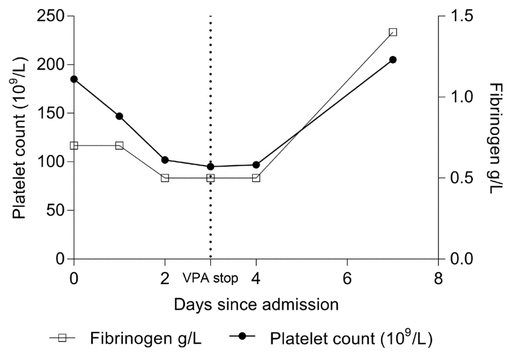
FIGURE 1 Platelet count and fibrinogen levels throughout the first week of hospital admission. The dotted line reflects the day when valproic acid (VPA) was discontinued.
Tanveer Brar, Sherif Hanafy Mahmoud
Valproic acid (VPA) is an antiepileptic drug used for the treatment of various seizure types, psychiatric disorders, and migraines.1 It has a complex mechanism of action, most likely associated with potentiating the effect of γ-aminobutyric acid (GABA) in the central nervous system and thus inhibiting neuronal excitation.2,3 VPA has good oral bioavailability, is widely distributed in the body, and is highly protein bound (up to 90%), mainly to albumin.4,5 It is metabolized in the liver primarily through glucuronidation and β-oxidation and should be used cautiously in those at risk of liver dysfunction.6
Given its narrow reference range, wide intra- and inter-patient variability, and diverse toxicity profile, VPA is a prime candidate for therapeutic drug monitoring. Current therapeutic drug monitoring practices involve measuring total VPA levels in the serum, even though it is the free drug that exhibits pharmacologic effect. Because of its high protein binding, conditions that result in an acute decrease in albumin would thus shift the equilibrium to a higher free fraction of VPA, resulting in more drug being available to elicit effect and increasing the risk of toxicity. Furthermore, VPA toxicity has a range of presentations, making its clinical diagnosis challenging.7–9
This case report describes a unique presentation of VPA toxicity and emphasizes the importance of determining free VPA levels, especially in the setting of hypoalbuminemia.
A patient in their late 40s was admitted to hospital from a long-term care facility with severe agitation and hypotension.* The patient had vague abdominal pain, decreased oral intake, and weight loss of about 4.5 kg over the course of a month. Two years before the current presentation, the patient had experienced a left middle cerebral artery ischemic stroke, which caused significant cognitive deficits and right-side hemiparesis. Shortly after the stroke, the patient had a first episode of an isolated tonic-clonic seizure, and VPA 500 mg orally, twice a day, was initiated. During the year preceding admission to hospital, the patient had been maintained on the same dose, with documentation of therapeutic total VPA level and normal albumin, and there was no documented recurrence of seizure activity or adverse effects.
The patient’s past medical history was pertinent for celiac disease, thrombocytosis (baseline platelet count 500 to 700 × 109/L), type 2 diabetes mellitus, and recurrent episodes of aspiration pneumonia; one of these episodes led to a hospital admission 3 months before the current presentation. The regimen of medications before admission was stable, with no drug–drug interactions noted. In addition to VPA, the pre-existing home medications were atorvastatin, apixaban, topiramate, gabapentin, pantoprazole, calcium, and vitamins.
On admission, the patient had an albumin level of 10 g/L (reference range 34–54 g/L), profound hypotension, and lactate of 4.7 mmol/L (reference range 0.5–1 mmol/L). Fluid resuscitation was accomplished with 7 L of IV crystalloids; in addition, 25% albumin was given by the IV route every 6 hours for 24 hours, and norepinephrine and vasopressin were initiated. Piperacillin–tazobactam, vancomycin, and hydrocortisone were initiated empirically for possible septic shock, and the patient was transferred to the intensive care unit (ICU) for ongoing management.
The results of laboratory tests at the time of admission showed the following coagulation abnormalities: platelet count 185 × 109/L (reference range 150–400 × 109/L) and fibrinogen 0.7 g/L (reference range 2.0–4.0 g/L). Several electrolyte derangements were also noted: potassium 3.1 mmol/L (reference range 3.5–5.1 mmol/L), sodium 125 mmol/L (reference range 135–145 mmol/L), magnesium 0.59 mmol/L (reference range 0.75–1.2 mmol/L), phosphate 0.67 mmol/L (reference range 0.8–1.45 mmol/L), and ionized calcium 0.97 mmol/L (reference range 1.13–1.4 mmol/L). The electrolyte abnormalities were believed to reflect poor nutritional status. Measurement of total VPA serum trough concentration was ordered to rule out acute VPA toxicity; the level was slightly subtherapeutic, at 341 μmol/L (reference range 350–850 μmol/L), and VPA was therefore continued.
Cultures of blood, sputum, and urine samples obtained on admission did not grow any pathogens. Computed tomography (CT) of the head, to rule out structural causes of agitation, showed no acute abnormalities. CT of the abdomen and pelvis was also done to investigate potential organic causes of abdominal pain; this imaging revealed diffusely increased subcutaneous fat throughout the torso and thighs suggestive of anasarca, multifocal and extensive thickening of the colon suggesting colitis, and mild sigmoid diverticulosis. At this point, a gastroenterology consultation was requested.
Testing for tissue transglutaminase immunoglobulin A yielded a positive result, and it was noted (following confirmation with the long-term care facility) that the patient had not been receiving a gluten-free diet since the most recent discharge from hospital about 3 months beforehand. A protein-losing enteropathy was diagnosed, which explained the severely low albumin and associated anasarca. Throughout the admission, coagulation markers continued to decline, with fibrinogen and platelet nadirs of 0.5 g/L and 95 × 109/L, respectively. A hematology opinion was requested, and hemolysis was ruled out.
On day 3 of the ICU admission, measurement of serum ammonia level was requested, and a send-out test for free VPA level was arranged. These tests showed elevation of both ammonia and free VPA (274 μmol/L; reference range 17–76 μmol/L). At this time, the VPA was discontinued and levetiracetam 500 mg orally, twice daily, was started. Approximately 1 week after discontinuation of VPA, the patient’s fibrinogen and platelet counts began to recover (Figure 1), and the agitation resolved.
|
| ||
|
FIGURE 1 Platelet count and fibrinogen levels throughout the first week of hospital admission. The dotted line reflects the day when valproic acid (VPA) was discontinued. | ||
VPA has complex pharmacokinetics, including a low hepatic extraction ratio and nonlinear profile due to saturable protein binding.1 When the dose of VPA is increased, the change in total serum concentration is not proportional to the change in dose. Rather, the change in total serum concentration is less than might be expected with a dose increase, whereas the change in free VPA is much greater than expected, because albumin binding sites are already occupied. Therefore, in situations involving alterations in albumin, there can be a substantial effect on the free fraction of VPA, disproportionate with total concentration.
Akin to its pharmacological effect, unbound VPA causes toxic effects, and the incidence and severity of these adverse effects depends on the concentration. The clinical presentation of toxicity is variable and can affect many body systems, making it challenging to predict what will occur. Commonly observed adverse effects include nausea, dyspepsia, tremor, and visual disturbances. In rare circumstances, patients may experience neurotoxicity, which manifests as myoclonus, miosis, severe agitation, or seizures, and hematological toxicities, including hypofibrinogenemia and thrombocytopenia. In cases of severe toxicity or overdose, anion gap metabolic acidosis, respiratory depression, hypotension, acute respiratory distress syndrome, and cerebral edema may also occur.7–11
Significant elevation of free VPA in patients with hypoalbuminemia has been highlighted previously in several case reports and observational studies.12–15 However, little has been reported concerning critically ill patients. Elevated free VPA in critically ill patients has been reported in 4 case reports and a case series of 15 patients (median percentage of free VPA 48%, interquartile range 15%–89%).16–20 However, not all patients exhibited probable VPA toxicity. Reported adverse drug events that were possibly related to VPA were hyperammonemia, elevation of liver enzymes, thrombocytopenia, and lethargy.16–20
To our knowledge, the case reported here is the first to describe diverse presentation of VPA toxicity secondary to the elevated free fraction (hematological abnormalities combined with agitation) in a critically ill patient. In addition, this case highlights the prompt reversal of such adverse effects upon discontinuation of VPA. In this case, the patient had thrombocytosis at baseline and presented with a remarkable drop in platelet count. Additionally, the patient’s fibrinogen levels were low at the time of admission and continued to decline over the first few days of the hospital stay. Upon discontinuation of VPA, both platelet count and fibrinogen level began to recover.
A modest amount of literature suggests that VPA can affect fibrinogen, including a case report of intracranial hemorrhage secondary to VPA-induced hypofibrinogenemia.21,22 The mechanism of this effect has not been well documented, and recovery is generally quick (within 5 days of VPA discontinuation).21 In our case, other differential diagnoses for the patient’s hematological abnormalities were excluded by our hematology colleagues. This patient also presented with severe agitation, which is an unusual manifestation of VPA toxicity. Given the proposed action of VPA on GABA receptors, we would typically expect patients with VPA toxicity to present with depressed mental status. However, some previous literature suggests that VPA neurotoxicity can present as agitation and in some instances can also lead to further seizures.10,23 Ultimately, this patient’s laboratory abnormalities and clinical symptoms began to normalize after discontinuation of VPA. On the basis of the patient’s presentation and recovery, we calculated the Naranjo score to help determine the likelihood that the symptoms were a result of VPA.24 The Naranjo score was 6, suggesting that the presenting abnormalities were “probably” due to VPA. No other medication changes were made at the same time as the VPA discontinuation, aside from hydrocortisone discontinuation, which was less likely than VPA to have contributed to the current finding.
Current practice for therapeutic drug monitoring of VPA is to measure total trough concentrations, because of the limited availability and greater cost of measuring free levels (although the cost varies from one centre to another). In this case, testing of free VPA was not available in any of the local centres, and the sample was therefore sent outside the province for analysis, which led to additional shipping costs. Unfortunately, no equations are available that allow accurate calculation of free VPA concentration from total serum concentration, and the greatest discordance appears to occur in critically ill patients.25,26
This case highlights that total VPA concentration can be misleading in patients with hypoalbuminemia, and it emphasizes the importance of monitoring free, or unbound, VPA concentration. Because albumin is a negative acute-phase reactant, patients who are acutely ill and receiving VPA are at the highest risk of experiencing toxic effects, and VPA should therefore be used with caution and concentrations should be monitored carefully. Patients with low albumin levels often have various other comorbidities (including chronic kidney disease, older age, acute illness, and malnourishment) that put them at greater risk of adverse drug reactions; measuring only the total VPA serum concentration in these patients may be misleading.
This report is limited by the inherent nature of case reports. The description of a single patient case limits the generalizability of the findings. In addition, there is a risk of bias secondary to overinterpretation of the patient’s clinical progress. Nonetheless, this case serves to emphasize that clinicians must be vigilant in monitoring free VPA in critically ill patients with hypoalbuminemia.
VPA toxicity can present in many ways, and it can therefore be challenging to diagnose clinically. Total VPA levels may underestimate the true drug exposure in patients with hypoalbuminemia and can potentially result in failure to recognize toxicity. Measuring free VPA levels in patients with hypoalbuminemia, particularly in settings of acute changes in albumin, is a better predictor of VPA exposure.
1 Abou-Khalil BW. Antiepileptic drugs. Continuum (Minneap Minn). 2016;22(1 Epilepsy):132–56.
2 Rosenberg G. The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cell Mol Life Sci. 2007;64(16):2090–103.
Crossref PubMed
3 Silva MFB, Aires CCP, Luis PBM, Ruiter JPN, IJlst L, Duran M, et al. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: a review. J Inherit Metab Dis. 2008;31(2):205–16.
Crossref PubMed
4 Chan K, Beran RG. Value of therapeutic drug level monitoring and unbound (free) levels. Seizure. 2008;17(6):572–5.
Crossref PubMed
5 Wulff K, Flachs H, Würtz-Jorgensen A, Gram L. Clinical pharmacological aspects of valproate sodium. Epilepsia. 1977;18(2):149–57.
Crossref PubMed
6 Gugler R, von Unruh GE. Clinical pharmacokinetics of valproic acid. Clin Pharmacokinet. 1980;5(1):67–83.
Crossref PubMed
7 Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol. 2000;22(1):62–5.
Crossref PubMed
8 Gerstner T, Teich M, Bell N, Longin E, Dempfle CE, Brand J, et al. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia. 2006;47(7):1136–43.
Crossref PubMed
9 Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323–38.
Crossref PubMed
10 Dupuis RE, Lichtman SN, Pollack GM. Acute valproic acid overdose. Clinical course and pharmacokinetic disposition of valproic acid and metabolites. Drug Saf. 1990;5(1):65–71.
Crossref PubMed
11 Gerstner T, Buesing D, Longin E, Bendl C, Wenzel D, Scheid B, et al. Valproic acid induced encephalopathy—19 new cases in Germany from 1994 to 2003—a side effect associated to VPA-therapy not only in young children. Seizure. 2006;15(6):443–8.
Crossref PubMed
12 Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40(10):986–93.
Crossref PubMed
13 Gidal BE, Collins DM, Beinlich BR. Apparent valproic acid neurotoxicity in a hypoalbuminemic patient. Ann Pharmacother. 1993;27(1):32–5.
Crossref PubMed
14 Dautzenberg G, Nederlof M, Beekman A, Egberts T, Heerdink ER. Severe cognitive impairment associated with a high free but therapeutic total concentration of valproic acid due to hypoalbuminemia in an older patient with bipolar disorder. J Clin Psychopharmacol. 2018;38(3):265–8.
Crossref PubMed
15 Doré M, San Juan AE, Frenette AJ, Williamson D. Clinical importance of monitoring unbound valproic acid concentration in patients with hypoalbuminemia. Pharmacotherapy. 2017;37(8):900–7.
Crossref PubMed
16 Jansen AJG, Hunfeld NGM, van Bommel J, Koch BCP, van Gelder T. Therapeutic drug monitoring of free fraction valproic acid in patients with hypoalbuminaemia. Neth J Med. 2012;70(7):329.
PubMed
17 de Maat MM, van Leeuwen HJ, Edelbroek PM. High unbound fraction of valproic acid in a hypoalbuminemic critically ill patient on renal replacement therapy. Ann Pharmacother. 2011;45(3):e18.
Crossref PubMed
18 Haroldson JA, Kramer LE, Wolff DL, Lake KD. Elevated free fractions of valproic acid in a heart transplant patient with hypoalbuminemia. Ann Pharmacother. 2000;34(2):183–7.
Crossref PubMed
19 Lagneau F, Perbet S, Delefosse D, Wernet A, Stocco J, Marty J. Drugs pharmacokinetics in ICU patients: consequences of hypoalbuminemia upon drugs monitoring and dosing scheme. Intensive Care Med. 2004;30(6):1247.
Crossref PubMed
20 Riker RR, Gagnon DJ, Hatton C, May T, Seder DB, Stokem K, et al. Valproate protein binding is highly variable in ICU patients and not predicted by total serum concentrations: a case series and literature review. Pharmacotherapy. 2017;37(4):500–8.
Crossref PubMed
21 Chen HF, Xu LP, Luo ZY, Yu ZQ, Li ZY, Cui QY, et al. Valproic acid-associated low fibrinogen and delayed intracranial hemorrhage: case report and mini literature review. Drug Des Devel Ther. 2013;7:767–70.
Crossref PubMed PMC
22 Karakayali B, Onsal Ozturk D, Yazar AS, Guven S, Islek I. Hypofibrinogenemia and intra-articular hemorrhage due to valproic acid. Pediatr Int. 2016;58(12):1358–9.
Crossref PubMed
23 Sztajnkrycer MD. Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol. 2002;40(6):789–801.
Crossref PubMed
24 Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Crossref PubMed
25 Fisch U, Baumann SM, Semmlack S, Marsch S, Rüegg S, Sutter R. Accuracy of calculated free valproate levels in adult patients with status epilepticus. Neurology. 2021;96(1):e102–e110.
Crossref
26 Nasreddine W, Dirani M, Atweh S, Makki A, Beydoun A. Determinants of free serum valproate concentration: a prospective study in patients on divalproex sodium monotherapy. Seizure. 2018;59:24–7.
Crossref PubMed
Competing interests: None declared. ( Return to Text )
*The authors were unable to obtain patient consent. Therefore, potentially identifiable information not relevant to the case has been omitted from the manuscript. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 75, NUMBER 3, Summer 2022