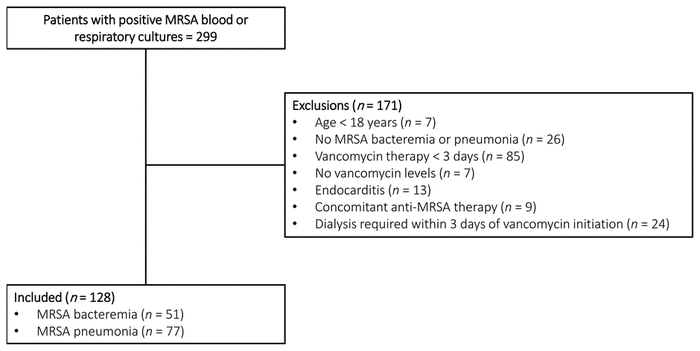
FIGURE 1 Flow diagram for patient inclusion. MRSA = methicillin-resistant Staphylococcus aureus .
Ryan Marko , Julia Hajjar , Vanessa Nzeribe , Michelle Pittman , Vincent Deslandes , Nadia Sant , Juthaporn Cowan , Kwadwo Kyermentang , Tim Ramsay , Sheryl Zelenitsky , Salmaan KanjiABSTRACT
Background
Vancomycin remains widely used for methicillin-resistant Staphylococcus aureus (MRSA) infections; however, treatment failure rates up to 50% have been reported. At the authors’ institution, monitoring of trough concentration is the standard of care for therapeutic drug monitoring of vancomycin. New guidelines support use of the ratio of 24-hour area under the concentration–time curve to minimum inhibitory concentration (AUC 24 /MIC) as the pharmacodynamic index most likely to predict outcomes in patients with MRSA-associated infections.
Objectives
To determine the discordance rate between trough levels and AUC 24 /MIC values and how treatment failure and nephrotoxicity outcomes compare between those achieving and not achieving their pharmacodynamic targets.
Methods
This retrospective cohort study involved patients with MRSA bacteremia or pneumonia admitted to the study hospital between March 1, 2014, and December 31, 2018, and treated with vancomycin. Data for trough concentrations were collected, and minimum concentrations ( C min ) were extrapolated. The AUC 24 /MIC values were determined using validated population pharmacokinetic models. The C min and AUC 24 /MIC values were characterized as below, within, or above pharmacodynamic targets (15–20 mg/L and 400–600, respectively). Discordance was defined as any instance where a patient’s paired C min and AUC 24 /MIC values fell in different ranges (i.e., below, within, or above) relative to the target ranges. Predictors of treatment failure and nephrotoxicity were determined using logistic regression.
Results
A total of 128 patients were included in the analyses. Of these, 73 (57%) received an initial vancomycin dose less than 15 mg/kg. The discordance rate between C min and AUC 24 /MIC values was 21% (27/128). Rates of treatment failure and nephrotoxicity were 34% (43/128) and 18% (23/128), respectively. No clinical variables were found to predict discordance. Logistic regression identified initiation of vancomycin after a positive culture result (odds ratio [OR] 4.41, 95% confidence interval [CI] 1.36–14.3) and achievement of target AUC 24 /MIC after 4 days (OR 3.48, 95% CI 1.39–8.70) as modifiable predictors of treatment failure.
Conclusions
The relationship between vancomycin monitoring and outcome is likely confounded by inadequate empiric or initial dosing. Before any modification of practice with respect to vancomycin monitoring, empiric vancomycin dosing should be optimized.
KEYWORDS: vancomycin , therapeutic drug monitoring , area under the concentration-time curve , trough , methicillin-resistant Staphylococcus aureus
RÉSUMÉ
Contexte
La vancomycine reste largement utilisée contre les infections dues au Staphylococcus aureus méthicillinorésistant (SAMR); cependant, on rapporte des taux d’échec de traitement allant jusqu’à 50 %. Dans l’institution où travaillent les auteurs, la surveillance de la concentration minimale constitue la norme de soins du suivi thérapeutique pharmacologique de la vancomycine. De nouvelles lignes directrices soutiennent l’utilisation du ratio de 24 h de l’aire sous la courbe de concentration-temps à concentration minimale inhibitrice (AUC 24 /MIC) en tant qu’indice pharmacodynamique, vraisemblablement pour prédire certains résultats concernant les patients présentant des infections associées au SAMR.
Objectifs
Déterminer le taux de discordance entre la concentration minimale et les valeurs de l’AUC 24 /MIC et la manière dont les échecs de traitement et les résultats de néphrotoxicité se comparent entre les personnes atteignant leurs cibles pharmacodynamiques et celles qui ne l’atteignent pas.
Méthodes
Cette étude de cohorte rétrospective impliquait des patients atteints d’une bactériémie au SAMR ou d’une pneumonie au SAMR, admis à l’hôpital où se déroulait l’étude entre le 1 er mars 2014 et le 31 décembre 2018 et traités à l’aide de vancomycine. Les données relatives aux concentrations minimales ont été recueillies, et les concentrations minimales ( C min ) extrapolées. Les valeurs de l’AUC 24 /MIC ont été déterminées à l’aide de modèles de population pharmacocinétiques validés. La caractérisation des valeurs de la C min et des valeurs de l’AUC 24 /MIC se décrit comme suit: « en dessous », « à l’intérieur » ou « au-dessus » des cibles pharmacodynamiques (respectivement 15–20 mg/L et 400–600). La discordance était définie comme une situation où les valeurs associées de la C min et de l’AUC 24 /MIC tombaient dans des plages différentes (c.-à-d., en dessous, à l’intérieur ou au-dessus) par rapport aux plages cibles. Une régression logistique a permis de déterminer les prédicteurs d’échecs de traitement et de néphrotoxicité.
Résultats
Au total, 128 patients ont été inclus dans les analyses. De ceux-ci, 73 (57 %) ont reçu une dose initiale de vancomycine de moins de 15 mg/kg. Le taux de discordance entre les valeurs de la C min et de l’AUC 24 /MIC était de 21 % (27/128). Les taux d’échec de traitement et de néphrotoxicité se montaient respectivement à 34 % (43/128) et 18 % (23/128). Aucune variable clinique n’a pu prédire la discordance. La régression logistique a permis de déterminer le début de l’administration de la vancomycine après un résultat de culture positif (rapport de cotes [RC] 4,41, 95 % intervalle de confiance [IC] 1,36–14,3) et l’atteinte de la cible de l’AUC 24 /MIC après quatre jours (RC 3,48, 95 % IC 1,39–8,70) en tant que prédicteurs modifiables de l’échec du traitement.
Conclusions
Il existe probablement une confusion relative à la relation entre la surveillance de la vancomycine et le résultat à cause d’un dosage empirique ou initial inadéquat. Avant de modifier la pratique relative à la surveillance de la vancomycine, le pharmacien doit optimiser son dosage empirique.
MOTS CLÉS: vancomycine , suivi thérapeutique pharmacologique , aire sous la courbe concentration-temps , minimal , Staphylococcus aureus méthicillinorésistant
Methicillin-resistant Staphylococcus aureus (MRSA) is an important pathogen causing a wide variety of clinically significant and often life-threatening infections.1,2 MRSA infections are associated with significant morbidity and mortality, increased length of stay in hospital, and increased cost of care.3 Vancomycin by IV administration remains the drug of choice for MRSA4; however, the incidence of vancomycin treatment failure is reportedly as high as 50%.3 Given the burden of MRSA infections, the paucity of alternative anti-MRSA agents, and the wide pharmacokinetic variability of vancomycin, there is a need to better understand vancomycin therapeutic monitoring to improve its effectiveness and safety while also minimizing the emergence of resistant pathogenic organisms.5
Although vancomycin is one of the oldest and most studied antibiotics, the correlation between monitoring of serum levels of the drug and clinical efficacy continues to be controversial. The latest (2020) consensus guidelines for therapeutic drug monitoring of vancomycin identified the ratio of 24-hour area under the concentration–time curve to minimum inhibitory concentration (AUC 24 /MIC) as the pharmacodynamic parameter most likely to predict clinical efficacy, with bactericidal activity being achieved with AUC 24 /MIC values of 400 or above and an upper limit of 600 to minimize the risk of nephrotoxicity.6 However, because of the logistic challenges associated with calculating AUC in practice, attaining trough levels of 15 to 20 mg/L has historically been recommended as a surrogate for the AUC 24 /MIC in cases of serious MRSA infections. Despite these past recommendations, retrospective and simulation studies have shown that targeting trough levels of 15 to 20 mg/L is not always associated with attainment of the AUC 24 /MIC target of at least 400. Conversely, using Monte Carlo simulations, Patel and others7 demonstrated that trough levels over 15 mg/L were not always required to achieve AUC 24 /MIC values of 400 when the MIC was 1 mg/L or lower, whereas targeting trough levels of 15 to 20 mg/L did not consistently result in AUC 24 /MIC values of 400 or above for MIC values of at least 2 mg/L. These results have led to updated guidelines favouring AUC 24 /MIC monitoring.6–10 Furthermore, although both trough levels of at least 15 mg/L and AUC 24 /MIC above 600 are associated with an increased risk of nephrotoxicity,6,11,12 the trough target of 15 to 20 mg/L does not correlate with clinical efficacy.7,9,13,14
As evidence supporting AUC 24 /MIC monitoring grows and new tools emerge to aid with AUC estimation,15 institutions are engaging in practice shifts away from monitoring trough levels to align with proposed AUC 24 /MIC targets.16–18 The primary objective of this study was to explore discordance rates between vancomycin trough level and AUC 24 /MIC monitoring in a cohort of patients with MRSA bacteremia or pneumonia. Given the limited clinical data regarding the significance of trough level and AUC 24 /MIC exposure in terms of clinical outcomes, our secondary objectives were to determine how proportions of efficacy and safety outcomes compare between those achieving and those not achieving the pharmacodynamic targets defined by trough and AUC 24 /MIC monitoring.
This retrospective observational study was conducted at 2 campuses of The Ottawa Hospital, a public, university-affiliated teaching institution with approximately 1100 beds. Ethics approval for this study was obtained, before initiation, from the Ottawa Hospital Research Ethics Board (REB CRRF 1154/protocol 20190031-01H).
Consecutive patients admitted to either campus of The Ottawa Hospital between March 1, 2014, and December 31, 2018, who had blood or respiratory cultures that grew MRSA were identified from the local microbiology database and assessed for study eligibility.
Patients were included if they were at least 18 years of age and had MRSA bacteremia or MRSA pneumonia. MRSA bacteremia was defined by at least one blood culture that was positive for MRSA. MRSA pneumonia was defined as MRSA growth in respiratory cultures and consistent clinical presentation of pneumonia, defined as the presence of infiltrate on chest radiography and at least one of the following criteria: purulent tracheal secretions documented in nursing notes, documented temperature of 38°C or above, or leukocyte count of 10 000/μL (10 × 109/L) or higher. Patients with MRSA-positive results for both blood and respiratory cultures were included in the MRSA pneumonia group. Additional inclusion criteria were treatment with vancomycin for at least 3 days and at least 1 serum vancomycin level reported at steady state (i.e., before the third dose or later). Patients were excluded from the study if they had infective endocarditis; had received concomitant therapy for MRSA with linezolid, daptomycin, sulfamethoxazole-trimethoprim, or tigecycline; or required dialysis within the first 3 days of vancomycin therapy.
Data were manually collected from electronic medical records by 3 of the investigators (R.M., J.H., and V.N.). A standardized and piloted case report form was used to collect patients’ demographic information, comorbidities, sequential organ failure assessment (SOFA) scores, blood and respiratory culture results, concomitant antibiotic use during vancomycin therapy, vancomycin doses and serum levels, timing of doses and serum levels, patient weight, serum creatinine throughout the course of vancomycin therapy, and concomitant use of nephrotoxic drugs during vancomycin therapy (limited to IV contrast, amphotericin B, aminoglycosides, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, and IV acyclovir and ganciclovir).
Treatment failure was defined as one or more of the following: death from any cause within 30 days of the index MRSA culture; microbiologic failure, defined as a blood or sputum sample growing MRSA that was obtained 7 or more days after the initiation of vancomycin therapy; or recurrence of MRSA bacteremia within 60 days after discontinuation of vancomycin therapy.19,20
Nephrotoxicity was defined as an increase in serum creatinine by 50% across any 2 consecutive time points from day 3 of vancomycin initiation up to 5 days after the end of vancomycin therapy.21 Nephrotoxicity was also assessed using the RIFLE criteria at the time of discharge from the intensive care unit (ICU) (if applicable) and hospital discharge.22
For the purpose of this study, MRSA clinical isolates were recovered from frozen stocks when available. The MICs for vancomycin were further determined by an agar dilution method. Mueller Hinton agar (MHA) plates containing vancomycin concentrations of 0.0625 to 128 μg/mL were prepared. Select colonies from 18- to 24-hour overnight incubation on a blood agar plate were each suspended in 1 mL sterile saline, and the turbidity was adjusted to 0.5 McFarland standard. These suspensions were then diluted 1:10 in sterile saline to give an inoculum concentration of 107 colony-forming units (CFU)/mL. Bacterial suspensions were then inoculated onto the MHA-containing vancomycin plates with the help of multipoint inoculators with 37 points (3 mm in diameter), each pin being able to deposit approximately 1 to 2 μL on the agar surface (equivalent to 104 CFU in a spot 5–8 mm in diameter). Staphylococcus aureus ATCC 25923 and ATCC 29213 were included on all test plates as control organisms. The plates were incubated at 35°C to 37°C for 24 hours. Results were read as the presence or absence of growth, where the MIC of a strain was considered as the lowest concentration of the antibiotic at which there was no visible growth.
Only single serum vancomycin levels were expected to be available for most patients within each dosing interval. Therefore, validated 2-compartment population pharmacokinetic models of vancomycin for critically ill and non–critically ill patients were used to obtain pharmacokinetic parameters, including clearance, volume of distribution, and the elimination rate constant ( k e ) (see Supplement 1, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/206).23,24 First-order elimination equations were then used to determine C max and C min values for each dosing interval.
Discordance analysis was conducted using only the first trough level measured at steady state for each patient. The extrapolated C min value was then used in place of C trough to avoid variability related to timing of sampling. Paired C min and model-predicted AUC 24 /MIC values were categorized as falling below, within, or above their pharmacodynamic targets (15–20 mg/L and 400–600, respectively). Concordance was defined as both estimates falling within the same range relative to the respective pharmacodynamic targets (i.e., both below, both within, or both above target). Discordance was defined as paired estimates falling in different ranges relative to the respective pharmacodynamic targets (e.g., C min was within the therapeutic range, but the AUC 24 /MIC ratio was above or below the therapeutic range). This approach was thought to be clinically relevant, because discordance would result in different actions depending upon the method of monitoring used by the clinician.
Given that utilization of 2 vancomycin levels is considered the most accurate method of AUC determination, we identified cases in which 2 levels within a single dosing interval were available at any point during the course of therapy. In these cases, the AUC 24 /MIC was calculated using both the 2-point method and population pharmacokinetic models (Supplement 1).15,23,24 The AUC 24 /MIC values obtained using each method were then plotted, and the agreement between the 2 methods was determined using correlation statistics.
Predictors of treatment failure, nephrotoxicity, and discordance were determined using logistic regression analysis. Modifiable covariates included in the model for treatment failure were time to a therapeutic C trough level (≥ 15 mg/L), time to therapeutic AUC 24 /MIC (≥ 400), vancomycin dose (mg/kg), and initiation of vancomycin after the first positive culture result. Covariates included in the model for nephrotoxicity were the proportions of patients with C min values greater than 20 mg/L or AUC 24 /MIC greater than 600. Covariates included in the model for discordance were use of a vasopressor, serum creatinine at or above 100 μmol/L, and site of infection (bacteremia versus pneumonia). We employed a 10:1 rule for covariate inclusion, whereby 1 covariate could be included for every 10 events.
We identified 299 patients who had blood and/or respiratory cultures positive for MRSA between March 1, 2014, and December 31, 2018. A total of 128 patients met the inclusion criteria (Figure 1). Baseline and vancomycin treatment characteristics of these patients are summarized in Table 1. Of these 128 patients, 51 (40%) had MRSA bacteremia, whereas 77 (60%) had MRSA pneumonia. Rates of bacteremia and pneumonia were similar between the 2 participating sites. A higher proportion of patients in the pneumonia group than the bacteremia group were admitted to the ICU (43/77 [56%] versus 3/51 [6%]). The requirement for vasopressors was 31/77 (40%) among patients with pneumonia and 5/51 (10%) among those with bacteremia. Only 5 (4%) of the 128 patients received a loading dose of at least 25 mg/kg.
|
|
||
|
FIGURE 1 Flow diagram for patient inclusion. MRSA = methicillin-resistant Staphylococcus aureus . |
||
TABLE 1
Demographic and Vancomycin Treatment Characteristics
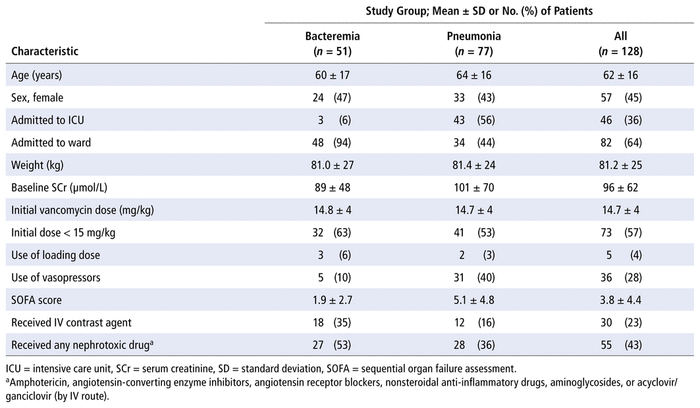
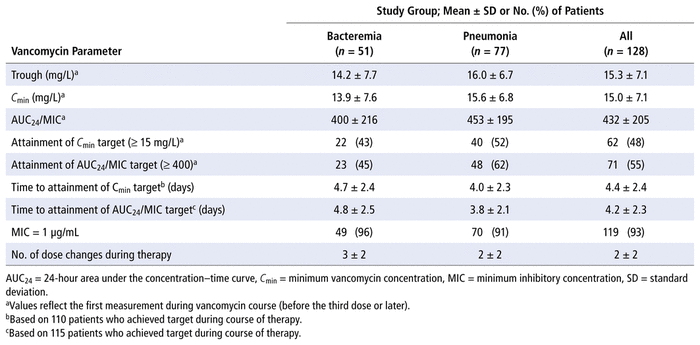
Table 2 summarizes therapeutic drug monitoring parameters for vancomycin. For all patients, the mean first steady-state value for C min was 15.0 (standard deviation [SD] 7.1) mg/L, and the corresponding mean AUC 24 /MIC value was 432 (SD 205). There were no instances in which 2 levels were available for AUC 24 /MIC determination for the first steady-state levels; therefore AUC 24 /MIC values were determined using only the population pharmacokinetic models for either critically ill patients ( n = 42) or non–critically ill patients ( n = 86). Of the 128 patients, 19 had multiple samples drawn for steady-state levels within a single dosing interval later during their course of therapy. For these patients, AUC 24 /MIC values calculated using both the 2-level method and the population pharmacokinetic models showed good correlation ( r 2 = 0.84; Figure 2).
TABLE 2
Parameters for Therapeutic Drug Monitoring of Vancomycin
|
|
||
|
FIGURE 2 Comparison of values for the ratio of area under the concentration–time curve to minimum inhibitory concentration (AUC/MIC), calculated using population pharmacokinetic models or using 2 vancomycin levels obtained during a single dosing interval ( r 2 = 0.84). For AUC/MIC values obtained using 2 levels, individual values for the elimination rate constant ( k e ) were calculated using first-order elimination kinetics (as described in the Methods). Once values for k e , maximum vancomycin concentration ( C max ), and minimum vancomycin concentration ( C min ) were determined, the corresponding AUC/MIC was calculated as described in the Methods. The plotted AUC/MIC values reflect exposures over various time intervals. |
||
The mean times to therapeutic C min (≥ 15 mg/L) and attainment of target AUC 24 /MIC (≥ 400) for the overall cohort were 4.4 (SD 2.4) and 4.2 (SD 2.3) days, respectively. Among the 128 initial MRSA-positive blood or respiratory cultures, 119 (93%) had an MIC of 1 μg/mL, whereas 8 (6%) and 1 (1%) had MIC values of 0.5 μg/mL and 2 μg/mL, respectively.
Results of the discordance analysis are presented in Figure 3. Among the 128 measured first steady-state trough levels, 27 (21%) exhibited discordance between C min and AUC 24 /MIC values. Of the 101 (79%) cases in which C min and AUC 24 /MIC values were found to be concordant, 72 (71%) were outside the therapeutic range. Figure 4 depicts good correlation between C min and AUC 24 /MIC values ( r 2 = 0.749).
|
|
||
|
FIGURE 3 Discordance analysis. Data are represented as number (%) of 128 patients. AUC 24 /MIC = ratio of 24-hour area under the concentration–time curve to minimum inhibitory concentration, C min = minimum vancomycin concentration. |
||
|
|
||
|
FIGURE 4 Discordance analysis ( r 2 = 0.749). AUC 24 /MIC = ratio of 24-hour area under the concentration–time curve to minimum inhibitory concentration, C min = minimum vancomycin concentration. |
||
Clinical outcomes are summarized in Table 3. Treatment failure occurred in 43 (34%) of the 128 patients. Treatment failure was more common in the group with pneumonia (35/77 [45%]) than the group with bacteremia (8/51 [16%]). Table 4 summarizes univariate analyses of treatment outcomes. Both initiation of vancomycin after the first positive culture result (odds ratio [OR] 4.41, 95% confidence interval [CI] 1.36–14.3) and time to attainment of target AUC 24 /MIC longer than 4 days (OR 3.48, 95% CI 1.39–8.70) were predictive of treatment failure according to logistic regression. Empiric dosing below 15 mg/kg (OR 1.06, 95% CI 0.46–2.42) and time to attainment of target C min longer than 4 days (OR 1.12, 95% CI 0.27–4.70) were not predictive of treatment failure.
TABLE 4
Univariate Analysis of Treatment Outcomes
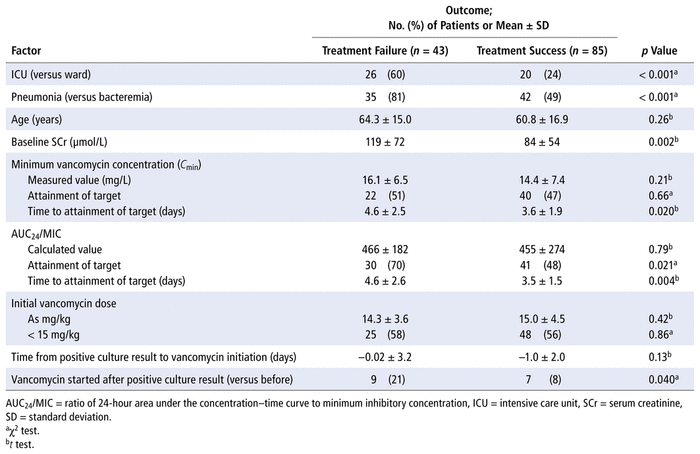
Nephrotoxicity was observed in 23 (18%) of the 128 patients, with similar frequency between the pneumonia and bacteremia groups. In a logistic regression model to determine predictors of nephrotoxicity, the proportions of patients with C min values greater than 20 mg/L (OR 0.74, 95% CI 0.20–2.69) or AUC 24 /MIC greater than 600 (OR 2.45, 95% CI 0.60–12.46) were included as covariates. Neither was found to be predictive of nephrotoxicity. Logistic regression did not identify any predictors of discordance associated with vasopressor usage (OR 0.81, 95% CI 0.29–2.21), serum creatinine at or above 100 μmol/L (OR 0.66, 95% CI 0.26–1.62), or infection site (bacteremia versus pneumonia) (OR 1.15, 95% CI 0.45–2.92) as covariates.
In this study, first steady-state trough levels were discordant with their corresponding AUC 24 /MIC values in 21% of cases. In the study cohort, treatment failure and nephrotoxicity rates were 34% and 18%, respectively. Although no clinical variables were found to predict discordance, we found that initiation of vancomycin after the first positive culture result and time to AUC 24 /MIC target attainment longer than 4 days were predictive of treatment failure. Notably, the average initial vancomycin dose was only 14.7 (SD 4) mg/kg, with only 4% of patients receiving a loading dose and 55% of patients reaching their AUC 24 /MIC target at the time of the first sample drawn for determination of steady-state vancomycin level. Collectively, the modifiable predictors of treatment failure described above highlight an opportunity to improve empiric dosing strategies by ensuring the timely initiation of appropriate therapy at adequate initial doses. These fundamental (and achievable) antimicrobial stewardship endeavours should precede any change in practice with respect to therapeutic drug monitoring of vancomycin. Whether it be the use of AUC 24 /MIC or the use of trough levels, the approach to drug monitoring will be hard pressed to improve patient outcomes without first optimizing the way in which vancomycin is prescribed.
Vancomycin trough levels have long been touted as an acceptable surrogate for the AUC 24 /MIC.21 However, published clinical studies describing the correlation between measured first steady-state trough levels and the corresponding calculated AUC 24 /MIC are limited, and the data that are available show variable degrees of correlation. Monte Carlo simulation studies by Patel and others7 demonstrated that trough values above 15 mg/L achieved through various dosing strategies are not required to consistently achieve AUC 24 /MIC values above 400. Similarly, simulation studies by Pai and others15 suggested poor correlation between trough levels and AUC 24 /MIC values, with an r 2 value of 0.409, which may be partly explained by MRSA isolates with MIC values greater than 1 mg/L. Jin and others25 compared AUC 24 /MIC values calculated using either creatinine clearance (as a surrogate for vancomycin clearance) or individual pharmacokinetic parameters with steady-state trough levels. These authors found that the AUC 24 /MIC values calculated using patient-specific pharmacokinetic parameters and commercial software showed a stronger correlation with trough levels ( r 2 = 0.964) than AUC 24 /MIC values calculated using creatinine clearance ( r 2 = 0.694). In a 2017 study exploring the relationship between the incidence of acute kidney injury and Bayesian-derived AUC 24 /MIC in MRSA bacteremia, Chavada and others26 found that 26.7% of patients with trough concentrations below 15 mg/L achieved AUC 24 /MIC values above 400. In that study, trough levels and AUC 24 /MIC values were found to be highly correlated ( r 2 = 0.88).26 In a retrospective study of 95 elderly patients receiving vancomycin, the correlation between Bayesian-derived AUC 24 and extrapolated C min levels was modest ( r 2 = 0.51).9 More than 30% of cases in which C min was below 15 mg/L actually achieved the AUC 24 target of 400.9
In the current study, C min and AUC 24 /MIC values were highly correlated, which suggests that using trough-based versus AUC 24 /MIC monitoring would have led to similar clinical decisions (i.e., dose adjustments) in the majority of cases. However, the observed rates of discordance were lower than anticipated, with a high proportion of vancomycin trough levels (44%; 56/128) falling below both C min and AUC 24 /MIC targets. The difference in the degrees of correlation between C min and AUC 24 /MIC values in our study compared with those described in the literature may be in part attributable to differences in methods for calculating AUC 24 /MIC. A higher degree of correlation might also be expected if there were a lower degree of variability in the distribution of MIC values. However, the proportion of isolates with MIC = 1 mg/L (93%) in the current study was consistent with other centres, and current guidelines advise that MIC of 1 mg/L be assumed in the event that the exact MIC value is not known.6 Considering that the average vancomycin dose was just below the recommended 15 mg/kg, these results suggest that our discordance analysis, and any subsequent relationship between target attainment and clinical outcomes, may be confounded by inadequate initial vancomycin dosing. We hypothesize that this is a consequence of the standard (one-size-fits-all) dosing strategies for vancomycin (and potentially dose rounding) that are commonly employed in our institution, rather than weight-based dosing.
The current accepted threshold for efficacy is AUC 24 /MIC above 400, with clinical data supporting this value limited primarily to single-centre retrospective analyses. In a recent meta-analysis, Dalton and others27 assessed the performance of the AUC 24 /MIC in predicting efficacy outcomes for MRSA infections. In addition to highlighting considerable heterogeneity in the study populations and methodologies of the included studies, the authors found that the sensitivity and specificity of the AUC 24 /MIC was suboptimal with respect to predicting efficacy outcomes. Furthermore, vancomycin efficacy thresholds in outcome assessment studies ranged from 211 to 667.27 When compared with our current study, in which we defined AUC 24 /MIC = 400 as the efficacy threshold, the variability in the literature suggests that optimal therapeutic efficacy thresholds are incompletely understood and depend on the type of MRSA infection and methods of both AUC 24 calculation and MIC determination. It is also important to consider that the median time to target attainment in our study was 4 days and that a time to attainment of target AUC 24 /MIC longer than 4 days was predictive of treatment failure. AUC 24 /MIC target attainment and its relationship with efficacy outcomes are reportedly dependent on achieving pharmacodynamic targets early (i.e., within days 1–2) in the course of therapy.20,28 In contrast, time to attainment of the C min target longer than 4 days did not predict treatment failure. Since C min and trough levels have been used as surrogates for the AUC 24 /MIC and have not been shown to be predictive of efficacy outcomes on their own, this result is not surprising. Our univariate analysis additionally showed that patients with treatment failure were more likely to have significantly higher baseline serum creatinine. It is possible that this is a marker of severity of illness, given that ICU admission was associated with treatment failure; however, it is also conceivable that clinicians take a more conservative approach to vancomycin dosing in patients with compromised renal function, though we note that patients with treatment failure were not more likely to have starting doses below 15 mg/kg relative to those with treatment success.
The main limitation of our study was that we were only able to determine the AUC 24 /MIC using one vancomycin level, while relying on previously published population-based pharmacokinetic models to estimate pharmacokinetic parameters such as the elimination rate constant and vancomycin clearance. Although the primary objective of our study was to determine discordance between first steady-state trough levels and AUC 24 /MIC values, we collected all vancomycin trough levels measured during each patient’s course of therapy and identified 19 instances in which multiple samples for postdistributional vancomycin levels were drawn within a single dosing interval, which allowed for comparison of AUC 24 /MIC values calculated using 2 levels and via population pharmacokinetic models, as described in the Methods. Although calculation of the AUC using 2 vancomycin levels allows determination of patient-specific pharmacokinetic parameters, the high degree of correlation between the 2 methods in our study supports our use of population pharmacokinetic models in the absence of multiple vancomycin levels. Additionally, the methods of AUC determination in our study have precedents in the literature29 and likely provide a more accurate estimation than simply calculating the AUC based on dividing the vancomycin dose by clearance, as has been used in numerous outcome studies that have informed the current AUC 24 /MIC therapeutic thresholds.30–32
Although the latest vancomycin guidelines for therapeutic drug monitoring recommend AUC 24 /MIC above 400 as the pharmacodynamic target for efficacy in cases of serious MRSA infection, the association between achievement of this threshold and improvement in patient outcomes depends on ensuring both appropriate selection of the empiric antibiotic and adequate initial dosing. Our findings suggest that the relationship between vancomycin monitoring and outcome is confounded by inadequate empiric dosing, which highlights an opportunity to improve vancomycin dosing strategies to ensure that therapeutic targets are achieved as soon as possible. In light of the new vancomycin drug monitoring guidelines, it is imperative to ensure that the approach to empirical dosing is optimized before any attempt to modify practice with respect to vancomycin monitoring.
1 Lodise TP, McKinnon PS. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2005;52(2):113–22.
Crossref PubMed
2 Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–74.
Crossref
3 Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–81.
Crossref
4 Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55.
Crossref
5 Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–9.
Crossref
6 Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–64.
Crossref
7 Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. Vancomycin: we can’t get there from here. Clin Infect Dis. 2011;52(8): 969–74.
Crossref
8 Hale CM, Seabury RW, Steele JM, Darko W, Miller CD. Are vancomycin trough concentrations of 15 to 20 mg/L associated with increased attainment of an AUC/MIC ≥ 400 in patients with presumed MRSA infection? J Pharm Pract. 2017;30(3):329–35.
Crossref
9 Bel Kamel A, Bourguignon L, Marcos M, Ducher M, Goutelle S. Is trough concentration of vancomycin predictive of the area under the curve? A clinical study in elderly patients. Ther Drug Monit. 2017; 39(1):83–7.
Crossref
10 Neely MN, Kato L, Youn G, Kraler L, Bayard D, van Guilder M, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042–17.
Crossref
11 van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734–44.
Crossref PMC
12 Wong-Beringer A, Joo J, Tse E, Beringer P. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents. 2011;37(2):95–101.
Crossref
13 DeRyke CA, Alexander DP. Optimizing vancomycin dosing through pharmacodynamic assessment targeting area under the concentration-time curve/minimum inhibitory concentration. Hosp Pharm. 2009; 44(9):751–65.
Crossref
14 Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58(1):309–16.
Crossref PMC
15 Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–7.
Crossref
16 Gregory ER, Burgess DR, Cotner SE, VanHoose JD, Flannery AH, Gardner B, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned. J Pharm Pract. 2019;33(6):774–8.
Crossref PubMed
17 Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant Staphylococcus aureus infection. J Pharm Pract. 2019;32(4):442–6.
Crossref
18 Heil EL, Claeys KC, Mynatt RP, Hopkins TL, Brade K, Watt I, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986–95.
Crossref
19 Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52(9):3315–20.
Crossref
20 Lodise TP, Drusano GL, Zasowski E, Dihmess A, Lazariu V, Cosler L, et al. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis. 2014;59(5):666–75.
Crossref
21 Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98.
Crossref
22 Endre ZH. Acute kidney injury: definitions and new paradigms. Adv Chronic Kidney Dis. 2008;15(3):213–21.
Crossref PubMed
23 Llopis-Salvia P, Jimenez-Torres NV. Population pharmacokinetic parameters of vancomycin in critically ill patients. J Clin Pharm Ther. 2006;31(5):447–54.
Crossref
24 Yamamoto M, Kuzuya T, Baba H, Yamada K, Nabeshima T. Population pharmacokinetic analysis of vancomycin in patients with gram-positive infections and the influence of infectious disease type. J Clin Pharm Ther. 2009;34(4):473–83.
Crossref PubMed
25 Jin SJ, Yoon JH, Ahn BS, Chung JA, Song YG. Underestimation of the calculated area under the concentration-time curve based on serum creatinine for vancomycin dosing. Infect Chemother. 2014;46(1):21–9.
Crossref PubMed PMC
26 Chavada R, Ghosh N, Sandaradura I, Maley M, Van Hal SJ. Establishment of an AUC0–24 threshold for nephrotoxicity is a step towards individualized vancomycin dosing for methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2017;61(5): e02535–16.
Crossref PubMed PMC
27 Dalton BR, Rajakumar I, Langevin A, Ondro C, Sabuda D, Griener TP, et al. Vancomycin area under the curve to minimum inhibitory concentration ratio predicting clinical outcome: a systematic review and meta-analysis with pooled sensitivity and specificity. Clin Microbiol Infect. 2020;26(4):436–46.
Crossref
28 Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54(5):621–9.
Crossref
29 Zelenitsky S, Rubinstein E, Ariano R, Iacovides H, Dodek P, Mirzanejad Y, et al. Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int J Antimicrob Agents. 2013;41(3):255–60.
Crossref
30 Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925–42.
Crossref
31 Kullar R, Leonard SN, Davis SL, Delgado G Jr, Pogue JM, Wahby KA, et al. Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15–20 mg/L suggested by the vancomycin consensus guidelines. Pharmacotherapy. 2011;31(5):441–8.
Crossref
32 Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O’Sullivan MV, et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57(4):1654–63.
Crossref PubMed PMC
Competing interests: None declared. ( Return to Text )
Note: This article contains supplementary material, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/206 ( Return to Text )
Funding: None received. ( Return to Text )
The authors would like to acknowledge the contributions of Dr Rakesh Patel to the development of the research protocol for this study.
Canadian Journal of Hospital Pharmacy , VOLUME 74 , NUMBER 4 , Fall 2021