Sukhpreet Poonia, Mazen Sharaf, Ric M Procyshyn, Randal White, Reza Rafizadeh
It has been estimated that 25% to 30% of individuals with a diagnosis of schizophrenia meet the criteria for treatment-resistant schizophrenia.1,2 This condition is commonly defined as the persistence of positive and negative symptoms despite 2 or more trials, of adequate dose and duration, of antipsychotic medication, with documented adherence.2 Currently, the only antipsychotic approved for use in treatment-resistant schizophrenia is clozapine. Of patients with this diagnosis, 30% to 60% will respond to an adequate trial of clozapine, defined as 12 weeks or more of treatment with plasma concentration of at least 1070 nmol/L (350 ng/mL).2 The remaining 40% to 70% of patients are considered to have clozapine resistance, and augmentation with electroconvulsive therapy (ECT) or other medications is often considered.3,4 Currently, the Canadian and US schizophrenia guidelines do not provide detailed guidance on augmentation strategies.1,5 However, an international expert working group recently achieved consensus on the use of amisulpride as an augmentation strategy in clozapine resistance for mixed positive and negative symptoms.6
Overall, previous prospective controlled studies have shown mixed results for amisulpride augmentation of clozapine, with both positive and negative findings.7–9 However, in clinical settings globally and in Canada, amisulpride is sometimes considered, given that its mechanism of action and receptor profile may be complementary to those of clozapine.
Amisulpride is a substituted benzamide antipsychotic with a selective affinity for the dopamine D2 and D3 receptors.10 At lower doses (50–300 mg/day), amisulpride enhances dopamine transmission by selectively antagonizing presynaptic autoreceptors, contributing to the drug’s efficacy in addressing primarily negative symptoms, whereas at higher doses (400–800 mg/day), amisulpride antagonizes postsynaptic D2 and D3 receptors in the limbic system, which is predictive of potent antipsychotic activity.10–12 Furthermore, amisulpride acts as a potent antagonist at the serotonin 5HT7 receptor, which is postulated to contribute to its antidepressant efficacy.13,14 The limbic selectivity of amisulpride is associated with a low propensity for causing extrapyramidal side effects.10 Amisulpride has little or no affinity for D1, D4, D5, serotonin, μ-adrenergic, H1 histaminergic, or anticholinergic receptors.15 Consequently, it is less likely than some other antipsychotics to increase the burden of side effects characteristic of clozapine, such as sedation, weight gain, and anticholinergic effects.15 Although amisulpride is not currently marketed or approved for use in clinical practice in North America, in Canada it can be obtained through Health Canada’s special access program for drugs.16
In this retrospective case series, we describe the practice of amisulpride augmentation of clozapine in a Canadian inpatient setting for treatment-resistant schizophrenia.
This retrospective case series involved patients admitted to a provincial tertiary inpatient program that provides specialized treatment and services to patients with treatment-resistant schizophrenia in a multidisciplinary setting, with an average length of stay of 25 weeks. At the study institution, patients’ symptom severity is measured by applying the Positive and Negative Syndrome Scale (PANSS) on admission and at discharge. The PANSS is a validated psychiatric rating scale and is considered the gold standard for quantifying symptoms related to schizophrenia in clinical trials. It is also used in clinical practice for monitoring treatment outcomes.17 At both admission and discharge, the PANSS is administered by the most responsible psychiatrist. Most admitted patients who meet the criteria for clozapine resistance are offered ECT; if this therapy is declined, patient-specific pharmacological strategies are typically offered. The criteria for clozapine resistance are 12 weeks of clozapine therapy at a therapeutic level (measured at least once and preferably twice over the course of treatment) with persistence of positive symptoms according to the PANSS, with 1 item rated at 6 or 2 items rated at 4.
Two of the authors (S.P. and M.S.) conducted a chart review, using electronic records, to identify patients with clozapine-resistant schizophrenia who underwent amisulpride augmentation of clozapine over the period January 1, 2017, to May 31, 2020. The starting date was chosen because our program did not access amisulpride (through the Health Canada special access program for drugs) before 2017. Of the 6 cases identified through this search, S.P. abstracted data for the first 3 cases, and M.S. abstracted data for the last 3 cases. The information collected for each case was reviewed independently by R.R.
This retrospective case series was approved by the Clinical Research Ethics Boards of the University of British Columbia and Vancouver Coastal Health Authority and employed principles highlighted in the Declaration of Helsinki. Given the retrospective nature of this study, patient consent was waived as per the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans and guidance from the local institutional review board.
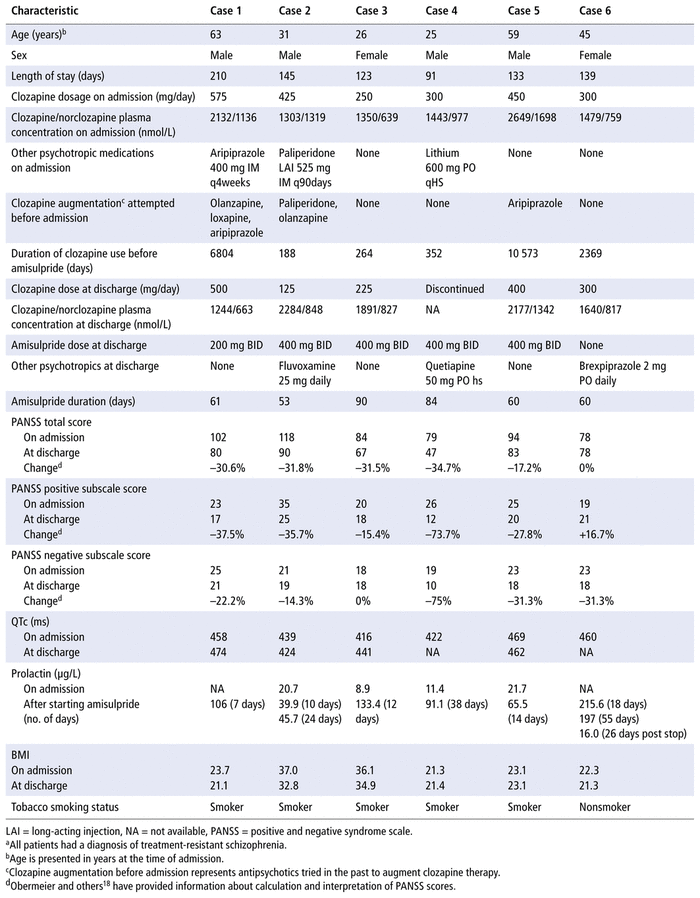
The 6 cases in which patients with clozapine resistance received amisulpride augmentation of their clozapine therapy are summarized in Table 1. All 6 patients were Caucasian. ECT was offered to all of the patients before initiation of amisulpride, and all declined. The mean clozapine dose at the time of admission was 383.3 mg/day, and the mean serum level of clozapine/norclozapine concentration was 1726/1088 nmol/L before initiation of amisulpride. Although the PANSS rating was obtained at the time of admission, rather than the time when amisulpride was started, the psychiatrists’ progress notes indicated no marked improvement in clinical presentation from the time of admission to the initiation of amisulpride.
TABLE 1 Summary of Casesa

Four of the 5 patients who tolerated amisulpride had a greater than 20% reduction in total PANSS score at the time of discharge (Table 1). In terms of the negative symptoms subscale, 3 of the 5 patients who tolerated amisulpride had a greater than 20% reduction in this symptom domain, despite a higher dosage range of amisulpride. In case 6, amisulpride was discontinued because of symptomatic hyperprolactinemia (amenorrhea); cases 1 to 5 displayed asymptomatic hyperprolactinemia at the time of discharge. Prolactin increases were noted as early as 1 to 2 weeks after starting the amisulpride treatment.
In case 4, the patient’s clozapine therapy was discontinued 7 days before discharge as a precautionary measure, because of abnormal ventricular septal motion detected by echocardiography. The patient was maintained on amisulpride monotherapy until discharge.
In case 2, the patient’s clozapine dose was decreased because of initiation of fluvoxamine 25 mg/day. Fluvoxamine is a potent inhibitor of cytochrome P450 1A2 (CYP1A2), which is the main enzyme involved in clozapine metabolism.19 Addition of fluvoxamine to clozapine will result in phenoconversion to poor metabolizer status at CYP1A2.19 In this patient, fluvoxamine was initiated after assessment of the clozapine–norclozapine ratio by the treating psychiatrist, which indicated extensive metabolizer status at CYP1A2. The serum levels from admission to discharge depicted a change in this ratio from 0.99 to 2.7.
Extrapyramidal symptoms (EPS) associated with the augmentation strategy were recorded if there was any use of anti-EPS medication after initiation of amisulpride or verified symptoms were clearly documented in the patient’s chart (or both). No significant changes in EPS were noted in any of the cases, nor was there a significant weight increase for any patient. There was a clinically insignificant increase in QTc interval after initiation of amisulpride in cases 1 and 3 (16 ms and 25 ms from baseline, respectively). Similarly, there was a decrease in QTc interval after initiation of amisulpride in cases 2 and 5 (15 ms and 7 ms from baseline, respectively). The remaining 2 patients did not undergo electrocardiography at a time close to discharge.
This case series supports the notion that augmentation of clozapine with amisulpride in patients with clozapine resistance may result in measurable improvements in both positive and negative symptom domains. Multiple confounding factors can influence a reduction in PANSS score in a specialized inpatient setting; consequently, we cannot attribute observed improvements exclusively to amisulpride augmentation. These variables include (but are not limited to) increased contact time with the health care team in a controlled environment and increased availability of cognitive behavioural therapy. Consequently, our data might not be generalizable to outpatient settings. Furthermore, in case 2, augmentation with fluvoxamine occurred concomitantly with amisulpride, which might have contributed to the measurable improvements at the time of discharge.
Although for each patient both admission and discharge PANSS scores were determined by the same psychiatrist, not a masked rater, we could not ensure retrospectively whether the scoring and calculations had been conducted without error or whether observer bias had been avoided. In addition, because the initial PANSS scores were obtained at the time of admission, not at the time of amisulpride initiation, we had to use progress notes to determine if there were marked improvements in the clinical presentation. Consequently, we do not know whether the PANSS scores had already declined, before initiation of amisulpride. Furthermore, given the retrospective nature of this study, data collection was limited to information that was available in the chart.
We strengthened the chart review process by utilizing data abstractors who were clinical pharmacists with specialization in mental health and strong familiarity with the organization of the electronic chart system. Abstracted data for each case were also reviewed independently by another clinical pharmacist.
Hyperprolactinemia was a consistent finding across all 6 patients. In a 2-week study of patients with schizophrenia, prolactin elevation was markedly greater with amisulpride than with other atypical agents.20 Elevated prolactin levels have been associated with oligomenorrhea, amenorrhea, galactorrhea, decreased libido, infertility, breast cancer in women,21 and decreased bone mass; these outcomes should be taken into account when augmentation is being considered.22 Although amisulpride has been associated with dose-dependent QT prolongation,23 the patients described in our case series did not experience clinically relevant increases in QT at therapeutic doses of amisulpride.
As mentioned above, amisulpride is not readily available in Canada and requires approval from Health Canada. Such approval generally takes about 24 hours, and shipping to the practitioner’s office or pharmacy takes another 6 to 8 weeks. Consequently, advance planning is important. Amisulpride also poses cost concerns, as it is available from only 1 manufacturer, and a single amisulpride 400-mg tablet costs twice as much as a clozapine 200-mg tablet. A cost–utility analysis would be of benefit to evaluate the overall cost effectiveness of this intervention.
To our knowledge, this is the first study examining the practice of augmentation of clozapine therapy with amisulpride in Canada, based on obtaining the medication through Health Canada’s special access program for drugs. Given the extended duration of stay in our tertiary facility, we have been able to provide details about the tolerability and efficacy of this augmentation strategy for the cases reported here. In light of mixed evidence of benefit, elevated costs, and barriers to access, the decision to prescribe amisulpride in Canada should be taken with caution and proactive planning. Further studies are needed to better understand the long-term efficacy and tolerability of this augmentation strategy. Future studies should focus on better characterization of subgroups of patients with treatment-resistant schizophrenia, to allow for greater precision in the selection of patients most likely to benefit from such an augmentation strategy.
1 Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. 2017; 62(9):604–16.
Crossref PubMed PMC
2 Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216–29.
Crossref
3 Potkin SG, Kane JM, Correll CU, Lindenmayer JP, Agid O, Marder SR, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6:Article 1.
Crossref PubMed PMC
4 Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772–7.
Crossref PubMed PMC
5 Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2020;177(9):868–72.
Crossref PubMed
6 Wagner E, Kane JM, Correll CU, Howes O, Siskind D, Honer WG, et al.; TRIPP Working Group. Clozapine combination and augmentation strategies in patients with schizophrenia—recommendations from an international expert survey among the Treatment Response and Resistance in Psychosis (TRRIP) Working Group. Schizophr Bull. 2020; 46(6):1459–70.
Crossref PubMed PMC
7 Assion HJ, Reinbold H, Lemanski S, Basilowski M, Juckel G. Amisulpride augmentation in patients with schizophrenia partially responsive or unresponsive to clozapine. A randomized, double-blind, placebo-controlled trial. Pharmacopsychiatry. 2008;41(1):24–8.
Crossref PubMed
8 Barnes TRE, Leeson V, Paton C, Marston L, Osborn DP, Kumar R, et al. Amisulpride augmentation of clozapine for treatment-refractory schizophrenia: a double-blind, placebo-controlled trial. Ther Adv Psychopharmacol. 2018;8(7):185–97.
Crossref PubMed PMC
9 Genç Y, Taner E, Candansayar S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther. 2007;24(1):1–13.
Crossref PubMed
10 Perrault G, Depoortere R, Morel E, Sanger DJ, Scatton B. Psychopharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther. 1997;280(1):73–82.
PubMed
11 Boyer P, Puech AJ, Lecrubier Y. Double blind trial versus placebo of low dose amisulpride (Solian 50) in schizophrenia with exclusively negative symptoms. Preliminary analysis of results. Ann Psychiatr. 1988; 3(3):321–5.
12 da Mota Neto JI, Soares BGO, de Lima MS. Amisulpride for schizophrenia. Cochrane Database Syst Rev. 2002:2002(2):CD001357.
13 Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL. Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berl). 2009;205(1):119–28.
Crossref
14 Smeraldi E. Amisulpride versus fluoxetine in patients with dysthymia or major depression in partial remission: a double-blind, comparative study. J Affect Disord. 1998;48(1):47–56.
Crossref PubMed
15 Pani L, Villagrán JM, Kontaxakis VP, Alptekin K. Practical issues with amisulpride in the management of patients with schizophrenia. Clin Drug Investig. 2008;28(8):465–77.
Crossref PubMed
16 Health Canada’s special access programs: overview. Government of Canada; 2020 [cited 2021 Feb 11]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/special-access.html
17 Fleischhacker W. The usefulness of rating scales in patients with schizophrenia. Acta Psychiatr Scand. 2016;133(6):435.
Crossref
18 Obermeier M, Schennach-Wolff R, Meyer S, Möller HJ, Riedel M, Krause D, et al. Is the PANSS used correctly? A systematic review. BMC Psychiatry. 2011;11:Article 113.
Crossref PubMed PMC
19 Kashuba AD, Nafziger AN, Kearns GL, Leeder JS, Gotschall R, Rocci ML Jr, et al. Effect of fluvoxamine therapy on the activities of CYP1A2, CYP2D6, and CYP3A as determined by phenotyping. Clin Pharmacol Ther. 1998;64(3):257–68.
Crossref PubMed
20 Fric M, Laux G. [Prolactin levels and symptoms of hyperprolactinemia in patients treated with amisulpride, risperidone, olanzapine and quetiapine]. Psychiatr Prax. 2003;30(Suppl 2):97–101. Article in German.
Crossref PubMed
21 Taipale H, Solmi M, Lähteenvuo M, Tanskanen A, Correll CU, Tiihonen J. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case–control study in Finland. Lancet Psychiatry. 2021;8(10):883–91.
Crossref PubMed
22 Majumdar A, Mangal NS. Hyperprolactinemia. In: Ghumman S, editor. Principles and practice of controlled ovarian stimulation in ART. p. 319–28. Springer; 2015 [cited 2022 May 20]. Available from: https://link.springer.com/chapter/10.1007/978-81-322-1686-5_29
Crossref
23 Isbister GK, Balit CR, Macleod D, Duffull SB. Amisulpride overdose is frequently associated with QT prolongation and torsades de pointes. J Clin Psychopharmacol. 2020;30(4):391–5.
Crossref
Competing interests: For activities not directly related to the case series reported here, Sukhpreet Poonia and Reza Rafizadeh have received payment for leading workshops on opioid-use disorder from the British Columbia Pharmacy Association; Ric Procyshyn has received royalties from the publisher of Clinical Handbook of Psychotropic Drugs, honoraria for advisory board service from Janssen, Lundbeck, and Otsuka, and honoraria for participation on speakers’ bureaus from Janssen, Lundbeck, Otsuka, and Eisai; and Randal White has received honoraria from HLS Therapeutics and the Canadian Agency for Drugs and Technology in Health. No other competing interests were declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 75, NUMBER 3, Summer 2022