TLC-Act: A Novel Tool for Managing Drug Interactions
Casara Hong, Michael Legal, Harkaryn Bagri, Louise Lau, Karen Dahri
ABSTRACT
Background
Clinical decision support systems (CDSS) are used by pharmacists to assist in managing drug–drug interactions (DDIs). However, previous research suggests that such systems may perform suboptimally in providing clinically relevant information in practice.
Objectives
The primary objective of this study was to develop a novel DDI management tool to reflect the clinical thought process that a pharmacist uses when assessing a DDI. The secondary objective was to investigate practitioners’ perceptions of this tool.
Methods
This study was conducted in 3 phases: development of the DDI management tool, implementation of the tool in clinical practice, and collection of practitioners’ opinions of the tool through an online qualitative survey (although because of circumstances related to the COVID-19 pandemic, the study population for the survey phase included only pharmacy residents). A comprehensive literature search and analysis by an expert panel provided underlying context for the DDI management tool. The tool was validated through simulation against a known list of DDIs before implementation into practice by hospital pharmacists and pharmacy residents. Participating pharmacy residents were invited to provide feedback on the tool. Survey results were analyzed using descriptive statistics.
Results
The novel tool that was developed in this study (called TLC-Act) consisted of components important to a pharmacist when assessing a DDI, including the duration of concomitant use of the interacting medications and patient-specific risk factors. Study participants implemented the tool in clinical practice for a total of 6 weeks. Of the 28 pharmacy residents surveyed, 15 (54%) submitted a response, of whom 11 (73%) found the TLC-Act tool to be slightly more useful for assessing a DDI than usual care with the CDSS alone.
Conclusions
The TLC-Act tool maps out a pharmacist’s clinical thought process when assessing a DDI in practice. This novel tool may be more useful than a CDSS alone for managing DDIs, as it takes into account other important factors pertinent to the assessment of a DDI.
KEYWORDS: drug interactions, algorithm, tool, pharmacists, resident, hospital
RÉSUMÉ
Contexte
Les systèmes d’aide à la décision clinique (SADC) sont utilisés par les pharmaciens pour les aider à gérer les interactions médicamenteuses (IM). Cependant, des recherches antérieures indiquent que ces systèmes peuvent fonctionner de manière sous-optimale pour fournir des informations cliniquement pertinentes dans la pratique.
Objectifs
L’objectif principal de cette étude consistait à développer un nouvel outil de gestion des IM pour reproduire le processus de réflexion clinique adopté par un pharmacien quand il les évalue. L’objectif secondaire consistait, quant à lui, à enquêter sur les perceptions des praticiens à l’égard de cet outil.
Méthodes
Cette étude a été menée en 3 phases : développement de l’outil de gestion des IM; sa mise en place dans la pratique clinique; et recueil des avis des praticiens sur celui-ci au moyen d’une enquête qualitative en ligne (bien qu’en raison des circonstances liées à la pandémie de COVID-19, la population étudiée pour la phase de l’enquête ne comprenne que des résidents en pharmacie). Une recherche documentaire et une analyse approfondies effectuées par un groupe d’experts ont fourni le contexte sous-jacent de l’outil de gestion des IM. L’outil a été validé par simulation par rapport à une liste connue d’IM avant sa mise en pratique par les pharmaciens hospitaliers et les résidents en pharmacie. Les résidents en pharmacie qui participaient à l’étude ont été invités à donner leur avis sur l’outil. Les résultats de l’enquête ont été analysés à l’aide de statistiques descriptives.
Résultats
Le nouvel outil développé dans le cadre de cette étude (le « TLC-Act ») se composait d’éléments d’évaluation des IM importants pour un pharmacien, y compris la durée de l’utilisation concomitante des médicaments en interaction et les facteurs de risque propres au patient. Les participants à l’étude ont mis en œuvre l’outil dans la pratique clinique pendant un total de 6 semaines. Sur les 28 résidents en pharmacie interrogés, 15 (54 %) ont soumis une réponse, et 11 (73 %) d’entre eux ont trouvé que l’outil TLC-Act était légèrement plus utile pour évaluer les IM que le SADC seul utilisé habituellement.
Conclusions
L’outil TLC-Act cherche à reproduire le processus de réflexion clinique d’un pharmacien lorsqu’il évalue les IM dans la pratique. Ce nouvel outil peut être plus utile qu’un SADC utilisé seul pour gérer les IM, car il prend en compte d’autres facteurs importants qui sont pertinents pour leur évaluation.
MOTS CLÉS: interactions médicamenteuses, algorithme, outil, pharmaciens, résident, hôpital
INTRODUCTION
Pharmacists are responsible for identifying and assessing drug–drug interactions (DDIs); however, the sheer volume of potential interactions requires a degree of reliance on computer systems, which have their own limitations. A DDI occurs when one drug changes the pharmacokinetic or pharmacodynamic properties of another drug, resulting in alterations in physiological processes or activity.1,2 Adverse drug events caused by DDIs are preventable errors, which account for 2% to 3% of hospital admissions.3 The prevalence of DDIs in hospitalized patients ranges from 15% to 45%.1 Additionally, patients who are admitted to hospital because of DDIs often experience longer lengths of stay than patients admitted for other reasons.4
As medication specialists, pharmacists have a unique role on the interdisciplinary health care team, intervening on DDIs to prevent adverse drug events as part of their professional practice. Given the vast number of known DDIs, clinical decision support systems (CDSS) and computerized DDI checkers are used by pharmacists to help identify DDIs of clinical importance. However, CDSS have been shown to have suboptimal performance in the clinical management of DDIs.2 For example, CDSS may be limited in their ability to detect updated and evidence-based clinically significant DDIs.2 Moreover, the comprehensiveness of the databases used for the alerts can vary considerably.2,5 Previous research has suggested that pharmacists perceive considerable discrepancies in the severity rankings of DDIs among various CDSS, which makes these systems difficult to view as reliable sources.6,7 Additionally, when different pharmacists were given exactly the same CDSS-generated severity classification for various DDIs, there was poor agreement among the management strategies that they recommended.6,7 These challenges can make it difficult for pharmacists to determine how to assess and properly manage DDIs encountered in practice.
Pharmacists are trained to develop and use a well- defined thought process to thoroughly manage DDIs as part of their provision of comprehensive patient care. A clinical thought process is a complex cognitive practice that involves clinical reasoning and critical problem-solving.8,9 Pharmacy regulatory authorities in various countries have developed practice standards, which state that pharmacists are expected to critically analyze and apply information to make evidence-informed decisions within their practice.10–12 The ability to competently fulfil these practice standards relies on the strength of a pharmacist’s clinical thought process. Little is currently known or published about the challenges that newly qualified pharmacy practitioners encounter when managing DDIs in practice.6,7,13
Ultimately, the goal of developing a novel DDI management tool is to outline a clinical decision-making thought process to help pharmacists critically analyze and manage DDIs. The primary objective of our study was to develop a novel DDI management tool, and the secondary objective was to investigate practitioners’ perceptions of this tool. The information from this study will provide insight into the clinical thought process that pharmacists utilize when assessing and managing DDIs in clinical practice.
METHODS
This study was conducted in 3 phases: development of the DDI management tool, implementation of the tool into clinical practice by study participants, and collection of participants’ perceptions regarding the tool. The original study population included clinical and dispensary pharmacists working at 3 hospitals in British Columbia’s Lower Mainland Pharmacy Services (LMPS) health authority (Vancouver General Hospital, St Paul’s Hospital, and Surrey Memorial Hospital) and 28 LMPS hospital pharmacy residents training in the 2019/20 academic year. However, because of circumstances related to the COVID-19 pandemic, the study population for the third (survey) phase was amended to include only pharmacy residents. Pharmacists working at other hospitals, advanced pharmacy practice residents, pharmacy technicians or assistants, and community pharmacists were excluded from this study. Once developed, the DDI management tool was introduced and implemented at all study sites. Ethics approval for this study was granted by the University of British Columbia’s Behavioural Research Ethics Board.
Phase 1: Development of the Tool
Development of the novel DDI management tool was accomplished through 4 distinct stages. Stage 1 focused on a comprehensive literature search to provide context for the DDI management tool. During stage 2, the study investigators created a preliminary version of the tool. Stage 3 involved analysis of the preliminary DDI management tool by an expert panel of pharmacist stakeholders. Stage 4 involved refinement of the tool and validation through simulations with sample DDIs.
Stage 1: Literature Search
A literature search was conducted to identify any previously published studies examining the development or evaluation of a DDI management tool for clinicians, as well as any studies examining clinical decision-making processes in the assessment of DDIs. Multiple databases were searched, specifically Ovid MEDLINE, Embase, PubMed, and Google Scholar, using the search terms “drug interaction”, “algorithm” or “tool” or “initiative” or “software”, “decision making” or “clinical decision” or “management” or “thought process”, and “pharmacy” or “pharmacist” (with date limits from 1960 to 2020). Context for the tool was developed from the literature search and previous work by our study group examining the perceptions and management of DDIs in hospital pharmacy over the course of several years.6,7,14 The members of the study group had extensive expertise in advanced clinical pharmacy practice, having worked in hospital practice in various roles for over 10 years. Given the capacity of these team members and knowledge gained from previous research, the study group was well positioned to develop the DDI management tool.
Stage 2: Creation of a Preliminary Tool
The study investigators first developed a preliminary version of the tool by mirroring the various steps and clinical checkpoints that pharmacists complete when assessing a DDI. This process was outlined as an algorithm using workflow mapping techniques. Pertinent categories for DDI assessment were organized and a scoring system was developed on the basis of clinical relevance, at the study investigators’ discretion. For this study, CDSS were defined as the clinical decision support systems or computerized DDI programs (e.g., Lexicomp, Wolters Kluwer Health; Micromedex, IBM) used by pharmacists when assessing DDIs to provide management suggestions.
Stage 3: Expert Evaluation of the Preliminary Tool
To minimize subjectivity and bias of the newly developed tool, an independent expert panel of key pharmacist stakeholders was invited to evaluate and provide feedback on the preliminary version. The panel consisted of content-matter experts and pharmacists with various levels of education who were practising in hospital settings. Each panelist independently reviewed the preliminary tool and provided feedback, which was then used by the study team to guide modifications for refining the tool.
Stage 4: Tool Finalization and Initial Testing
The final version of the tool underwent initial testing and validation through simulations using a list of 15 known DDIs previously evaluated by the study team and found to have differences between CDSS recommendations and pharmacists’ actions in practice.7 For each DDI, the management recommendations generated by the novel tool were compared with the data from pharmacists surveyed in our previous research.7 On the basis of simulation results, additional revisions were considered, and the components of the tool were finalized through discussion with the study team.
Phase 2: Implementation of the Tool
Multiple modalities were used to disseminate the tool. An electronic PDF document of the tool and a video overview created by the investigators were sent by email to study participants through pharmacy administrative staff at each site. Paper handouts were also made available to the pharmacists. An infographic poster containing a QR code linked to a PDF version of the tool was posted in the pharmacies at each site to alert staff to the new tool. Additionally, in-person educational presentations were provided at the sites to further explain both the use of the tool and the study more generally. A total duration of 6 weeks was allowed for study participants to implement the TLC-Act tool in their practice before dissemination of the feedback survey.
Phase 3: Assessment of the Tool in Clinical Practice
Feedback about the TLC-Act tool was solicited by means of a survey made available through the online platform Qualtrics (version February 2020). The residency program coordinator sent information about the survey to pharmacy residents by email, on behalf of the research team. Additionally, because the COVID-19 pandemic resulted in suspension of clinical rotations for the pharmacy residents during the 6-week implementation period, 2 additional paper-based practice cases were provided to survey participants to supplement their experience with use of the tool. The survey questions were developed by consensus among the investigators, and included questions pertaining to the organization and use of the tool. Informed consent to participate in the survey was implied by participation in the survey. The survey responses were collected through the Qualtrics platform (version February 2020) and analyzed with Excel spreadsheet software (version 16.41; Microsoft Corporation) using descriptive statistics.
RESULTS
Development and Final Components of the Novel DDI Management Tool (TLC-Act)
The novel DDI management tool was generated to map the clinical thought process of a pharmacist and to establish a systematic approach to managing DDIs. The tool incorporated a scoring system of components pertinent to assessing a DDI, as established by study investigators on the basis of their previous clinical experience. The literature search yielded no studies directly relevant to our search criteria, and no DDI management strategies or tools were identified. Therefore, the final components of the TLC-Act tool were decided upon through discussion by the study team, evaluation of the tool by the expert panel, and simulated use against a list of DDIs known from our previous research. A total of 9 hospital pharmacists with various roles at different sites constituted the expert panel, which independently evaluated the tool and provided recommendations for revision. The tool underwent a total of 8 revisions during the development phase, with each revision being completed through review and discussion among members of the study team. There was a high level of agreement between the DDI management strategies recommended by the TLC-Act tool and the preferred actions taken by pharmacists in clinical practice, as determined in a previous survey study regarding DDI management (with matching for 14 of 15 recommendations).7
The 3 main sections of the tool used for scoring a DDI referred to the time frame for onset of effects from the DDI, the severity rating generated by the CDSS, and the level of available evidence for the interaction. Information related to time of onset of effects and level of interaction severity (i.e., the T and L sections of the tool), generated a combined total score that was then used to determine the suggested strategy for managing the DDI (Figure 1A). If the total score was between 1 and 3 points, the user was instructed to complete the section for “current available evidence” (i.e., the C section of the tool shown in Figure 1A). This section was based on available evidence (summarized from the CDSS or obtained through a literature search performed by the pharmacist) and generated a letter grade.
| |

| |
|
FIGURE 1A TLC-Act, a novel tool for management of drug–drug interactions (DDIs), outlining a pharmacist’s clinical thought process for assessing a DDI (part 1 of 2). Components of the tool include time and onset of effects from the DDI, severity of the interaction, and currently available evidence for the DDI. For each DDI, the tool yields a total score and letter grade that are then used to generate a management strategy (second page of the tool: see Figure 1B). PK = pharmacokinetic, PRN = as needed, RCT = randomized controlled trial. © 2019 Lower Mainland Pharmacy Services, British Columbia. Reproduced by permission. |
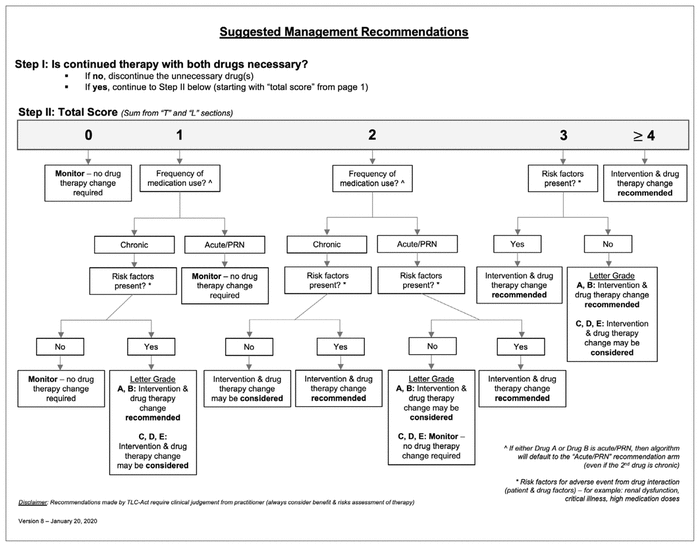
Using both the combined total score (from the T and L sections) and, if applicable, the letter grade (from the C section), the tool then suggested a management strategy for the DDI (i.e., the “Act” section of the tool). For this section of the tool, the user had to complete documentation for actions or interventions undertaken to manage the DDI, including communication with the patient’s care team and education provided to the patient (Figure 1A). Suggested management recommendations determined from the total score and letter grade were provided on the second page of the tool (Figure 1B). Based on the user’s consideration of additional case-specific factors, including the frequency of administration for the interacting medications and the presence of other patient-specific factors that could increase the risk of an adverse effect from the DDI, the tool generated recommendations for management approaches. Examples of other patient-specific risk factors might include renal or liver dysfunction, critical illness, or high medication dosages. Evaluation of a patient’s risk factors was based on the practitioner’s clinical judgment, which was not limited by the tool. According to the information used to assess the interaction, the TLC-Act tool recommended one of the following management approaches: monitoring (no change in drug therapy required), consideration of an intervention and drug therapy change, or recommendation for an intervention and drug therapy change (Figure 1B).
| |
| |
|
FIGURE 1B TLC-Act, a novel tool for management of drug–drug interactions (DDIs), outlining a pharmacist’s clinical thought process for assessing a DDI (part 2 of 2). For each DDI, the tool yields a total score and letter grade (as shown in Figure 1A), which are then used to generate a suggested management strategy. Additional components, specifically the frequency of drug administration and the presence of patient-specific risk factors, are considered to determine the suggested management strategy. PRN = as needed. © 2019 Lower Mainland Pharmacy Services, British Columbia. Reproduced by permission. |
Implementation of the Tool
The novel DDI management tool was introduced and implemented at all 3 study sites in February 2020, with a target study population of more than 500 hospital pharmacists. However, because of circumstances surrounding the COVID-19 pandemic, many pharmacists working at the 3 target sites were unable to participate in our assessment survey. We were unable to quantify the total number of pharmacists who used the tool in clinical practice during our study or the duration of use. Pharmacy residents had an average of 4 weeks to implement the tool, which was shortened from the planned implementation period of 6 weeks when clinical rotations were suspended as a result of the COVID-19 pandemic.
Assessment of the Tool in Clinical Practice
Although both pharmacists and pharmacy residents had an opportunity to implement the TLC-Act tool in their clinical practice, assessment of the tool focused solely on the perspectives of pharmacy residents, for the reasons outlined above. The assessment survey consisted of 13 Likert-style questions to reflect the usability, feasibility, and utility of the tool (Supplement 1, available from https://www.cjhp-online.ca/index.php/cjhp/issue/view/209 ). Of the 28 pharmacy residents who participated in implementing the novel DDI management tool, 15 provided feedback (response rate 54%).
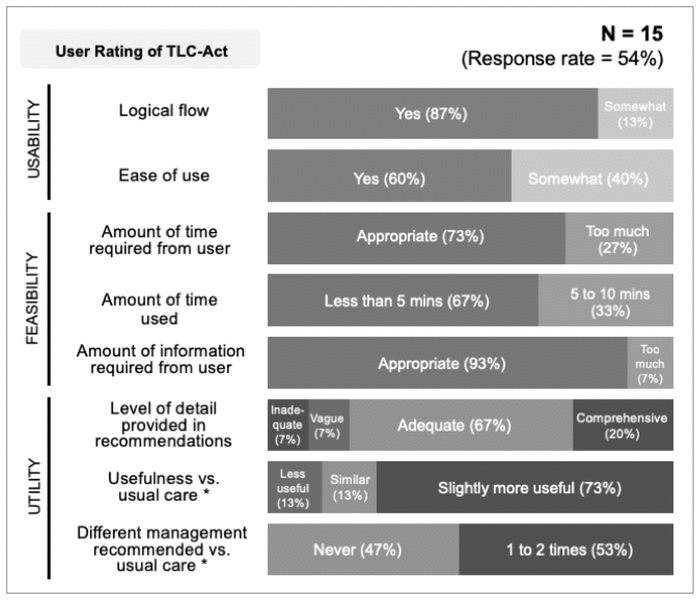
Overall, the TLC-Act tool was rated by survey respondents as slightly more useful than usual care, where usual care was defined as the use of CDSS or DDI computerized software (e.g., Lexicomp, Micromedex) alone for managing a DDI (Figure 2). When asked to rate the usability of the tool, 13 (87%) of the survey respondents found that the tool had logical flow, and 9 (60%) found it easy to use. In addition, the majority of respondents felt that the amounts of time and information required to use the tool were appropriate. On average, the time required to assess a single DDI with the TLC-Act tool was less than 5 minutes for two-thirds of survey respondents. When asked to rate the utility of the tool for practice, two-thirds of respondents believed that the level of detail provided by the recommendations was adequate.
| |
| |
|
FIGURE 2 Results of the online feedback survey evaluating TLC-Act, the novel tool for management of drug–drug interactions (DDIs). The survey was organized to assess the usability, feasibility, and utility of the tool. The survey response rate was 54% (15/28). The figure displays only survey options that were selected by respondents. *Usual care was defined as use of a clinical decision support system or DDI software (e.g., Lexicomp, Micromedex) alone, without reference to the TLC-Act tool. |
All of the pharmacy residents who responded to the feedback survey reported that they would consider recommending the tool to other pharmacists for assessing DDIs. However, only 5 (33%) said they would consider recommending the tool to other pharmacists in its current form, without further revisions. Suggested revisions for the tool (which were provided as free text) included reducing the time to use the tool and minimizing the need for manual calculations. Additionally, 9 (60%) respondents were not using the tool in clinical practice when they responded to the survey. Instead, these participants evaluated the TLC-Act tool using the 2 practice clinical cases provided to the residency class. The other 6 (40%) respondents had each used the TLC-Act tool in clinical practice from 1 to 3 times during the implementation phase.
DISCUSSION
The findings of this study suggest that use of the TLC-Act tool, in combination with already-established CDSS, may support clinical decision-making by hospital pharmacy residents when they are assessing DDIs. Previous research has suggested inconsistencies between how DDIs are categorized by CDSS and how pharmacists use the information supplied by CDSS in clinical practice, which raises questions about the utility of these computerized systems.6,7
Although the interacting medications themselves have substantial effects on the severity of DDIs and the urgency of acting upon them, many other factors influence how an interaction should be managed. Our novel algorithm sought to incorporate these additional factors to better guide clinicians in their decision-making processes. How an interaction is managed is largely influenced by factors specific to the patient who is taking the interacting medications, for example, risk factors that might increase the probability of experiencing an adverse effect or the frequency of administration of the medications. Furthermore, clinicians may consider the level of evidence available about the specific DDI. Because of the relatively low incidence of DDIs reported in the literature, clinicians must critically evaluate whether the available evidence is applicable to the specific patient or the interacting medications being assessed. For example, the interaction between escitalopram and enoxaparin is considered a moderate-severity DDI, and computerized interaction systems typically recommend that the clinician consider modifying the patient’s drug therapy according to the manufacturers’ prescribing information related to increased bleeding complications with medications that have antiplatelet properties.15 However, no specific evidence pertaining directly to the interaction between escitalopram and enoxaparin is available in the literature. Therefore, for patients receiving prophylactic doses of enoxaparin for prevention of venous thromboembolism (VTE) during an acute care inpatient admission, it may be more appropriate to monitor for bleeding complications than to alter the patient’s long-term antidepressant therapy. Overall, because recommendations generated by CDSS are generic in nature and do not account for patient- or case-specific factors (e.g., medication dosages, clinical context, or duration of therapy), such management strategies may be more conservative than necessary.
With those considerations in mind, our research team developed the TLC-Act tool to provide an alternative approach to managing DDIs. Returning to the example of escitalopram and enoxaparin, the TLC-Act tool categorizes the interaction as “1E” (i.e., unknown reaction onset, CDSS rating of moderate severity, and theoretical interaction based on mechanism), for which the recommendation is to monitor for an adverse drug event (i.e., no change in drug therapy required) if the patient is using the concomitant medications for an acute indication such as VTE prophylaxis during an inpatient admission. Conversely, if the patient is expected to use both medications for chronic indications (e.g., VTE treatment) and patient-specific factors are present that could increase the possibility of an adverse effect (i.e., bleeding complication), an intervention and drug therapy change may be considered. As such, the recommendations provided by the TLC-Act tool may represent a more realistic management approach for practice than traditional CDSS recommendations.
Preliminary assessment of the TLC-Act tool based on feedback gathered by surveying hospital pharmacy residents suggested a reasonable degree of usability and utility. Limitations of the current version of the tool include the constraint of testing one DDI at a time and the need for manual calculations. Improvements might be aimed at transitioning to an electronic application, to allow for automatic calculations and assessment of multiple interacting medications, thus improving usability. Another limitation was the small cohort of pharmacy residents who participated in the evaluation survey, given that the original study population of pharmacists had to be excluded from the survey because of workload constraints related to the COVID-19 pandemic. As a result, use of TLC-Act as a clinical tool requires validation with a larger population of pharmacists. The TLC-Act tool primarily functions as a step-by-step method for assessing a DDI; it may have less utility for experienced clinicians for whom these steps have become more automatic. However, based on implementation of the TLC-Act tool in this study and the information gathered, there may be value in using the tool as a teaching aid to support new clinicians, such as pharmacy residents or students, as they develop clinical decision-making skills pertaining to DDI management.
CONCLUSION
The TLC-Act is a novel DDI management tool designed for pharmacists and new clinicians, which may support development of a clinician’s clinical thought process and a systematic approach to assessing DDIs. Survey responses from hospital pharmacy residents provide a preliminary understanding of the usability and utility of the TLC-Act tool. Most survey respondents perceived the TLC-Act tool to be slightly more useful than usual care for managing DDIs. Because pharmacy residents and students are learning to build their clinical decision-making skills, the TLC-Act tool may have value as a teaching aid to support development of a systematic thought process.
Future directions include implementing the TLC-Act tool into an entry-to-practice Doctor of Pharmacy program curriculum to allow exploration of its utility as a clinical decision teaching aid for pharmacy students. By training pharmacy learners and new pharmacists to use these steps in their clinical reasoning and thought process, our study team hopes the tool will lead to eventual improvements in clinical outcomes for patients by minimizing adverse effects from DDIs.
References
1 Espinosa-Bosch M, Santos-Ramos B, Gil-Navarro MV, Santos-Rubio MD, Marín-Gil R, Villacorta-Linaza P. Prevalence of drug interactions in hospital healthcare. Int J Clin Pharm. 2012;34(6):807–17.
Crossref PubMed
2 Malone DC, Abarca J, Skrepnek GH, Murphy JE, Armstrong EP, Grizzle AJ, et al. Pharmacist workload and pharmacy characteristics associated with the dispensing of potentially clinically important drug-drug interactions. Med Care. 2007;45(5):456–62.
Crossref PubMed
3 Tragni E, Casula M, Pieri V, Favato G, Marcobelli A, Trotta MG, et al. Prevalence of the prescription of potentially interacting drugs. PLoS One. 2013;8(10):e78827.
Crossref PubMed PMC
4 Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678–86.
Crossref PubMed PMC
5 Tilson H, Hines LE, McEvoy G, Weinstein DM, Hansten PD, Matuszewski K, et al. Recommendations for selecting drug-drug interactions for clinical decision support. Am J Health Sys Pharm. 2016;73(8):576–85.
Crossref
6 Bagri H, Dahri K, Legal M. Hospital pharmacists’ perceptions and decision-making related to drug-drug interactions. Can J Hosp Pharm. 2019;72(4):288–94.
PubMed PMC
7 Lau L, Bagri H, Legal M, Dahri K. Comparison of clinical importance of drug interactions identified by hospital pharmacists and a local clinical decision support system. Can J Hosp Pharm. 2021;74(3):203–10.
PubMed PMC
8 Faucher C. Differentiating the elements of clinical thinking. Optom Educ. 2011;36(3):140–5.
9 Young M, Thomas A, Gordon D, Gruppen L, Lubarsky S, Rencic J, et al. The terminology of clinical reasoning in health professions education: implications and considerations. Med Teach. 2019;41(11):1277–84.
Crossref PubMed
10 Pharmacists’ patient care process. Joint Commission of Pharmacy Practitioners; 2014 [cited 2022 Apr 30]. Available from: https://jcpp.net/wp-content/uploads/2016/03/PatientCareProcess-with-supporting-organizations.pdf
11 Professional competencies for Canadian pharmacists at entry to practice. National Association of Pharmacy Regulatory Authorities [Canada]; 2014 [cited 2020 Nov 28]. Available from: https://napra.ca/pharmacists/professional-competencies-canadian-pharmacists-entry-practice-2014
12 Hepler C, Strand L. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533–43.
PubMed
13 Mourh J, Newby B. Barriers and strategies for transition from student to successful hospital pharmacist. Can J Hosp Pharm. 2019;72(3):219–26.
PubMed PMC
14 Poon D, Legal M, Lau L, Bagri H, Dahri K. Ambulatory heart function and transplant patients’ perceptions of drug–drug interactions: a qualitative study. Can J Hosp Pharm. 2022;75(2):71–8.
PubMed PMC
15 Enoxaparin/agents with antiplatelet properties. In: Lexicomp drug interactions. Wolters Kluwer Clinical Drug Information Inc; 2020.
Casara Hong, BSc, PharmD, ACPR, is with St Paul’s Hospital, Providence Health Care, Vancouver, British Columbia
Michael Legal, BSc(Pharm), PharmD, ACPR, FCSHP, is with St Paul’s Hospital, Providence Health Care, and the Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, British Columbia
Harkaryn Bagri, BSc, BSc(Pharm), ACPR, is with Surrey Memorial Hospital, Fraser Health Authority, Surrey, British Columbia
Louise Lau, BSc, BSc(Pharm), ACPR, is with Vancouver General Hospital, Vancouver Coastal Health, Vancouver, British Columbia
Karen Dahri, BSc, BSc(Pharm), PharmD, ACPR, FCSHP, is with Vancouver General Hospital, Vancouver Coastal Health, and the Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, British Columbia
Competing interests: None declared. ( Return to Text )
Address correspondence to: Dr Karen Dahri, Vancouver General Hospital, Vancouver Coastal Health, 855 West 12th Avenue, Vancouver BC V5Z 1M9, email: karen.dahri@vch.ca
(Return to Top)
Note: This article contains supplementary material (Supplement 1), available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/209 ( Return to Text )
Funding: None received. ( Return to Text )
Acknowledgements
The authors would like to thank the panel of content-matter experts and pharmacists who provided discussion and feedback during the developmental stages of the TLC-Act tool.
Canadian Journal of Hospital Pharmacy, VOLUME 75, NUMBER 3, Summer 2022


