marrow suppression: a case report. BMC Clin Pharmacol. 2003;3:Article 2.
Crossref PubMed PMC
20 Murphy MF, Metcalfe P, Grint PC, Green AR, Knowles S, Amess JA, et al. Cephalosporin-induced immune neutropenia. Br J Haematol. 1985;59(1):9–14.
Crossref PubMed
Competing interests: For activities not directly related to the study reported here, Frédéric Calon has received grants from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Fonds de la recherche du Québec en santé, the Canada Foundation for Innovation, Parkinson Canada, and the Alzheimer Society of Canada; consulting fees from various public and private entities; and payments for expert testimony given in the province of Quebec. No other competing interests were declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 75, NUMBER 3, Summer 2022